Abstract
Introduction: Many older adults (≥ 60) with AML treated with intensive or non-intensive chemotherapy have a poor prognosis and spend a significant portion of their life from diagnosis until death in the hospital. We hypothesized that the burden of illness, rather than its treatment, plays a major role in increasing health care utilization. Therefore, we examined health care utilization and end-of-life (EOL) outcomes for older adults with AML receiving supportive care alone.
Methods: We conducted a retrospective analysis of 98 consecutive older patients diagnosed with AML between 9/9/1993 and 12/23/2011 and treated with supportive care alone at two tertiary care hospitals. We examined their health care utilization including frequency of hospitalizations, clinic visits, and intensive care unit admissions and their place of death. We also assessed health care utilization during the last 30 days of life including palliative care consultations, hospice referrals, hospitalizations, and blood transfusions.
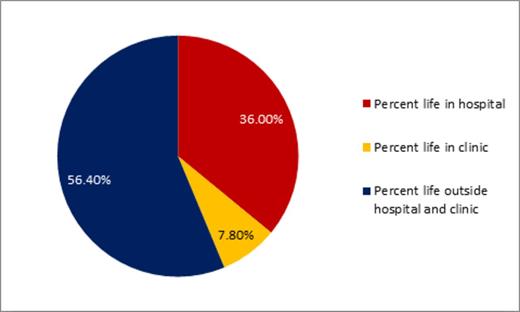
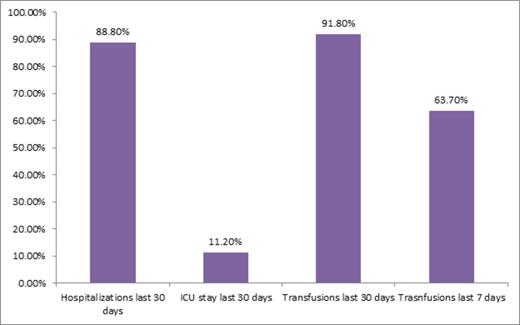
Results: The study included mostly white (n= 93/98, 95%) and male (n=52/98, 53%) patients with a median age of 77 (range 60-94). Supportive care treatments included transfusions (n=59/98, 60%), hydroxurea and transfusions (n=23/98, 24%), hydroxyurea without transfusion support (n=9/98, 9%), transfusions and growth factor support (n=7/98, 7%), or growth factor support alone (n=1/98, 1%). The median number of hospitalizations for the entire cohort was 1 (range 0-5). All patients died with a median number of days from diagnosis until death of 36.0 days (range 1-389). Patients spent a mean of 36% of their life from diagnosis in the hospital and 8% of their life attending outpatient clinic appointments. Only 18% (n=18/98) and 30% (n=29/98) of patients utilized palliative care or hospice services, respectively. A minority of patients (n=13/98, 13%) were admitted to the intensive care unit with 11% (n=11/98) having an intensive care unit stay during the last 30 days of life. Within 30 days of death, 88% (n=87/98) of patients were hospitalized with 46% (N=45/98) dying in the hospital. The majority (n=89/98, 92%) of patients received a blood transfusion within the last 30 days of life and 64% (n=58/91) received a transfusion in the last 7 days of life.
Conclusion: Despite a decision to not receive chemotherapy, older patients with AML receiving supportive care alone have high health care utilization reflecting the burden of this disease. Despite this populations' poor prognosis, palliative care and hospice services are rarely utilized. Future work should study novel health-care delivery models designed to meet the needs of this population, such as transfusion support and other intensive supportive care measures at home toward the EOL.
LeBlanc:Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees; Flatiron: Consultancy; Epi-Q: Consultancy; Helsinn Therapeutics: Honoraria, Research Funding. Steensma:Incyte: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Onconova: Consultancy. Fathi:Seattle Genetics: Other: Advisory Board participation, Research Funding; Agios Pharmaceuticals: Other: Advisory Board participation; Merck: Other: Advisory Board participation. DeAngelo:Celgene: Consultancy; Amgen: Consultancy; Ariad: Consultancy; Bristol Myers Squibb: Consultancy; Agios: Consultancy; Incyte: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Stone:Merck: Consultancy; Agios: Consultancy; AROG: Consultancy; Roche/Genetech: Consultancy; Pfizer: Consultancy; Karyopharm: Consultancy; Sunesis: Consultancy, Other: DSMB for clinical trial; Abbvie: Consultancy; Celator: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Novartis: Research Funding; Juno: Consultancy. Chen:Bayer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal