Abstract
Introduction: Patients' individual preferences for specific treatment attributes are an important factor to consider in treatment decisions. This area of research is relatively underexplored for patients with multiple myeloma (MM).
Aims: To understand MM patients' strength of preference for method of administration and for avoiding specific adverse events (AEs).
Methods: AEs were selected from trials of MM treatments used globally across the disease course: lenalidomide (FIRST, MM-009/010), bortezomib (VISTA, APEX, MMY-3021), thalidomide (IFM 99-06), pomalidomide (MM-003), and carfilzomib (PX-171-003, -004, -005). AEs selected for evaluation were narrowed down to 12, based on discussions with MM patients, from a list of hematologic and non-hematologic AEs with a grade 3/4 incidence > 5% and the greatest difference in rate of occurrence across trials: bone pain, febrile neutropenia, hypokalemia, hyponatremia, infection, lymphopenia, neuralgia, neutropenia, peripheral neuropathy, renal adverse reaction, and thrombocytopenia and thromboembolic events. MM patients were recruited to complete an online survey. Following an introductory tutorial, patients completed 14 discrete choice cards on which they selected their preferred option between 2 hypothetical treatments with varying combinations of AEs (absent/present), route of administration (oral, subcutaneous [SC], intravenous [IV]), and progression-free survival (PFS; 22, 24, or 26 months, based on evidence of first-line MM treatment). Results were expressed as odds ratios (ORs) and coefficients. Strength of preference was converted into a willingness to trade (WTT) PFS months to receive preferred choice of treatment.
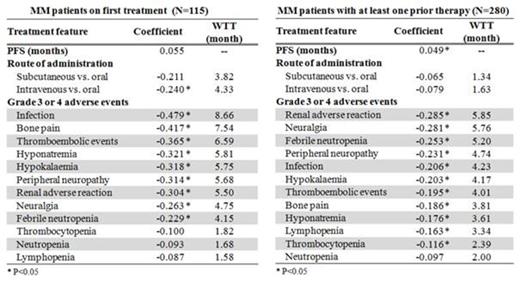
Results: Four hundred patients from 8 countries participated in the survey: Canada (13; 3.3%), Denmark (9; 2.3%), France (68; 17.0%), Germany (65; 16.3%), Italy (89; 22.3%), Spain (81; 20.3%), Sweden (11; 2.8%), and the United Kingdom (64; 16.0%). Of the respondents, 28.8% were on their first treatment, 70.0% of patients reported having switched treatment. The majority (58.7%) were male, with a mean age of 40 years. Patients showed a preference for oral vs IV administration (OR, 0.875 [95% CI, 0.78-0.98]; P = .020), and there was a trend toward preferring oral over SC administration (OR, 0.897 [95% CI, 0.80-1.01]; P = .067). Strength of preference declined in patients with prior treatments. Patients expressed a statistically significant preference (P < .01) to avoid (OR < 1) all presented grade 3/4 AEs, except for hematologic AEs: thrombocytopenia (OR [P value]: 0.904 [.23]), neutropenia (0.911 [.30]), and lymphopenia (0.916 [.39]) for first treatment patients, and neutropenia (0.907 [.08]) for patients with prior therapy. The relative importance of bone pain, infection, and thromboembolic events was lower in patients with prior therapies, while the relative importance of grade 3/4 neuralgia, febrile neutropenia, and renal adverse reaction increased. The table shows patient preferences as coefficients, and by months of PFS WTT. Example: Patients on their first treatment would be WTT 4.33 mos of PFS to receive oral vs IV administration.
Conclusions: Study results display important findings concerning preferences of younger, working-age MM patients on individual AEs and methods of administration. Patients expressed smaller preference for avoiding hematologic AEs, such as neutropenia, lymphopenia, and thrombocytopenia, and an increasing relative importance to avoiding some symptomatic AEs (eg, neuropathy, neuralgia, renal adverse reaction, and febrile neutropenia) over the course of their disease. Patient preference should be considered when making treatment decisions. Future analyses could explore subgroups based on demographics and disease history, including prior AEs.
Leleu:Amgen: Patents & Royalties; Novartis: Honoraria; Celgene Corporation: Honoraria; Janssen: Honoraria; BMS: Honoraria. Mateos:Janssen-Cilag: Consultancy, Honoraria; Onyx: Consultancy; Celgene: Consultancy, Honoraria; Takeda: Consultancy. Delforge:Novartis: Honoraria; Celgene Corporation: Honoraria; Janssen: Honoraria; Amgen: Honoraria. Lewis:Celgene Corporation: Employment, Equity Ownership. Schindler:Celgene Corporation: Employment, Equity Ownership. Gibson:Celgene Corporation: Employment, Equity Ownership. Yang:Analysis Group: Employment. Weisel:Amgen: Consultancy, Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novartis: Other: Travel Support; Onyx: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Other: Travel Support; Janssen Pharmaceuticals: Consultancy, Honoraria, Other: Travel Support, Research Funding; Noxxon: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal