Abstract
Introduction: T-cell lymphomas are lymphoid malignancies with aggressive clinical course and poor prognosis. The etiology remains to be elucidated, and no uniformed therapeutic strategies have been achieved. Increasing evidence has shown that aberrant activation of signal transducer and activator of transcription-3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3) may be associated with the development of T-cell lymphomas. The Hedgehog (Hh) pathway, aberrantly activated in a number of tumors, has been extensively studied with respect to its interaction with other oncogenic pathways. It has been established that inhibition of Hh pathway has antitumor efficacy in T-cell lymphoma cells. Gli1 inhibitor GANT61 is a promising Hh pathway inhibitor against cancer therapy. We speculated that GANT61 has antitumor activity for T-cell Lymphoma cells and the activity is associated with down-regulation of phosphorylated STAT3 (p-STAT3) and SOCS3. This study investigated the inhibitory effects and the mechanism of action of GANT61 on T-cell lymphoma cells.
Methods: The paraffin-embedded tissues from thirty-five T-cell lymphoma patients were collected prior to therapeutic intervention. The expression of Gli1, p-STAT3 and SOCS3 in T-cell lymphoma tissues and cell lines (Jurkat, Karpass299 and Myla3676 cells) was investigated by Immunohistochemistry (IHC) and Western-blot respectively. Reactive hyperplasia of lymph node tissues and peripheral blood T cells from healthy volunteers served as control respectively. Cytotoxicity of GANT61 on the three cells was assessed by the cell counting kit-8 (CCK-8) assay after a 48-hour GANT61 treatment at different concentrations, and half-maximal inhibitory concentration (IC50) values were calculated respectively. Effect of GANT61 on apoptosis of these cells was evaluated by AnexinV-PE/7AAD assay, and protein expression of Gli1, p-STAT3, STAT3 and SOCS3 was detected by Western-blot after a 24-hour GANT61 treatment at different concentrations. To confirm the specific role of Gli1, lentivirus-mediated RNA interference was carried out to knockdown Gli1 gene in the three cells. Proliferation and apoptosis of normal and stable transfected siGli1 cells were assessed by CCK8 assay and AnexinV/7AAD assay respectively. Protein expression of Gli1, p-STAT3 and SOCS3 was assessed by Western-blot analysis after the genetically intervention.
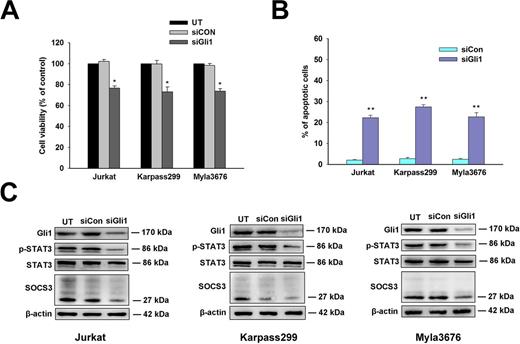
Results: Western-blot and IHC analysis showed that the expression of Gli1, p-STAT3 and SOCS3 protein was up-regulated in T-cell lymphoma cells and tissues compared with peripheral blood T cells from healthy volunteers and tissues from patients with reactive hyperplasia of lymph node respectively (Figure 1A-B). Moreover, the interaction of p-STAT3 and Gli1 in Jurkat, Myla3676 and Karpass299 cells were observed in the Western- blot analysis of Co-Immunoprecipitation (Co-IP) (Figure 1C). Viability of Jurkat, Karpass299 and Myla3676 cells decreased in a dose-dependent manner after a 48-hour incubation of GANT61 (Figure 2A). GANT61 induced apoptosis and decreased the protein expression of Gli1, p-STAT3 and SOCS3 in the three cells after a 24-hour treatment of GANT61 in a dose-dependent manner (Figure 2B-C). Furthermore, knockdown of Gli1 by lentivirus-mediated RNA interference inhibited the proliferation and induced apoptosis (Figure 3A-B), as well as downregulated the protein expression of Gli1, p-STAT3 and SOCS3 in these cells (Figure 3C).
Conclusions: These results showed that protein expression of Gli1, p-STAT3 and SOCS3 was up-regulated in T-cell lymphoma tissues and cell lines. Inhibition of Gli1 by GANT61 or RNA interference could attenuate proliferation and induce apoptosis of Jurkat, Karpass299 and Myla3676 cells. Moreover, the p-STAT3 and SOCS3 were decreased accompanied with the inhibition of Gli1. Our findings indicated a potential mechanism for the antitumor activity of GANT61 which might inhibit viability of T-cell lymphoma cells at least partially by down-regulating p-STAT3 and SOCS3 pathways. The crosstalk between Hh pathway and STAT3 pathway in T cell lymphomas deserves more exploration. Targeting the Hh pathway may be a novel therapeutic strategy for T-cell lymphomas.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal