Abstract
Background: Karyotype classification is one of the strongest independent prognostic indicators in AML. The majority of recurring chromosomal aberrations are associated with an individual prognosis, other less frequent like the Del (20q), have been minimally evaluated and classified as intermediate risk in AML. Multiple studies established isolated 20q deletion as a good prognostic marker in MDS, with lower AML transformation rates and longer median overall survival (OS) in comparison with complex 20q deletion.
Objective: The aim of this study is to determine the frequency and the impact on outcome of 20q deletion alone or with additional cytogenetic abnormalities in adult patients with AML.
Patients and Methods: AML patients with chromosome 20 abnormalities were identified between 2000 and 2012 through the MD Anderson Cancer Center AML database (n=1741). Collected data included baseline demographics, number and type of additional cytogenetic abnormalities, disease characteristics, treatment and outcome. OS was defined as time from hematological diagnosis to death or last follow-up and relapse-free survival (RFS) was measured from time of hematological response to relapse. The Kaplan-Meier product limit method was used to estimate overall survival and the log-rank tests were employed for statistical comparisons between the OS curves.
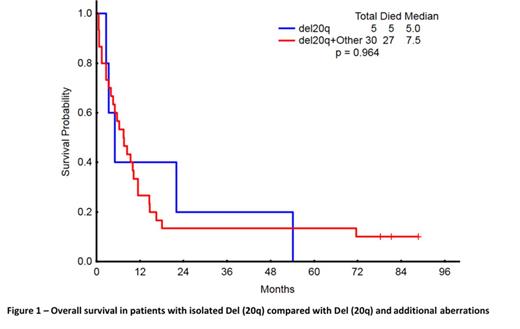
Results: From a total of 1741 adult AML patients, we identified 35 with Del (20q), representing 2% of our cohort. The distribution of cytogenetic abnormalities was as follows: isolated Del (20q) in 5 (14%), +8 in 3 (9%), +8 complex in 2 (6%), -5 complex in 8 (23%), -7 complex in 5 (14%), -7 not complex in 1 (3%), -5 and -7 complex in 6 (17%), other complex in 1 (3%), and other not complex in 4 (11%). Patients with Del (20q) were older (p=0.04), with lower bone marrow blast numbers (p<0.001), and lower WBC (p=0.001) compared to patients without Del (20q) (Table 1). Median RFS and OS for patients with Del (20q) were 16.8 and 7.5 months (mos), respectively. Objective response rates were 43% and 65% for patients with and without Del (20q), respectively (p=0.04) and the CR rates were 36% and 58%, respectively (p=0.01). Significant benefit was observed for OS in patients without Del (20q) (13.5 mos; 95% CI, 13.45-13.49; p=0.011), but not in RFS (19.52 mos; 95% CI, 19.48-19.55; p=0.376) in comparison with patients with Del (20q) (16.8 mos; 95% CI, 16.41-17.23; and 7.5 mos; 95% CI, 7.26-7.73). Patients with Del (20q) were compared to the remaining patients with leukemia classified as unfavorable cytogenetic status; the median survival for Del (20q) patients was similar by OS (OS 6.9 mos, 95% CI, 6.82-6.91). On the other hand, patients with Del (20q) had a significantly decreased overall survival (7.5 mos; 95% CI, 7.26-7.73, p=0.002) in comparison to patients with normal karyotype (17.7 mos; 95% CI, 17.64-17.71). No difference in survival was observed between patients with isolated Del (20q) and those with additional cytogenetic abnormalities: the median OS were 5 and 7.5 mos, respectively (p=0.964) (Figure 1).
Conclusion: Our data demonstrated that Del (20q) occurs in 2% of previously untreated AML patients, with around 63% of these patients showing complex karyotype. Patients with Del (20q) have lower response rate and worse outcome, similar to patients with unfavorable cytogenetics.
Clinical descriptors, hematologic parameters and outcome of each set of patients
| . | Del 20q in Karyotype . | All others non-Del 20q . | P . |
|---|---|---|---|
| N = 35 | N = 1706 | ||
| Age (y), median (range) | 65 (35-83) | 61 (12-89) | 0.04 |
| Baseline hematologic data median (range) | |||
| WBC × 109/L | 2.7 (0.7-32.6) | 5.8 (0.3-433) | 0.001 |
| Hemoglobin, g/dL | 8.1 (5.6-14.2) | 8.7 (2-93.3) | 0.29 |
| Platelet count, × 109/L | 40 (7-254) | 49 (2-676) | 0.21 |
| Neutrophil | 29 (4-88) | 16 (0-94) | <0.001 |
| PB blasts | 9 (0-50) | 16 (0-99) | 0.02 |
| BM blasts | 30 (7-98) | 47 (0-99) | <0.001 |
| Treatment Response and Survival | |||
| Prior Chemo/XRT | 7 | 301 | 0.72 |
| CR | 13 (37%) | 990 (58%) | 0.01 |
| CRp | 3 (9%) | 88 (5%) | 0.03 |
| Cri | 0 | 18 (1%) | - |
| RFS median, mo (95% CI) | 17.22 (16.81-17.62) | 25.59 (25.56-25-62) | 0.55 |
| OS median, mo (95% CI) | 7.49 (7.26-7.73) | 13.47 (13.45-13.49) | 0.01 |
| . | Del 20q in Karyotype . | All others non-Del 20q . | P . |
|---|---|---|---|
| N = 35 | N = 1706 | ||
| Age (y), median (range) | 65 (35-83) | 61 (12-89) | 0.04 |
| Baseline hematologic data median (range) | |||
| WBC × 109/L | 2.7 (0.7-32.6) | 5.8 (0.3-433) | 0.001 |
| Hemoglobin, g/dL | 8.1 (5.6-14.2) | 8.7 (2-93.3) | 0.29 |
| Platelet count, × 109/L | 40 (7-254) | 49 (2-676) | 0.21 |
| Neutrophil | 29 (4-88) | 16 (0-94) | <0.001 |
| PB blasts | 9 (0-50) | 16 (0-99) | 0.02 |
| BM blasts | 30 (7-98) | 47 (0-99) | <0.001 |
| Treatment Response and Survival | |||
| Prior Chemo/XRT | 7 | 301 | 0.72 |
| CR | 13 (37%) | 990 (58%) | 0.01 |
| CRp | 3 (9%) | 88 (5%) | 0.03 |
| Cri | 0 | 18 (1%) | - |
| RFS median, mo (95% CI) | 17.22 (16.81-17.62) | 25.59 (25.56-25-62) | 0.55 |
| OS median, mo (95% CI) | 7.49 (7.26-7.73) | 13.47 (13.45-13.49) | 0.01 |
Chahoud:American Society of Hematology (ASH): Other: 2015 HONORS Award recipient. Off Label Use: Inotuzumab.. Cortes:Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; BerGenBio AS: Research Funding; Teva: Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal