Abstract
Background. Risk assessment in chronic lymphocytic leukemia (CLL) is determined by the presence/absence of several negative prognostic factors, including the unmutated (UM) IGHV mutational status, TP53 deregulation by mutation and/or deletion of the 17p (17p-) chromosome, and high CD49d expression. Nevertheless, clinical heterogeneity can be observed also in the context of low-risk CLL, identified according to the absence of the aforementioned negative prognosticators. Thus, identifying novel markers that may predict an indolent clinical course in the context of low-risk CLL cases can be of key clinical relevance. The CD150/SLAMF1, CD305/LAIR1, and CD307b/FCRL2 molecules have been independently reported as molecules associated with a mutated IGHV status, and with longer time to first treatment in CLL. However, the prognostic relevance of the combined CD150/CD305/CD307b expression in predicting overall survival (OS) in the context of low-risk CLL remains to be explored.
Aim. To assess the prognostic value of CD150, CD305, CD307b as OS predictors in the context of low-risk CLL, as defined according to the canonical prognostic factors.
Methods. The study included 330 CLL cases all characterized for Rai stage (stages 0-I: 254 cases), CD49d expression (CD49d- CLL, <30% of positive cells by flow cytometry: 191), IGHV mutational status (mutated, M: 191), karyotype abnormalities according to the hierarchical stratification (normal/13q-/+12: 206 cases), all tested at diagnosis. Median follow-up of patients was 77 months with 67 deaths. Immunophenotypic analysis was performed in thawed samples using a combination of anti-CD5 FITC, -CD19PE-Cy7, -CD150PE, -CD305PerCPCy5.5, CD307bAPC mAbs, and DAPI to exclude dead cells. Both percent of positive cells and mean fluorescence intensity (MFI) data were recorded.
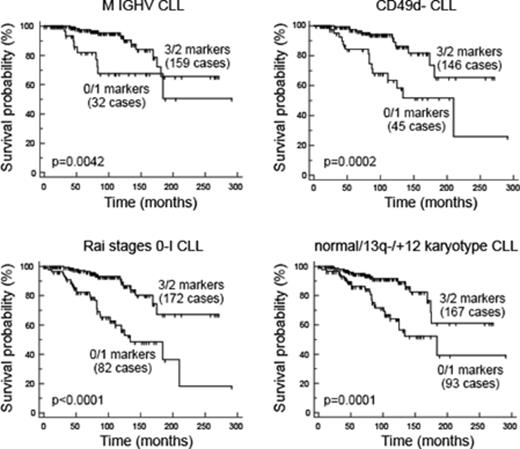
Results. A significantly higher (p<0.0001) CD150, CD305 and CD307b expression in M versus UM CLL was documented, with mean % of expression/MFI of 65%/498MFI vs. 25%/160MFI (CD150), 60%/1208MFI vs. 33%/583MFI (CD305), 87%/495MFI vs. 76%/383MFI (CD307b). The best cut-off levels for OS, calculated using a ROC analysis, were: 50%/290MFI for CD150; 10%/230MFI for CD305; 80%/360MFI for CD307b. Given the overall concordance in the definition of positive cases by using MFI or % values, the latter was chosen for further analyses. Using the % cut-offs, 154 (46.7%), 234 (70.9%) and 212(64.2%) were classified positive for CD150, CD305 and CD307b respectively. The clinical impact of the three markers as OS predictors was confirmed in both univariate (hazard ratio/confidence interval (HR/CI)= 0.20/0.11-0.37; p<0.0001 for CD150; HR/CI= 0.40/0.25-0.65; p=0.0002 for CD305; HR/CI= 0.27/0.16-0.44; p<0.0001 for CD307b), and multivariate (HR/CI=0.34/0.17-0.66; p=0.0015 for CD150; HR/CI=0.47/0.29-0.76; p=0.0023 for CD305;HR/CI=0.42/0.24-0.73; p=0.0022 for CD307b) analyses. Therefore, we combined their expression in a 0 (all the three markers below the cut-off) to 3 (all the three markers above the cut-off) score, and dichotomized CLL cases according to the expression of 3/2 markers (n=205) versus 0/1 markers (n=125). The prognostic impact of this combined markers expression was tested in univariate analysis (HR/CI=0.24/0.14-0.40; p<0.0001 ) and in a multivariate model including: IGHV mutational status, CD49d expression, Rai stage (stage 0-I versus stages II-IV), karyotype abnormalities (normal/del13/+12 versus del11/del17). The combined markers expression retained its prognostic impact (HR/CI=0.47/0.26-0.87; p=0.0172), along with the UM IGHV (HR/CI=2.66/1.44-4.89; p=0.0018), CD49d+ expression (HR/CI=2.14/1.25-3.67; p=0.0058) del11/del17 (HR/CI=2.08/1.23-3.50; p=0.0063). Moreover, expression of ≥2 markers was associated with a better prognosis in the context of the M IGHV (p=0.0042), CD49d- (p=0.0002), Rai 0-I stage (p<0.0001) and normal/del13/+12 karyotype (p=0.0001) groups (see Figure).
Conclusions: High expression of at least two of the CD150, CD305 and CD307b molecules predicts longer OS in CLL, also in the context of low-risk prognostic categories. A synergic effect of the CD150, CD305 and CD307b molecules, all inhibitors of the B-cell receptor signaling, may be taken into account to functionally explain this peculiar clinical behavior.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal