Key Points
MYC-IG translocation partner gene is a negative predictor of survival in DLBCL patients.
Abstract
Diffuse large B-cell lymphoma (DLBCL) with MYC rearrangement (MYC-R) carries an unfavorable outcome. We explored the prognostic value of the MYC translocation partner gene in a series of MYC-R de novo DLBCL patients enrolled in first-line prospective clinical trials (Groupe d’Etudes des Lymphomes de l’Adulte/Lymphoma Study Association) and treated with rituximab-anthracycline–based chemotherapy. A total of 774 DLBCL cases characterized for cell of origin by the Hans classifier were analyzed using fluorescence in situ hybridization with BCL2, BCL6, MYC, immunoglobulin (IG)K, and IGL break-apart and IGH/MYC, IGK/MYC, and IGL/MYC fusion probes. MYC-R was observed in 51/574 (8.9%) evaluable DLBCL cases. MYC-R cases were predominantly of the germinal center B-cell–like subtype 37/51 (74%) with no distinctive morphologic and phenotypic features. Nineteen cases were MYC single-hit and 32 cases were MYC double-hit (MYC plus BCL2 and/or BCL6) DLBCL. MYC translocation partner was an IG gene in 24 cases (MYC-IG) and a non-IG gene (MYC-non-IG) in 26 of 50 evaluable cases. Noteworthy, MYC-IG patients had shorter overall survival (OS) (P = .0002) compared with MYC-negative patients, whereas no survival difference was observed between MYC-non-IG and MYC-negative patients. In multivariate analyses, MYC-IG predicted poor progression-free survival (P = .0051) and OS (P = .0006) independently from the International Prognostic Index and the Hans classifier. In conclusion, we show in this prospective randomized trial that the adverse prognostic impact of MYC-R is correlated to the MYC-IG translocation partner gene in DLBCL patients treated with immunochemotherapy. These results may have an important impact on the clinical management of DLBCL patients with MYC-R who should be routinely characterized according to MYC partner gene. These trials are individually registered at www.clinicaltrials.gov as #NCT00144807, #NCT01087424, #NCT00169143, #NCT00144755, #NCT00140660, #NCT00140595, and #NCT00135499.
Introduction
MYC is an oncogene involved in the pathogenesis of Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).1 MYC transcription factor plays a dual role as gene amplifier but also downregulates tumor cell proliferation by regulating the P53 apoptotic pathway.2,3 According to published studies, MYC rearrangement (MYC-R) is observed in 7% to 21% of DLBCL cases.4-15 MYC break may occur de novo as a sole genetic event or in combination with BCL2 and/or BCL6 translocations defining so-called “double-hit” (DHL) or “triple-hit” lymphomas (THL).16 Most MYC-R DLBCL have a germinal center B-cell–like (GCB) phenotype as defined by the Hans immunohistochemical classifier.16,17 In contrast to BL where MYC is classically translocated within the immunoglobulin (IG) loci, either the IG heavy chain gene (IGH) or more rarely the Ig light chain genes κ (IGK) or λ (IGL), up to half of MYC-R DLBCL involve non-IG translocation partner genes including BCL6, PAX5, BCL11A, or IKAROS.17-21
When considering the clinical relevance of MYC-R in DLBCL, there remain several unresolved issues. First, many groups have focused on the prognostic value of MYC gene deregulation in a variety of “aggressive” B-cell lymphomas with controversial results. In DLBCL, most studies have reported MYC-R as a strong adverse prognostic factor,4-10,22 whereas others failed to demonstrate any significant impact of MYC-R alone on survival.11,12,19,20 Secondly, DHL have been reported to have an extremely poor prognosis, but most studies were based on the retrospective analysis of patients presenting with very aggressive clinical features, thereby precluding their inclusion in any prospective clinical trials.16,17,23-28 Finally, the potential role of the MYC translocation partner gene has been addressed in a few recent studies. Interestingly, although heterogeneous in terms of histologies and therapy, they suggested a possible prognostic impact of an IG partner gene.17,19,20
This prompted us to investigate the prognostic value of the MYC translocation partner gene (IG vs non-IG) in de novo DLBCL patients enrolled in first-line prospective Groupe d’Etudes des Lymphomes de l’Adulte (GELA)/Lymphoma Study Association (LYSA) clinical trials, and treated with rituximab and anthracycline-based chemotherapy. In this study, we also focused on the clinical relevance of MYC-R–associated parameters such as single-hit (SH) or double-hit (DH) status in this clinical context.
Patients and methods
Patient selection
A total of 1696 patients with previously untreated de novo CD20+ DLBCL were enrolled in the GELA/LYSA LNH01-5B and LNH03-B clinical trials. LNH01-5B was a randomized trial initiated in 2001 that included de novo CD20+ DLBCL patients (age-adjusted International Prognosis Index [aaIPI] = 2 to 3, age 60 to 65 years) randomly assigned to treatment with R-CHOP or rituximab plus doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP). The LNH03-B trial initiated in 2003 was dedicated to patients with de novo previously untreated CD20+ DLBCL. Patients were stratified according to age and aaIPI, and were assigned to the following randomized trials: LNH03-1B (aaIPI = 0, <60 years), LNH03-2B (aaIPI = 1, <60 years), LNH03-3B and 39B (aaIPI = 2 to 3, <60 years), LNH03-6B (aaIPI = 0 to 3, age 60 to 80 years), and LNH03-7B (>80 years). Details regarding the design and data management of the LNH03-2B, 03-3B, and 03-7B trials have been published.29-32 Treatments included R-CHOP21/14 (03-6B, 03-2B, 01-5B), R-mini-CHOP21 (03-7B), and R-ACVBP or ACVBP (03-1B and 03-3B, 03-39B) regimens. The trials are individually registered at clinicaltrials.gov as noted previously. For the purpose of the current study, only patients with DLBCL treated with rituximab-associated chemotherapy were selected. These studies complied with all provisions of the Declaration of Helsinki and were conducted in accordance with good clinical practice guidelines. All patients gave written informed consent to participate and to provide tissue material for biological studies.
Morphology
Tumor samples from CD20+ DLBCL patients enrolled in the trials were centrally reviewed by at least two hematopathologists from LYSA to confirm the diagnosis of CD20-positive DLBCL according to the World Health Organization classification.1 Tissue microarrays (TMA) containing 3 representative 0.6-mm cores of routinely processed tissues from DLBCL cases were prepared (Beecher Instruments, Silver Spring, MD). Only patients with large tumor samples were selected for TMA (excluding needle biopsy). Among patients with available tissue blocks, 854 were subjected to TMA. The quality of each tissue core was evaluated for morphology using hematoxylin and eosin staining and for the percentage of CD20+ tumor cells. Only tissue cores with more than 50% CD20+ tumor cells were considered evaluable for fluorescence in situ hybridization (FISH) and immunohistochemical studies. Consequently, the eligible population included 774 patients with a pre-analytical validation of TMA cores.
Immunohistochemistry
Paraffin tissue sections from TMA blocks (3 μm thick) were subjected to antigen retrieval and immunostained on a BenchMark Ultra automated stainer (Roche Ventana, Tucson, AZ) for CD20, CD5, CD10, BCL6, and MUM1 as previously described.33 In addition, Mib1 (Ki67), BCL2 (clone 124; Dako Cytomation, Glostrup Denmark) and MYC (Epitomics, Burlingame, CA) were performed on full slides of MYC-R DLBCL. The cell of origin classification was based on the Hans algorithm.34 A detailed additional pathological review of all MYC-R cases was performed by 4 expert hematopathologists (C.C.B., T.J.M., J.B., and P.G.) with additional immunostainings for Ki67, BCL2, and MYC, and with the knowledge of FISH results to search for any specific pathological features (ie, cytological appearance, starry sky pattern, Ki67 proliferative index, and level of MYC protein expression).
Interphase FISH analysis
FISH analysis was performed on 3 µm TMA tissue sections using break-apart FISH DNA probes for cMYC/8q24, BCL2/18q21, and BCL6/3q27 (probes Y5410, Y5407, and Y5408; Dako A/S) as previously described.35 All cases with MYC-R were further analyzed using Vysis LSI IGH/MYC/CEP 8 Tri-Color Dual Fusion Probes (Abbott Laboratories, Chicago, IL) and IGK and IGL break-apart FISH DNA probes (Y5416 and Y5412; Dako A/S). In cases where FISH with break-apart probes suggested breakpoints affecting the MYC locus as well as one of the IG light-chain loci, IGK or IGL, interphase FISH was performed using IGK-MYC and IGL-MYC double-color fusion assays.4,36 Slides were evaluated under a fluorescence microscope (Zeiss, Göttingen, Germany) equipped with appropriate filter sets.
Statistical analysis
Patients’ characteristics and response rates were compared using the χ2 or Fisher’s exact tests (depending on the number of observations) for categorical parameters and the Mann–Whitney test for continuous parameters. Overall survival (OS) was measured from date of randomization to death from any cause and progression-free survival (PFS) from the date of randomization to the date of disease progression, relapse, or death from any cause. Survival analyses were performed using the log-rank test and expressed as Kaplan–Meier plots with appropriate 95% CIs. Multivariate analyses were performed with a Cox proportional hazards regression model. Differences between the results of comparative tests were considered significant if the 2-sided P value was < .05. Statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC).
Results
Clinical characteristics and outcome of the global population
Of the 774 DLBCL patients treated by R-chemo and with evaluable TMA tissue cores, 574 (74%) had interpretable FISH signals using a MYC break-apart probe and constituted our study population (Figure 1). The clinical characteristics of the patients are listed in Table 1. The median age was 62 years (range, 18 to 93 years). The median follow-up was 43 months (range, 0.3 to 83.2 months). The response rate at the end of the treatment was 80.5% (complete remission/unconfirmed complete remission). The 5-year PFS for the 574 patients was 64% and the 5-year OS was 72.3%. The majority of the patients (63.2%) received R-CHOP (319) or R-mini-CHOP (44), and 211 patients (36.8%) received R-ACVBP. The clinical features of the 574 patients of this study were similar to those of the entire cohort of patients (n = 1696) included in the LNH-01-5B and LNH03 trials (data not shown).
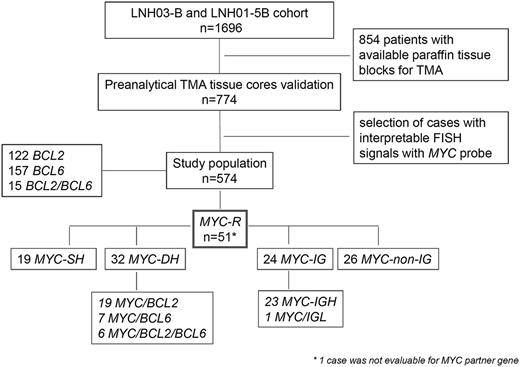
Flow-chart of LNH03-B and LNH01-5B cohort, case selection, and FISH results.BCL2-R, DLBCL with BCL2 gene rearrangement; BCL6-R, DLBCL with BCL6 gene rearrangement; MYC-R, DLBCL with MYC gene rearrangement; MYC-IG, MYC gene rearrangement with IG partner gene; MYC-non-IG, MYC gene rearrangement with non-IG partner gene.
Flow-chart of LNH03-B and LNH01-5B cohort, case selection, and FISH results.BCL2-R, DLBCL with BCL2 gene rearrangement; BCL6-R, DLBCL with BCL6 gene rearrangement; MYC-R, DLBCL with MYC gene rearrangement; MYC-IG, MYC gene rearrangement with IG partner gene; MYC-non-IG, MYC gene rearrangement with non-IG partner gene.
Clinical features of MYC-R vs MYC-neg, MYC-SH vs MYC-DH, and MYC-IG vs MYC-non-IG partner gene
| . | All patients (n = 574) . | MYC-neg (n = 523) . | MYC-R (n = 51) . | . | MYC-SH (n = 19) . | MYC-DH (n = 32) . | . | MYC-IG (n = 24) . | MYC-non-IG (n = 26) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | n (%) . | n (%) . | n (%) . | P . | n (%) . | n (%) . | P . | n (%) . | n (%) . | P . |
| Median age (range), y | 62 (18-93) | 62 (18-93) | 64 (29-84) | .213 | 65 (29-84) | 62.5 (35-84) | .546 | 69 (29-84) | 60.5 (32-84) | .027 |
| Sex | .285 | .789 | .817 | |||||||
| Male | 308 (53.7) | 277 (53.0) | 31 (60.8) | 12 (63.2) | 19(59.4) | 14 (58.3) | 16 (61.5) | |||
| Female | 266 (46.3) | 246 (47.0) | 20 (39.2) | 7 (36.8) | 13(40.6) | 10 (41.7) | 10 (38.5) | |||
| IPI score | < .001 | .077 | .324 | |||||||
| 0 | 50 (8.7) | 47 (9.0) | 3 (5.9) | 2 (10.5) | 1 (3.1) | 0 (0.0) | 3 (11.5) | |||
| 1 | 119 (20.7) | 115 (22.0) | 4 (7.8) | 1 (5.3) | 3 (9.4) | 1 (4.2) | 3 (11.5) | |||
| 2 | 143 (24.9) | 133 (25.4) | 10 (19.6) | 7 (36.8) | 3 (9.4) | 5 (20.8) | 5 (19.2) | |||
| 3 | 143 (24.9) | 124 (23.7) | 19 (37.3) | 3 (15.8) | 16 (50.0) | 8 (33.3) | 10 (38.5) | |||
| 4 | 91 (15.9) | 85 (16.3) | 6 (11.8) | 2 (10.5) | 4 (12.5) | 4 (16.7) | 2 (7.7) | |||
| 5 | 28 (4.9) | 19 (3.6) | 9 (17.6) | 4 (21.1) | 5 (15.6) | 6 (25.0) | 3 (11.5) | |||
| Age adjusted IPI | .015 | .237 | .338 | |||||||
| 0 | 69 (12.0) | 66 (12.6) | 3 (5.9) | 2 (10.5) | 1 (3.1) | 0 (0.0) | 3 (11.5) | |||
| 1 | 246 (42.9) | 230 (44.0) | 16 (31.4) | 8 (42.1) | 8 (25.0) | 8 (33.3) | 8 (30.8) | |||
| 2 | 205 (35.7) | 183 (35.0) | 22 (43.1) | 5 (26.3) | 17 (53.1) | 10 (41.7) | 11 (42.3) | |||
| 3 | 54 (9.4) | 44 (8.4) | 10 (19.6) | 4 (21.1) | 6 (18.8) | 6 (25.0) | 4 (15.4) | |||
| Ann Arbor stage | .005 | .268 | .187 | |||||||
| I-II | 152 (26.5) | 147 (28.1) | 5 (9.8) | 3 (15.8) | 2 (6.3) | 1 (4.2) | 4 (15.4) | |||
| III-IV | 422 (73.5) | 376 (71.9) | 46 (90.2) | 16 (84.2) | 30 (93.8) | 23 (95.8) | 22 (84.6) | |||
| Performance status (ECOG) | .224 | .860 | .691 | |||||||
| 0-1 | 499 (86.9) | 458 (87.5) | 41 (80.4) | 15 (79.0) | 26 (81.2) | 18 (75.0) | 21 (84.6) | |||
| ≥2 | 75 (13.0) | 65 (12.4) | 10 (19.6) | 4 (21.0) | 6 (18.8) | 6 (25.0) | 4 (15.4) | |||
| LDH > normal | 320 (55.8) | 286 (54.8) | 34 (66.7) | .103 | 10 (52.6) | 24 (75.0) | .101 | 17 (70.8) | 16 (61.5) | .488 |
| Extranodal site >1 | 209 (36.4) | 181 (34.6) | 28 (54.9) | .004 | 10 (52.6) | 18 (56.3) | .802 | 17 (70.8) | 11 (42.3) | .042 |
| Bone marrow involvement | 88 (16.6) | 74 (15.3) | 14 (29.7) | .011 | 5 (29.4) | 9 (30.0) | .966 | 9 (40.9) | 5 (20.8) | .139 |
| Mass >10 cm | 83 (15.3) | 77 (15.6) | 6 (12.8) | .612 | 1 (5.3) | 5 (17.9) | .204 | 5 (20.8) | 1 (4.5) | .101 |
| B symptoms | 183 (31.9) | 169 (32.4) | 14 (27.5) | .472 | 3 (15.8) | 11(34.4) | .150 | 3 (12.5) | 11 (42.3) | .019 |
| β 2 microglobulin (≥3 mg/L) | 160 (33.8) | 141(33.0) | 19 (40.4) | .308 | 6 (33.3) | 13 (44.8) | .435 | 11 (45.8) | 8 (34.8) | .440 |
| Albumin (g/L) ≤35 g/L | 123 (24.6) | 110 (24.2) | 13 (28.3) | .540 | 5 (31.3) | 8 (26.7) | .742 | 9 (39.1) | 4 (18.2) | .121 |
| LNH trials | — | — | — | |||||||
| LNH03-1B | 51 (8.9) | 48 (9.2) | 3 (5.9) | 2 (10.5) | 1 (3.1) | 0 (0.0) | 3 (11.5) | |||
| LNH03-2B | 139 (24.2) | 129 (24.7) | 10 (19.6) | 5 (26.3) | 5 (15.6) | 4 (16.7) | 6 (23.1) | |||
| LNH03-3B | 47 (8.2) | 44 (8.4) | 3 (5.9) | 0(0) | 3 (9.4) | 1 (4.2) | 2 (7.7) | |||
| LNH03-39B | 22 (3.8) | 20 (3.8) | 2 (3.9) | 0(0) | 2 (6.3) | 1 (4.2) | 1 (3.8) | |||
| LNH03-6B | 240 (41.8) | 216 (41.3) | 24 (47.1) | 9 (47.4) | 15 (46.9) | 15 (62.5) | 8 (30.8) | |||
| LNH03-7B | 44 (7.7) | 39 (7.5) | 5 (9.8) | 3 (15.8) | 2 (6.3) | 3 (12.5) | 2 (7.7) | |||
| LNH01-5B | 31 (5.4) | 27 (5.2) | 4 (7.8) | 0 (0) | 4 (12.5) | 0 (0.0) | 4 (15.4) | |||
| Arm of treatment | — | — | — | |||||||
| R-ACVBP | 189 (32.9) | 176 (33.7) | 13 (25.5) | 2 (10.5) | 11 (34.4) | 2 (8.3) | 11 (42.3) | |||
| R-ACVBP+ASCT | 22 (3.8) | 20 (3.8) | 2 (3.9) | 0 (0) | 2 (6.3) | 1 (4.2) | 1 (3.8) | |||
| R-CHOP21 | 200 (34.8) | 179 (34.2) | 21 (41.2) | 9 (47.4) | 12 (37.5) | 10 (41.7) | 11 (42.3) | |||
| R-CHOP14 | 119 (20.7) | 109 (20.8) | 10 (19.6) | 5 (26.3) | 5 (15.6) | 8 (33.3) | 1 (3.8) | |||
| R-mini-CHOP21 | 44 (7.7) | 39 (7.5) | 5 (9.8) | 3 (15.8) | 2 (6.3) | 3 (12.5) | 2 (7.7) | |||
| Arm of treatment | — | — | — | |||||||
| R-ACVBP | 211 (36.8) | 196 (37.5) | 15 (29.4) | 2 (10.5) | 13 (40.6) | 3 (12.5) | 12 (46.2) | |||
| R-CHOP | 363 (63.2) | 327 (62.5) | 36 (70.6) | 17 (89.5) | 19 (59.4) | 21 (87.5) | 14 (53.8) |
| . | All patients (n = 574) . | MYC-neg (n = 523) . | MYC-R (n = 51) . | . | MYC-SH (n = 19) . | MYC-DH (n = 32) . | . | MYC-IG (n = 24) . | MYC-non-IG (n = 26) . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | n (%) . | n (%) . | n (%) . | P . | n (%) . | n (%) . | P . | n (%) . | n (%) . | P . |
| Median age (range), y | 62 (18-93) | 62 (18-93) | 64 (29-84) | .213 | 65 (29-84) | 62.5 (35-84) | .546 | 69 (29-84) | 60.5 (32-84) | .027 |
| Sex | .285 | .789 | .817 | |||||||
| Male | 308 (53.7) | 277 (53.0) | 31 (60.8) | 12 (63.2) | 19(59.4) | 14 (58.3) | 16 (61.5) | |||
| Female | 266 (46.3) | 246 (47.0) | 20 (39.2) | 7 (36.8) | 13(40.6) | 10 (41.7) | 10 (38.5) | |||
| IPI score | < .001 | .077 | .324 | |||||||
| 0 | 50 (8.7) | 47 (9.0) | 3 (5.9) | 2 (10.5) | 1 (3.1) | 0 (0.0) | 3 (11.5) | |||
| 1 | 119 (20.7) | 115 (22.0) | 4 (7.8) | 1 (5.3) | 3 (9.4) | 1 (4.2) | 3 (11.5) | |||
| 2 | 143 (24.9) | 133 (25.4) | 10 (19.6) | 7 (36.8) | 3 (9.4) | 5 (20.8) | 5 (19.2) | |||
| 3 | 143 (24.9) | 124 (23.7) | 19 (37.3) | 3 (15.8) | 16 (50.0) | 8 (33.3) | 10 (38.5) | |||
| 4 | 91 (15.9) | 85 (16.3) | 6 (11.8) | 2 (10.5) | 4 (12.5) | 4 (16.7) | 2 (7.7) | |||
| 5 | 28 (4.9) | 19 (3.6) | 9 (17.6) | 4 (21.1) | 5 (15.6) | 6 (25.0) | 3 (11.5) | |||
| Age adjusted IPI | .015 | .237 | .338 | |||||||
| 0 | 69 (12.0) | 66 (12.6) | 3 (5.9) | 2 (10.5) | 1 (3.1) | 0 (0.0) | 3 (11.5) | |||
| 1 | 246 (42.9) | 230 (44.0) | 16 (31.4) | 8 (42.1) | 8 (25.0) | 8 (33.3) | 8 (30.8) | |||
| 2 | 205 (35.7) | 183 (35.0) | 22 (43.1) | 5 (26.3) | 17 (53.1) | 10 (41.7) | 11 (42.3) | |||
| 3 | 54 (9.4) | 44 (8.4) | 10 (19.6) | 4 (21.1) | 6 (18.8) | 6 (25.0) | 4 (15.4) | |||
| Ann Arbor stage | .005 | .268 | .187 | |||||||
| I-II | 152 (26.5) | 147 (28.1) | 5 (9.8) | 3 (15.8) | 2 (6.3) | 1 (4.2) | 4 (15.4) | |||
| III-IV | 422 (73.5) | 376 (71.9) | 46 (90.2) | 16 (84.2) | 30 (93.8) | 23 (95.8) | 22 (84.6) | |||
| Performance status (ECOG) | .224 | .860 | .691 | |||||||
| 0-1 | 499 (86.9) | 458 (87.5) | 41 (80.4) | 15 (79.0) | 26 (81.2) | 18 (75.0) | 21 (84.6) | |||
| ≥2 | 75 (13.0) | 65 (12.4) | 10 (19.6) | 4 (21.0) | 6 (18.8) | 6 (25.0) | 4 (15.4) | |||
| LDH > normal | 320 (55.8) | 286 (54.8) | 34 (66.7) | .103 | 10 (52.6) | 24 (75.0) | .101 | 17 (70.8) | 16 (61.5) | .488 |
| Extranodal site >1 | 209 (36.4) | 181 (34.6) | 28 (54.9) | .004 | 10 (52.6) | 18 (56.3) | .802 | 17 (70.8) | 11 (42.3) | .042 |
| Bone marrow involvement | 88 (16.6) | 74 (15.3) | 14 (29.7) | .011 | 5 (29.4) | 9 (30.0) | .966 | 9 (40.9) | 5 (20.8) | .139 |
| Mass >10 cm | 83 (15.3) | 77 (15.6) | 6 (12.8) | .612 | 1 (5.3) | 5 (17.9) | .204 | 5 (20.8) | 1 (4.5) | .101 |
| B symptoms | 183 (31.9) | 169 (32.4) | 14 (27.5) | .472 | 3 (15.8) | 11(34.4) | .150 | 3 (12.5) | 11 (42.3) | .019 |
| β 2 microglobulin (≥3 mg/L) | 160 (33.8) | 141(33.0) | 19 (40.4) | .308 | 6 (33.3) | 13 (44.8) | .435 | 11 (45.8) | 8 (34.8) | .440 |
| Albumin (g/L) ≤35 g/L | 123 (24.6) | 110 (24.2) | 13 (28.3) | .540 | 5 (31.3) | 8 (26.7) | .742 | 9 (39.1) | 4 (18.2) | .121 |
| LNH trials | — | — | — | |||||||
| LNH03-1B | 51 (8.9) | 48 (9.2) | 3 (5.9) | 2 (10.5) | 1 (3.1) | 0 (0.0) | 3 (11.5) | |||
| LNH03-2B | 139 (24.2) | 129 (24.7) | 10 (19.6) | 5 (26.3) | 5 (15.6) | 4 (16.7) | 6 (23.1) | |||
| LNH03-3B | 47 (8.2) | 44 (8.4) | 3 (5.9) | 0(0) | 3 (9.4) | 1 (4.2) | 2 (7.7) | |||
| LNH03-39B | 22 (3.8) | 20 (3.8) | 2 (3.9) | 0(0) | 2 (6.3) | 1 (4.2) | 1 (3.8) | |||
| LNH03-6B | 240 (41.8) | 216 (41.3) | 24 (47.1) | 9 (47.4) | 15 (46.9) | 15 (62.5) | 8 (30.8) | |||
| LNH03-7B | 44 (7.7) | 39 (7.5) | 5 (9.8) | 3 (15.8) | 2 (6.3) | 3 (12.5) | 2 (7.7) | |||
| LNH01-5B | 31 (5.4) | 27 (5.2) | 4 (7.8) | 0 (0) | 4 (12.5) | 0 (0.0) | 4 (15.4) | |||
| Arm of treatment | — | — | — | |||||||
| R-ACVBP | 189 (32.9) | 176 (33.7) | 13 (25.5) | 2 (10.5) | 11 (34.4) | 2 (8.3) | 11 (42.3) | |||
| R-ACVBP+ASCT | 22 (3.8) | 20 (3.8) | 2 (3.9) | 0 (0) | 2 (6.3) | 1 (4.2) | 1 (3.8) | |||
| R-CHOP21 | 200 (34.8) | 179 (34.2) | 21 (41.2) | 9 (47.4) | 12 (37.5) | 10 (41.7) | 11 (42.3) | |||
| R-CHOP14 | 119 (20.7) | 109 (20.8) | 10 (19.6) | 5 (26.3) | 5 (15.6) | 8 (33.3) | 1 (3.8) | |||
| R-mini-CHOP21 | 44 (7.7) | 39 (7.5) | 5 (9.8) | 3 (15.8) | 2 (6.3) | 3 (12.5) | 2 (7.7) | |||
| Arm of treatment | — | — | — | |||||||
| R-ACVBP | 211 (36.8) | 196 (37.5) | 15 (29.4) | 2 (10.5) | 13 (40.6) | 3 (12.5) | 12 (46.2) | |||
| R-CHOP | 363 (63.2) | 327 (62.5) | 36 (70.6) | 17 (89.5) | 19 (59.4) | 21 (87.5) | 14 (53.8) |
ECOG, Eastern Cooperative Oncology Group; LNH, non-Hodgkin lymphoma; MYC-neg, DLBCL without MYC gene rearrangement.
FISH results
BCL2/18q21, BCL6/3q27, and MYC/8q24 gene rearrangements were observed in 21.2% (122/574), 27.4% (157/573), and 8.9% (51/574) of the cases, respectively (Figure 1). Fifteen cases harbored both BCL2 and BCL6 gene rearrangements (15/573 = 2.6%).
MYC-R cases were subdivided into MYC-SH (19 cases) when there was no concomitant rearrangement of both BCL2 and BCL6, and MYC-DH (32 cases) when concurrent BCL2 and/or BCL6 breakpoints were observed. For simplification, the term “double-hit” was used for all cases with MYC-R and additional breakpoints, including triple-hit cases. In total, MYC-DH included 19 MYC/BCL2, 7 MYC/BCL6, and 6 MYC/BCL2/BCL6 cases.
MYC-R tumor samples were evaluable for MYC Ig partner genes using MYC/IgH/CEN8 fusion probe, and IGK and IGL break-apart probes: 23 cases showed MYC/IGH fusion signals and 5 cases IGK (n = 3) or IGL (n = 2) breaks. In order to demonstrate whether these IGK or IGL were fused to the MYC gene, 4 of these 5 cases with sufficient material available were further evaluated with MYC-IGK or MYC-IGL fusion probes. Only 1 of these 4 cases with IGK or IGL breaks displayed MYC-IGL fusion signals. Therefore, the other 3 were assigned to the non-IG-MYC group.
Altogether, among the 50 MYC-R DLBCL evaluable for MYC-IG partner gene, MYC translocation partner genes involved IG loci in 24 cases (23 IGH and 1 IGL) and were called MYC-IG, whereas the partner gene was a non-IG gene in 26 cases (MYC-non-IG).
Pathological features of MYC-R DLBCL cases
To search for potential peculiar morphologic and phenotypic features, the 51 MYC-R DLBCL cases were re-reviewed with additional immunostainings for Ki67, MYC, and BCL2 performed on full slides, and the FISH results were as follows: 47 cases were confirmed as DLBCL not otherwise specified (DLBCL NOS) and included 43 centroblastic variants and 4 immunoblastic variants of DLBCL (3 MYC-IG and 1 MYC-non-IG), 2 DLBCL NOS were associated with a minor focal follicular lymphoma component, whereas only 4 cases could retrospectively be considered as having some features of B-cell lymphoma, unclassifiable, or intermediate between DLBCL and BL (BCLu) (Table 2). Among the 51 cases, only a minority showed high numbers of apoptotic bodies (8/49 = 16.3%), or mitosis (13/49 = 26.5%) and a starry sky pattern (7/49 = 14.3%). No significant differences based on these morphologic criteria were observed between SH/DH or IG/non-IG subgroups.
Pathological features of the 51 MYC-R DLBCL cases according to the SH/DH or IG/non-IG status
| . | MYC-R (n = 51) . | MYC-SH (n = 19) . | MYC-DH (n = 32) . | . | MYC- IG (n = 24) . | MYC-non-IG (n = 26) . | . |
|---|---|---|---|---|---|---|---|
| . | n (%) . | n (%) . | n (%) . | P (MYC-SH vs MYC-DH) . | n (%) . | n (%) . | P (MYC-IG vs MYC-non-IG) . |
| Histologic subtype | .452 | .221 | |||||
| DLBCL NOS (*) | 47 (92) | 18 (94.7) | 29 (90) | 21 (87.5) | 25 (96.1) | ||
| Consistent with BCLu | 4 (7.8) | 1 (5.3) | 3 (9.4) | 3 (12.5) | 1 (3.8) | ||
| Apoptotic bodies (n = 49) | .0935 | 1.0 | |||||
| 0-2 | 41 (83.7) | 16 (84.2) | 25 (83.3) | 20 (83.3) | 20 (83.3) | ||
| 3 | 8 (16.3) | 3 (15.8) | 5 (16.7) | 4 (16.7) | 4 (16.7) | ||
| Starry sky pattern (n = 49) | .550 | .683 | |||||
| 0-2 | 42 (85.7) | 17 (89.5) | 25 (83.3) | 21 (87.5) | 20 (83.3) | ||
| 3 | 7 (14.3) | 2 (10.5) | 5 (16.7) | 3 (12.5) | 4 (16.7) | ||
| Mitosis (n = 49) | .193 | .745 | |||||
| 0-2 | 36 (73.5) | 12 (63.2) | 24 (80.0) | 18 (75.0) | 17 (70.8) | ||
| 3 | 13 (26.5) | 7 (36.8) | 6 (20.0) | 6 (25.0) | 7 (29.2) | ||
| Hans score (n = 50) | .171 | .682 | |||||
| GCB | 37 (74.0) | 12 (63.2) | 25 (80.6) | 17 (70.8) | 19 (76.0) | ||
| Non-GCB | 13 (26.0) | 7 (36.8) | 6 (19.4) | 7 (29.2) | 6 (24.0) | ||
| Ki67 ≥80% (n = 49) | 30 (61.2) | 15 (78.9) | 15 (50.0) | .043 | 15 (65.2) | 14 (56.0) | .514 |
| BCL2 protein (>50% or 70%) (n = 50) | 37 (74.0) | 11 (57.9) | 26 (83.9) | .042 | 18 (64.3) | 19 (86.4) | .077 |
| MYC protein ≥90% (n = 50) | 23 (46) | 11 (57.9) | 12 (38.7) | .186 | 15 (62.5) | 8 (32) | .032 |
| MYC partner gene† | .038 | <.001 | |||||
| IG (IGH, K, L) | 24 (48) | 12 (63.2) | 12 (38.7) | 24 (100.0) | 0 (0) | ||
| Non-IG | 26 (52) | 7 (36.8) | 19 (61.3) | 0 (0) | 26 (100) |
| . | MYC-R (n = 51) . | MYC-SH (n = 19) . | MYC-DH (n = 32) . | . | MYC- IG (n = 24) . | MYC-non-IG (n = 26) . | . |
|---|---|---|---|---|---|---|---|
| . | n (%) . | n (%) . | n (%) . | P (MYC-SH vs MYC-DH) . | n (%) . | n (%) . | P (MYC-IG vs MYC-non-IG) . |
| Histologic subtype | .452 | .221 | |||||
| DLBCL NOS (*) | 47 (92) | 18 (94.7) | 29 (90) | 21 (87.5) | 25 (96.1) | ||
| Consistent with BCLu | 4 (7.8) | 1 (5.3) | 3 (9.4) | 3 (12.5) | 1 (3.8) | ||
| Apoptotic bodies (n = 49) | .0935 | 1.0 | |||||
| 0-2 | 41 (83.7) | 16 (84.2) | 25 (83.3) | 20 (83.3) | 20 (83.3) | ||
| 3 | 8 (16.3) | 3 (15.8) | 5 (16.7) | 4 (16.7) | 4 (16.7) | ||
| Starry sky pattern (n = 49) | .550 | .683 | |||||
| 0-2 | 42 (85.7) | 17 (89.5) | 25 (83.3) | 21 (87.5) | 20 (83.3) | ||
| 3 | 7 (14.3) | 2 (10.5) | 5 (16.7) | 3 (12.5) | 4 (16.7) | ||
| Mitosis (n = 49) | .193 | .745 | |||||
| 0-2 | 36 (73.5) | 12 (63.2) | 24 (80.0) | 18 (75.0) | 17 (70.8) | ||
| 3 | 13 (26.5) | 7 (36.8) | 6 (20.0) | 6 (25.0) | 7 (29.2) | ||
| Hans score (n = 50) | .171 | .682 | |||||
| GCB | 37 (74.0) | 12 (63.2) | 25 (80.6) | 17 (70.8) | 19 (76.0) | ||
| Non-GCB | 13 (26.0) | 7 (36.8) | 6 (19.4) | 7 (29.2) | 6 (24.0) | ||
| Ki67 ≥80% (n = 49) | 30 (61.2) | 15 (78.9) | 15 (50.0) | .043 | 15 (65.2) | 14 (56.0) | .514 |
| BCL2 protein (>50% or 70%) (n = 50) | 37 (74.0) | 11 (57.9) | 26 (83.9) | .042 | 18 (64.3) | 19 (86.4) | .077 |
| MYC protein ≥90% (n = 50) | 23 (46) | 11 (57.9) | 12 (38.7) | .186 | 15 (62.5) | 8 (32) | .032 |
| MYC partner gene† | .038 | <.001 | |||||
| IG (IGH, K, L) | 24 (48) | 12 (63.2) | 12 (38.7) | 24 (100.0) | 0 (0) | ||
| Non-IG | 26 (52) | 7 (36.8) | 19 (61.3) | 0 (0) | 26 (100) |
BCLu: B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL.
Including 2 cases with a minor follicular lymphoma component.
1 case was not evaluable for MYC partner gene.
According to the Hans algorithm, the majority of MYC-R cases were of the GCB subtype (74%). MYC-SH lymphomas included 12 GCB and 7 non-GCB cases. MYC-DH lymphomas included 25 GCB (18 MYC/BCL2, 6 THL, and 1 MYC/BCL6) and 6 non-GCB cases (6 MYC/BCL6). A proliferative index ≥80% evaluated with Ki67 immunostaining was significantly more frequent in MYC-SH compared with MYC-DH tumors (78.9% vs 50.0%; P = .043).
BCL2 protein (50% or 70% threshold) was expressed in 37/50 (74%) of MYC-R evaluable cases. High BCL2 expression (>50% or 70%) was significantly more frequent in MYC-DH compared with MYC-SH DLBCL (83.9% vs 57.9%; P = .042), whereas no significant differences were noted between MYC-IG and MYC-non-IG subgroups (64.3% vs 86.4%; P = .077).
MYC protein expression in virtually all tumor cells (≥90%) was observed in 23/50 (46%) of evaluable cases. No significant difference was observed between the SH and DH subgroups (57.9% vs 38.7%; P = .186), whereas high MYC protein expression was significantly more frequent in the MYC-Ig subgroup compared with MYC-non-IG DLBCL (62.5% vs 32%; P = .032).
Clinical features of MYC-R DLBCL patients
Comparison of the clinical characteristics of MYC-R vs MYC-negative, MYC-SH vs MYC-DH, MYC-IG vs MYC-non-IG DLBCL patients are presented in Table 1. Compared with MYC-negative DLBCL patients, MYC-R patients presented with higher International Prognosis Index (IPI) score (P < .001), aaIPI (P = .015), Ann Arbor stage (P = .005), number of extranodal sites (P = .004), and frequency of bone marrow involvement (P = .011). There were no significant clinical and biological differences between MYC-SH and MYC-DH subgroups of patients. MYC-IG compared with MYC-non-IG patients were significantly older (median age, 69 vs 60.5 years; P = .027) and presented a higher number of extranodal sites involvement (P = .042), whereas conversely, MYC-non-IG patients showed a higher frequency of B symptoms (P = .019).
Patient outcome
By univariate analysis, high IPI and non-GCB subtype were significantly associated with shorter PFS and OS (IPI: PFS and OS, P < .0001) (Cell of origin: PFS, P = .0001; OS, P = .0004). BCL2 and BCL6 gene alterations did not significantly predict survival (PFS and OS) (see supplemental Table 1A, available on the Blood Web site).
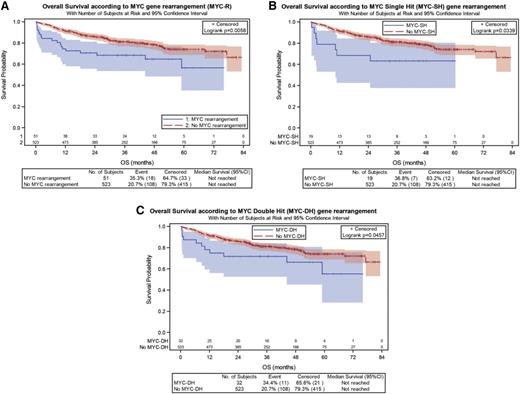
MYC-R, MYC-SH, and MYC-DH were associated with shorter OS in the global population (P = .0058; P = .0339; and P = .0457, respectively) (Figure 2; supplemental Table 1A). MYC-R, MYC-SH, and MYC-DH were associated with shorter PFS and OS in GCB DLBCL (PFS: P = .0014, P = .0269, and P = .0078, respectively; OS: P = .0001, P = .0153, and P = .0007, respectively), a finding that did not reach the level of significance in the non-GCB subtype (supplemental Figure 1; supplemental Table 1B). Interestingly, all but one GCB MYC-DH consisted of MYC/BCL2 cases (18 MYC/BCL2, 6 THL, and 1 MYC/BCL6), whereas all non-GCB MYC-DH were MYC/BCL6 cases.
Univariate analysis of MYC-R for OS. (A) The global population, (B) SH, and (C) subgroups of DLBCL patients.
Univariate analysis of MYC-R for OS. (A) The global population, (B) SH, and (C) subgroups of DLBCL patients.
The outcome of DH-BCL2 (including THL) (n = 25) and DH-BCL6 (n = 7) were compared. A trend toward a lower PFS and OS for DH-BCL2 was noted (supplemental Figure 3), which did not reach statistical significance within the limits of the number of cases in each subgroup (PFS: P = .1966; OS: P = .2839).
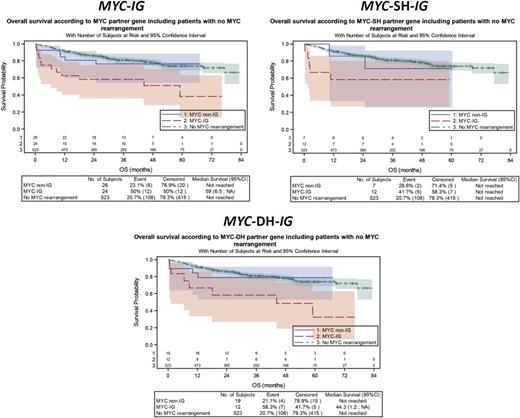
When considering the MYC translocation partner gene, MYC-IG patients had a significantly shorter PFS (P = .0023) and OS (P = .0002) when compared with MYC-negative DLBCL patients, whereas no significant differences were observed between MYC-non-IG and MYC negative patients on PFS (P = .9661) and OS (P = .6526) (Figure 3; supplemental Table 1A). A similar impact of MYC-IG on OS was observed in the MYC-SH (P = .0175) and MYC-DH (P = .0023) subgroups. MYC-IG translocations had an adverse prognostic effect on PFS (P < .0001) and OS (P < .0001) in GCB DLBCL, which did not reach statistical significance in the non-GCB subtype (supplemental Figure 2; supplemental Table 1B). A similar poor impact of MYC-IG partner gene on OS was observed in GCB SH/DH DLBCL subgroups (supplemental Figure 2; supplemental Table 1B). No interaction was found between MYC-R and treatment regimen (R-CHOP vs R-ACVBP) (data not shown).
Univariate analysis of MYC-IG for OS. (A) The global population, (B) SH, and (C) DH subgroups of DLBCL patients compared with MYC-non-IG and MYC-negative DLBCL patients.
Univariate analysis of MYC-IG for OS. (A) The global population, (B) SH, and (C) DH subgroups of DLBCL patients compared with MYC-non-IG and MYC-negative DLBCL patients.
In a multivariate analysis incorporating IPI, Hans score, and MYC-R, MYC-R remained statistically significant on OS (P = .0089) (supplemental Table 2A). In a multivariate analysis incorporating IPI, Hans score, and MYC-SH or MYC-DH status, only MYC-SH remained an independent prognostic factor on PFS (P = .0482) and OS (P = .0139) in addition to IPI and Hans score (supplemental Table 2B). However, when excluding MYC/BCL6 DH cases from the MYC-DH group, MYC/BCL2 DH status (including THL) had an independent poor prognostic impact on both PFS (P = .027) and OS (P = .0055) (supplemental Table 2C).
When considering the MYC translocation partner gene, MYC-IG predicted a poor PFS (P = .0051) and OS (P = .0006) in addition to IPI and the Hans score (supplemental Table 2D). Because the median age of MYC-IG patients was almost a decade older than MYC-non-IG patients, age was evaluated by multivariate analysis and remained an independent prognostic factor (data not shown).
Discussion
To the best of our knowledge, this is the first study addressing the impact of the MYC partner gene in a large series of unselected patients with de novo DLBCL and treated with rituximab-anthracycline–based chemotherapy in the setting of prospective clinical trials. In this series of 574 DLBCL patients, we show that MYC-IG had a significant negative impact on outcome (PFS and OS), whereas no significant difference in PFS and OS was observed between MYC-non-IG and MYC-negative DLBCL. These results may have an important impact on the clinical management of DLBCL patients with MYC-R who should be characterized according to MYC partner gene, because non-IG MYC partner genes appear to have little influence on survival.
The prognostic impact of the MYC partner gene in DLBCL associated with MYC-R is currently a major issue but has remained controversial.37 Indeed, this issue has been discussed in only 3 previous studies that included B-cell lymphoma patients with heterogeneous lymphoma entities, patients at diagnosis and at relapse, and/or patients treated before the era of rituximab resulting in discrepant results. Johnson et al were the first to point out the potential relevance of IG as a MYC partner in a retrospective series of 54 patients with DH MYC/BCL2 BCLs diagnosed between 1991 and 2007, selected on the basis of availability of karyotypic analysis.17 Histologies were variable including only 17 de novo DLBCL patients (n = 17) and the prognosis impact of the MYC partner gene could be evaluated in 40 patients including 11 patients receiving rituximab-based chemotherapy. The study by Pedersen et al was based on a prospective cohort comprising all patients diagnosed with DLBCL or BCLu in a single institution between 2009 and 2011.20 Histopathological subtypes included de novo DLBCL (n = 162), transformed DLBCL (n = 65), and relapse (n = 25). Treatment regimens were variable with 14% of patients not receiving rituximab. MYC translocation was observed in 51 cases but, unexpectedly, showed no correlation with OS, whereas MYC translocation with an IG partner gene was reported to correlate with a worse prognosis. However, in addition to the above limitations, the data presented in this study were also controversial because the authors considered 21 cases with concurrent MYC and IGK or IGL rearrangements detected using break-apart probes as MYC-IG cases, which most likely represents an overstatement since these cases were not investigated for MYC-IGK or MYC-IGL fusions with appropriate probes. Indeed, in our study, among the 4 cases showing concurrent MYC and IGK or IGL light chain rearrangements using break-apart probes, only 1 proved to be a “real” MYC-IG case with the MYC-IGL fusion probe and the remaining cases were assigned to the non-IG subgroup. Finally, Aukema et al focused on 80 MYC-R B-cell lymphomas of various histopathological subtypes and found no significant difference between MYC-SH vs MYC-DH and MYC-IG vs MYC-non-IG lymphoma subgroups regarding the molecular cytogenetic, array-CGH, mutational, gene-expression profiles, and survival.38 Overall, it can be speculated that the discrepancies observed between the above mentioned reports are related to the heterogeneity of the populations studied (which comprised various histopathological subtypes), making the results difficult to compare.
We observed an overall incidence of MYC-R of 8.9%, which is consistent with the literature by using a commercially available probe that covers most breakpoints commonly involved in MYC translocations, and similar to the majority of previous published series.10,17-19,34,39,40 We corroborate previous reports on the adverse prognostic impact of MYC-R in DLBCL, which remained an independent prognostic factor for OS in multivariate analysis incorporating IPI and the Hans score.4-10,14,22 However, we show that this prognostic impact is related to the MYC-Ig partner gene.
In this prospective study of de novo unselected DLBCL, the incidence of MYC-SH was 3.3% (19/574) and MYC-DH was 5.6% (32/574). In univariate analysis, MYC-SH and MYC-DH were associated with poor OS, but in multivariate analysis, only MYC-SH retained a poor prognostic significance on OS. These results might appear in contrast to a previous study by Hummel et al reporting “MYC-simple” aggressive B-cell lymphomas including BLs, as having a favorable outcome.4 However, our series differs from the previous study as we excluded BLs. In addition, “MYC-simple” according to Hummel et al was defined by the presence of MYC-IG fusions, a low chromosomal complexity score using array-based comparative genomic hybridization and absence of BCL2 or BCL6 breaks. The FISH approach adopted in our study explored 3 major oncogenic loci (BCL2, BCL6, and MYC) but did not address the genetic complexity of the tumor, and therefore does not preclude the presence of additional cytogenetic abnormalities.
Concerning MYC-DH, the absence of prognosis significance by multivariate analysis may appear unexpected as the majority of published reports describe DHL as highly aggressive tumors with poor outcome and resistance to conventional chemotherapy.16,17,23-28,41 Although further studies are needed to understand such differences, it is noteworthy that MYC-DH patients in our series probably differ from DHL patients of previous reports because we included only patients with newly diagnosed DLBCL enrolled in controlled clinical trials in contrast to all aforementioned studies, which were a retrospective series of aggressive BCLs. In addition, patients enrolled in clinical trials are usually biased toward more healthy patients. Moreover, most studies did not perform FISH for BCL6 and may have missed MYC/BCL6 DHL and THL. Interestingly, when excluding the MYC/BCL6 tumors from the MYC-DH group in the multivariate analysis, MYC/BCL2 DH status retained its independent poor prognosis significance on both PFS and OS, suggesting that the poor prognosis of DHL is most likely related to MYC/BCL2 DHs. In this respect, when comparing the outcome of MYC/BCL2 and MYC/BCL6 DHL patients, we observed a trend toward a worse PFS and OS for MYC/BCL2 patients, although this remained nonsignificant probably due to the limited number of cases. International efforts with large cohorts of de novo DLBCL patients enrolled in clinical trials would be needed to explore this issue. Altogether, de novo DHL DLBCL probably represents a heterogeneous group of diseases with variable outcomes, including patients with aggressive presentation and a very poor outcome, and a group of DLBCL NOS without major distinct clinical and pathological features.
The pathological review of MYC-R DLBCL showed that most cases were GCB DLBCL (74%) in keeping with previous studies.8,9,16,17 Interestingly, the features usually believed to be associated with aggressiveness as apoptotic bodies, starry sky pattern, and mitosis were not prominent. The proliferative index evaluated with Ki67 immunostaining was variable and found to be ≤80% in 40% of the cases, indicating that immunostaining for Ki67 is not relevant to prescreen for MYC-R DLBCL as already reported.14,42 BCL2 overexpression was significantly more frequent in MYC-DH than MYC-SH patients, which may be explained by the large number of MYC/BCL2 DHL in this series. MYC protein expression in virtually all tumor cells (≥90%) was observed only in 46% of MYC-R cases, suggesting that immunostaining for MYC may not be an efficient surrogate to detect MYC-R DLBCL. Altogether, there were no clear pathological features in routine practice that may indicate that a DLBCL may harbor MYC-R.
The biological basis for the dismal outcome of MYC-IG DLBCL patients needs to be clarified. We show that a high level of MYC protein expression (≥90%) correlated with the MYC-IG subgroup, in agreement with a higher level of MYC transcripts in MYC-IG cases compared with MYC-non-IG cases observed in previous reports.21,38 These findings suggest that a full “MYC-program” may not be reached with a non-IG promoter. However, MYC related oncogenesis is probably not sufficient to explain these differences, and additional genetic and environmental factors probably interact.
In conclusion, our study shows that MYC-Ig rearrangements are negative predictors of survival in DLBCL patients in the setting of prospective controlled clinical trials. Because immunostaining for MYC is not a robust approach to prescreen for DLBCL patients with MYC breaks, we believe that primary DLBCL patients should be investigated for MYC breaks by FISH, at least in the GCB subtype. In addition, MYC-R patients should be screened for the IG MYC partner gene. We also show that MYC-R predicted a worse prognosis with no interaction with treatment arm and chemotherapy regimen suggesting that conventional chemotherapy, such as R-CHOP or intensive R-ACVBP regimens are not the optimal approaches for these patients, and that potential targeted therapy acting on the MYC oncogenesis pathway should be investigated.
Presented in part as an oral presentation at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
Presented as a poster at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-22, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the entire staff of the Lymphoma Study Academic Research Organization (LYSA) for managing the study, coordinating the clinical trials, and especially the LYSA platform for their expert assistance with histologic techniques and statistical analyses, and reporting. The authors also thank Laurence de Leval and Catherine Thieblemont for their critical review of the manuscript.
This study was supported in part by research funding from: Programme Hospitalier de Recherche Clinique Assistance Publique–Hôpitaux de Paris (AOM 03060), the Association pour la Recherche Thérapeutique, Génétique et Immunologique dans les Lymphomes, INSERM U955 eq9, and an Institut National du Cancer grant through the Institut National du Cancer/Directorate-General for Healthcare (DGOS) Genetic HEterogeneity of DIffuse large B-cell lymphoma (GHEDI) Groupe d'Etude des Lymphomes de l'Adulte (GELA) project. R.S. is supported for research on MYC-positive lymphomas through the Bundesministerium für Bildung und Forschung via the Molecular Mechanisms in Malignant Lymphomas Driven by MYC (MMML-MYC-SYS) project (0316166B).
Authorship
Contribution: C.C.-B., G.S., C.H., F.J., K.L., J.-P.J., H.T., T.J.M., and P.G. were responsible for the conception and design of the study; C.C.-B., G.S., C.H., F.J., K.L., J.-P.J., H.T., P.C.-D., M.B., J.B., R.D., D.C., M.P., K.B., B.F., C.R., T.P., N.K., F.P., I.N., R.S., T.J.M., and P.G. performed the data analysis and interpretation; C.C.-B., P.G., F.J., and T.J.M. wrote the manuscript; and all authors provided administrative support, provided study materials or patients, collected and assembled data, and approved the final manuscript.
Conflict-of-interest disclosure: C.C.-B. had an advisory role for Celgene and had travel accommodations provided by Celgene. G.S. received honoraria for advisory boards or meetings from Amgen, Celgene, Gilead, Janssen, Mundipharma, and Roche. K.M. had an advisory role for Celgene and received honoraria from Janssen, Roche, and Celgene; travel accommodations were provided by Amgen, Celgene, and Janssen. C.R. had an advisory role for Celgene and Sunesis; received research funding from Celgene and Chugai; had intellectual property interest with Affichem; and had travel accommodations provided by Amgen and Novartis. C.H. received honoraria from Roche, Amgen, Janssen, and Gilead; and had an advisory role for Janssen, Gilead, Takeda, and Roche. I.N. had travel accommodations provided by Affymetrix. F.J. received honoraria from Celgene, Roche, and Janssen. K.L. received honoraria from Astra-Zeneca and Boehringer Ingelheim. H.T. received honoraria from Celgene, Roche, and Janssen; had an advisory role for Takeda; and received research funding from Celgene. T.J.M. received travel accommodations from Amgen, Mundipharma, and Gilead; and had an advisory role for Merck. P.G. had an advisory role for Takeda and had travel accommodations provided by Zenyaku Kogyo. The remaining authors declare no competing financial interests.
Correspondence: Christiane Copie-Bergman, Département de Pathologie Hôpital Henri Mondor, 51 avenue du Maréchal de Lattre de Tassigny, 94010 Créteil, France; e-mail: christiane.copie@hmn.aphp.fr.
References
Author notes
T.J.M. and P.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal