Abstract
Introduction: Novel treatments have significantly improved outcomes for multiple myeloma (MM) patients, and HRQoL is an increasingly important endpoint to measure outcomes. This study explores patients' HRQoL in Relapsed/Refractory MM (RRMM) patients receiving second or third line lenalidomide or bortezomib treatment and assesses HRQoL changes from baseline for patients completing the study and those discontinuing.
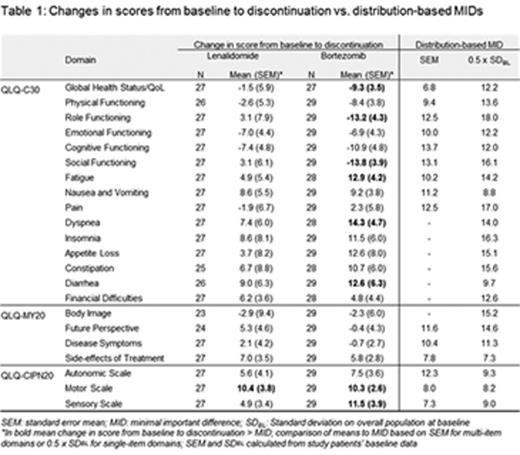
Methods: A multicenter, observational 6-month study was conducted in Italy, Germany, France, UK, Ireland, and Belgium in RRMM patients starting second- or third-line treatment with bortezomib or lenalidomide. HRQoL/functioning and symptoms of patients were measured via 3 EORTC patient questionnaires and changes from baseline were assessed at month 6 and study discontinuation. Clinical significance in HRQoL score changes was assessed against distribution-based minimal important differences (MID; Table). HRQoL domains considered included 15 domains from EORTC Quality of Life Core Questionnaire (QLQ-C30), 4 from EORTC QLQ-Multiple Myeloma (QLQ-MY20), and 3 from EORTC QLQ-Chemotherapy-Induced Neuropathy (CIPN20). Domain scores ranged from 0 to 100. For functional domains, higher HRQoL/functioning scores indicated better HRQoL/functioning; for symptom domains, higher symptom scores indicated greater symptom burden.
Results: A total of 258 patients (mean age, 70 yrs; 54% male) were included in the study by 33 sites from Dec 2010 to July 2014. At baseline, the median time since diagnosis was 2.8 years for lenalidomide and 3.9 years for bortezomib; an ECOG performance status >2 was reported in 6.2% of lenalidomide patients (n=10) vs 3.1% of bortezomib (n=3); 8.9% (n=23) reported starting third line treatment (lenalidomide: 6.2% (n=10); bortezomib: 13.5% (n=13)). Whereas 5.6% (n = 9) of patients had received dialysis prior to entering the lenalidomide cohort, none had received dialysis in the bortezomib cohort. Chronic heart failure at baseline was more often observed for lenalidomide (14.8%, n=24) vs bortezomib (8.3%, n=8). EORTC questionnaires were completed by 251 (97.3%), 137 (53.1%), and 56 (21.7%) of patients at baseline, month 6, and study discontinuation, respectively. Out of 162 pts receiving LEN, 64 (39.5%) discontinued the study before 6 months, 6.2% (n=10) due to disease progression, 16.0% (n=26) due to treatment discontinuation, and 17.3% (n=28) due to other reasons (including death, lost to follow-up, withdrawn consent). Out of 96 patients receiving bortezomib, 53 (55.2%) discontinued the study: 10.4% (n=10) due to disease progression, 27.1% (n=26) due to treatment discontinuation, and 17.7% (n=17) due to other reasons (including death, lost to follow-up, withdrawn consent).
At study completion (month 6), HRQoL reductions from baseline were observed for only 1 of 22 domains in each cohort: mean change (SEM) of 10.9 (2.8) for Diarrhoea domain in the lenalidomide cohort and -8.5 (3.5) for Global Health Status/QoL domain in the bortezomib cohort, indicating relative stability of HRQoL in patients on continued treatment within the 2 cohorts. For patients who discontinued the study prior to 6 months due to disease progression or treatment discontinuation, clinically meaningful declines in HRQoL exceeding the MID were more often observed in the bortezomib cohort (8 of 22 domains), than in the lenalidomide cohort (1 of 22 domains) (Table 1).
Conclusions: This observational study showed that HRQoL could be maintained under continued treatment in both the lenalidomide and bortezomib cohorts. However, higher progression and treatment discontinuation rates were observed in the bortezomib cohort and study discontinuation was often associated with clinically meaningful deteriorations in HRQoL for bortezomib-treated patients, but not for lenalidomide-treated patients.
The findings of this study are of potential relevance to future MM studies, both in the relapsed/ refractory and newly diagnosed setting. HRQoL of patients with early discontinuation has not always been reported separately from patients who discontinued fixed duration treatment in MM studies. Future studies should address the question of HRQoL separately at early discontinuation, for all current and future treatment alternatives approved in MM.
Kyriakou:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Murphy:Celgene: Honoraria. Petrucci:Celgene: Honoraria; Janssen-Cilag: Honoraria; Amgen: Honoraria; Mundipharma: Honoraria; BMS: Honoraria. Bacon:Celgene Corporation: Employment, Equity Ownership. Lewis:Celgene Corporation: Employment, Equity Ownership. Gilet:Celgene: Consultancy. Arnould:Celgene: Consultancy. Vande Broek:Celgene: Consultancy; Roche: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Leleu:Janssen: Honoraria; Novartis: Honoraria; Celgene: Honoraria; BMS: Honoraria; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal