Abstract
Innate immune receptors like pattern recognition receptors (PRRs) including toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD) like-receptors (NLR) on immune cells play an important role in initiating inflammatory responses to damage- and pathogen- associated molecular patterns (DAMPs and PAMPs) expressed on invading pathogens or released from damaged cells. Although it is well known that DAMPs directly modulate innate immune functions, it is less clear whether DAMPs directly regulate T cell intrinsic function. Members of the sialic acid binding Ig-like lectin (Siglec) family have immunoreceptor tyrosine-based inhibitory motifs (ITIM) or ITIM-like regions in their intracellular domain that negatively regulate immune activation induced by DAMPs. Our previous data suggested that the Siglec- G-CD24 interaction in host APCs plays an important role in the negative regulation of graft-versus host (GVH) responses. However, the T cell autonomous role of Siglec-G in the regulation of T cell responses is not known. Because Siglecs are important negative regulators of immune responses, we tested the hypothesis that the deficiency of Siglec-G in donor T cells would enhance GVHD.
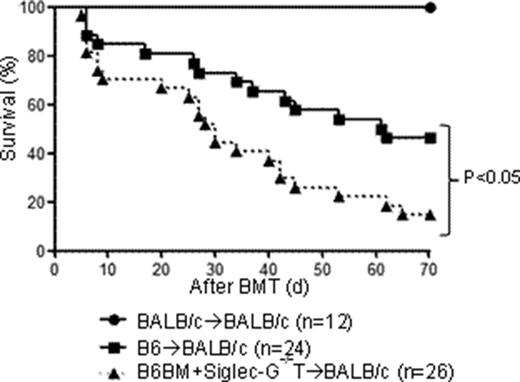
To test our hypothesis, we first examined detailed phenotypic analysis of various T cell subsets and activation markers in naïve Siglec-G-/- and wild-type (WT) B6 animals and found similar distribution of naïve, memory, effector and regulatory T cells. In order to examine whether the absence of Siglec-G in donors affects GVHD, WT-BALB/cmice were lethally irradiated (850cGy) and transplanted on day 0 with 5x106 bone marrow and 0.5x106 splenic CD90+ T cells from either syngeneic WT-BALB/c, allogeneic MHC-mismatched WT-B6 or Siglec-G-/- animals. The recipients receiving donor T cells from Siglec-G-/- animals showed a significantly worse survival compared to allogeneic WT-B6 animals (p<0.05). This increased mortality was also associated with more severe GVHD damage in target organs and a higher expansion of activated CD69+, IFN-r+, and IL-17A+ donor T cells in the spleen and target organs. Enhanced GVHD mortality and severity was also observed in MHC mismatched haploidentical matched B6 in to F1models (p<0.05).
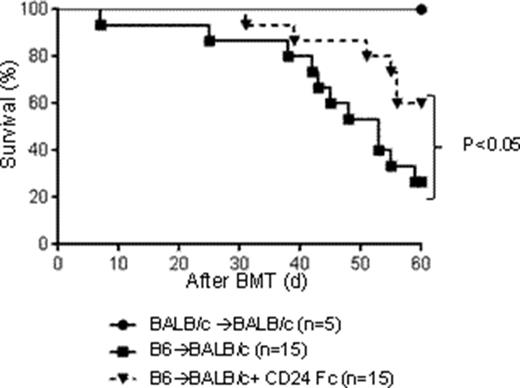
To explore the mechanism, we tested whether Siglec-G deficiency alters the naïve T cell responses in vitro after allogeneic or non-specific TCR stimulation in the absence of exogenous DAMPs. Interestingly Siglec-G-/- T cells showed similar proliferation in vitro, when compared to WT B6 T cells. In addition, Siglec-G-/- Tregs are equally suppressive in suppression assay and Siglec-G-/- T cells showed severe GVHD even Tregs are depleted in allo-BMT. However, Siglec-G-/- T cells showed a higher proliferation after direct TCR stimulation (CD3/CD28) with addition of DAMP (HMGB-1) when compared to WT T cells in vitro, suggesting direct T cell intrinsic effects. Consistent with this result, allogeneic Siglec-G-/- T cells caused similar mortality compared to WT controls in non-irradiated B6 into F1 model due to the absence of DAMPs from conditioning. To test the critical cellular mechanisms, we examined the function of endogenous Siglec-G ligand, CD24. We utilized BALB/c CD24-/- animals as hosts in same BMT model and found that CD24-/- animals showed an enhanced GVHD mortality and severity when compared to WT animals (p<0.05). To enhance Siglec-G-CD24 axis, we utilized a novel CD24 fusion protein (CD24Fc) in same BMT model and found that CD24 Fc ameliorated GVHD severity and mortality in not only allogeneic WT-B6 animals (p<0.05) but also CD24-/- animals (p<0.05). Next we explored DAMPs regulation by Siglec-G-CD24 axis in GVL. We utilized the same model of CD24Fc treatment but added P815 at the same time of allo-BMT and found that CD24Fc treated animals showed equivalent GVL to non-treated animals, suggesting that regulation of DAMPs with CD24Fc mitigates GVHD with maintaining GVL effect.
Collectively our data suggested that the expression of both Siglec-G on donor T cells and CD24 on hosts is critical for controlling GVHD in the context of DAMPs released from conditioning, and represents a novel strategy that CD24Fc can mitigates GVHD with maintaining GVL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal