Key Points
High ΔNp73/TAp73 expression ratio is associated with lower overall survival and higher cumulative incidence of relapse in APL.
ΔNp73/TAp73 expression ratio is an independent prognostic marker in APL.
Abstract
The TP73 gene transcript is alternatively spliced and translated into the transcriptionally active (TAp73) or inactive (ΔNp73) isoforms, with opposite effects on the expression of p53 target genes and on apoptosis induction. The imbalance between ΔNp73 and TAp73 may contribute to tumorigenesis and resistance to chemotherapy in human cancers, including hematologic malignancies. In acute promyelocytic leukemia (APL), both isoforms are expressed, but their relevance in determining response to therapy and contribution to leukemogenesis remains unknown. Here, we provide the first evidence that a higher ΔNp73/TAp73 RNA expression ratio is associated with lower survival, lower disease-free survival, and higher risk of relapse in patients with APL homogeneously treated with all-trans retinoic acid and anthracycline-based chemotherapy, according to the International Consortium on Acute Promyelocytic Leukemia (IC-APL) study. Cox proportional hazards modeling showed that a high ΔNp73/TAp73 ratio was independently associated with shorter overall survival (hazard ratio, 4.47; 95% confidence interval, 1.64-12.2; P = .0035). Our data support the hypothesis that the ΔNp73/TAp73 ratio is an important determinant of clinical response in APL and may offer a therapeutic target for enhancing chemosensitivity in blast cells.
Introduction
Accumulating evidence suggests that an imbalance between ΔNp73 and TAp73 proteins (both isoforms encoded by the TP73 gene) may contribute to both tumorigenesis and resistance to chemotherapy.1 Although the full-length TAp73 largely mimics p53 activities in experimental systems,2 the transactivation-deficient ΔNp73 isoform, by competition with p53 for DNA binding sites3 and/or ΔNp73 oligomerization with TAp73,4 exerts a dominant-negative effect on their functions. Overexpression of the ΔNp73 transcript has been associated with adverse prognosis and chemotherapy failure in several human tumors,5 including hematologic malignancies.6 Higher expression of ΔNp73 in relation to TAp73 transcripts (ΔNp73/TAp73 ratio) was previously associated with in vitro resistance to cytarabine-induced apoptosis in leukemic blast cells.7 Particularly in patients with acute promyelocytic leukemia (APL), the ΔNp73/TAp73 ratio varies substantially,7 but the clinical significance of this variation remains unclear. Here, we retrospectively quantified ΔNp73 and TAp73 transcript levels in samples from patients with APL treated in the International Consortium on Acute Promyelocytic Leukemia (IC-APL) study8 and correlated these findings with clinical and laboratory features, hematologic recovery, relapse, and survival.
Study design
Patients
A total of 129 consecutive patients with newly diagnosed APL who were enrolled in the IC-APL study were included. Details about the diagnosis, classification, and treatment protocol are published elsewhere.8 According to the Declaration of Helsinki, informed consent was obtained from all patients.
Gene expression profile of TP73 isoforms
The transcript levels of TP73 isoforms (ΔNp73 and TAp73) were quantified using the TaqMan Gene Expression Assay (Applied Biosystems), following the manufacturer’s instructions. Details can be found in supplemental Data (see supplemental Data available at the Blood Web site).
Statistical analysis
Patient baseline characteristics were reported descriptively. Using survival receiver operating characteristic curve analysis9 and the C index,10 we dichotomized patients into 2 groups according to ΔNp73/TAp73 ratio (low expression, <1.6; high expression, ≥1.6). All P values were 2 sided with a significance level of .05. Details of the statistical analysis and clinical end points can be found in supplemental Data.
Results and discussion
The ΔNp73/TAp73 ratio had a median value of 0.6 (range, 0-72.6) whereas the median values of the primary data from ΔNp73 and TAp73 were 11 (range, 0-3371) and 23.5 (range, 0-316), respectively. Supplemental Figure 1 shows the endogenous levels of ΔNp73 and TAp73 proteins in primary APL blasts. Baseline characteristics were similar between patients with low and high ΔNp73/TAp73 ratios (Table 1), except for higher white blood cell (WBC) counts in patients with high ΔNp73/TAp73 ratio (P = .0002). Considering Programa para el Tratamiento de Hemopatías Malignas/Gruppo Italiano Malattie Ematologiche in Adulti criteria,11 24% and 67% of patients assigned to the low and high ΔNp73/TAp73 groups were classified as having a high risk of relapse, respectively (P < .0001).
Clinical and baseline characteristics
| Characteristic . | All patients . | ΔNp73/TAp73 ratio . | P . | |
|---|---|---|---|---|
| Low expression . | High expression . | |||
| Sex, no. (%) | .07 | |||
| Female | 69 (53.5) | 56 (58.3) | 13 (39.4) | |
| Male | 60 (46.5) | 40 (41.7) | 20 (60.6) | |
| Age, median (range), y | 34 (9, 73) | 34 (16, 73) | 31 (9, 60) | .75 |
| ECOG performance status, no. (%) | .12 | |||
| 0 | 81 (62.8) | 64 (66.7) | 17 (51.5) | |
| 1 | 22 (17.1) | 16 (16.7) | 6 (18.2) | |
| 2 | 15 (11.6) | 11 (11.5) | 4 (12.1) | |
| ≥3 | 11 (8.5) | 5 (5.2) | 6 (18.2) | |
| Relapse-risk group, no. (%) | <.0001 | |||
| High | 45 (34.9) | 23 (24) | 22 (66.7) | |
| Intermediate | 61 (47.3) | 51 (53.1) | 10 (30.3) | |
| Low | 23 (17.8) | 22 (22.9) | 1 (3) | |
| Morphologic subtype, no. (%) | .37 | |||
| Hypergranular | 122 (94.6) | 92 (95.8) | 30 (90.9) | |
| Microgranular | 7 (5.4) | 4 (4.2) | 3 (9.1) | |
| PML breakpoint, no. (%) | .24 | |||
| BCR1 | 70 (54.3) | 54 (56.3) | 16 (48.5) | |
| BCR2 | 3 (2.3) | 3 (3.1) | — | |
| BCR3 | 44 (34.1) | 29 (30.2) | 15 (45.5) | |
| Unknown | 12 (9.3) | 10 (10.4) | 2 (6.1) | |
| Fever, no. (%) | .31 | |||
| No | 66 (51.2) | 52 (54.2) | 14 (42.4) | |
| Yes | 63 (48.8) | 44 (45.8) | 19 (57.6) | |
| WBC counts, no. (%), ×109/L | .0002 | |||
| ≤5 | 68 (52.7) | 59 (61.5) | 9 (27.3) | |
| 5-10 | 16 (12.4) | 14 (14.6) | 2 (6.1) | |
| 10-50 | 32 (24.8) | 17 (17.7) | 15 (45.5) | |
| >50 | 13 (1.1) | 6 (6.3) | 7 (21.2) | |
| Median (range) | 4.3 (0.2, 13.2) | 2.9 (0.2, 102.7) | 23.7 (0.5, 132.5) | |
| Platelet counts, no. (%), ×109/L | .49 | |||
| ≤40 | 96 (74.4) | 73 (76) | 23 (69.7) | |
| >40 | 33 (25.6) | 23 (24) | 10 (30.3) | |
| Median (range) | 27 (3, 128) | 27 (4, 128) | 30 (3, 99) | |
| Hemoglobin, no. (%), g/dL | .15 | |||
| ≤10 | 99 (76.7) | 77 (80.2) | 22 (66.7) | |
| >10 | 30 (23.3) | 19 (19.8) | 11 (33.3) | |
| Median (range) | 8.7 (3.4, 14.1) | 8.7 (4.4, 14.1) | 9.5 (3.4, 12.5) | |
| Creatinine, no. (%), mg/dL | .65 | |||
| ≤1.4 | 123 (95.3) | 92 (95.8) | 31 (93.9) | |
| >1.4 | 6 (4.7) | 4 (4.2) | 2 (6.1) | |
| Median (range) | 0.8 (0.3, 4.3) | 0.8 (0.3, 4.3) | 0.9 (0.4, 1.9) | |
| Uric acid, no. (%), mg/dL | .32 | |||
| ≤7 | 114 (88.4) | 82 (85.4) | 32 (97) | |
| >7 | 5 (3.9) | 5 (5.2) | — | |
| Unknown | 10 (7.8) | 9 (9.4) | 1 (3) | |
| Median (range) | 3.7 (0.8, 9.2) | 3.6 (0.8, 9.2) | 4.2 (2.1, 6.6) | |
| Fibrinogen, no. (%), mg/dL | .68 | |||
| ≤170 | 70 (54.3) | 51 (53.1) | 19 (57.6) | |
| >170 | 54 (41.9) | 42 (43.8) | 12 (36.4) | |
| Unknown | 5 (3.9) | 3 (3.1) | 2 (6.1) | |
| Median (range) | 159.5 (10, 605) | 160 (10, 605) | 159 (52.0, 373.0) | |
| Albumin, no. (%), g/dL | .39 | |||
| ≤3.5 | 19 (14.7) | 12 (12.5) | 7 (21.2) | |
| >3.5 | 81 (62.8) | 61 (63.5) | 20 (60.6) | |
| Unknown | 29 (22.5) | 23 (24) | 6 (18.2) | |
| Median (range) | 4 (2.2, 5.4) | 4.1 (2.2, 5.4) | 3.9 (2.3, 5.2) | |
| Coagulopathy, no. (%) | .70 | |||
| No | 47 (36.4) | 35 (36.5) | 12 (36.4) | |
| Yes | 80 (62) | 59 (61.5) | 21 (63.6) | |
| CR, % | 85 | 89 | 76 | .09 |
| 3-y OS, % (95% CI) | 84 (77, 90) | 91 (83, 95) | 67 (48, 80) | .0005 |
| 3-y DFS, % (95% CI) | 87 (78, 92) | 95 (88, 98) | 62 (39, 78) | <.0001 |
| 3-y CIR, % (95% CI) | 9 (4, 16) | 1.2 (0.1, 6) | 30 (13, 49) | <.0001 |
| 3-y CI of NRM, % (95% CI) | 4.6 (2, 10) | 3.5 (1, 9) | 8 (1.3, 23) | .36 |
| Characteristic . | All patients . | ΔNp73/TAp73 ratio . | P . | |
|---|---|---|---|---|
| Low expression . | High expression . | |||
| Sex, no. (%) | .07 | |||
| Female | 69 (53.5) | 56 (58.3) | 13 (39.4) | |
| Male | 60 (46.5) | 40 (41.7) | 20 (60.6) | |
| Age, median (range), y | 34 (9, 73) | 34 (16, 73) | 31 (9, 60) | .75 |
| ECOG performance status, no. (%) | .12 | |||
| 0 | 81 (62.8) | 64 (66.7) | 17 (51.5) | |
| 1 | 22 (17.1) | 16 (16.7) | 6 (18.2) | |
| 2 | 15 (11.6) | 11 (11.5) | 4 (12.1) | |
| ≥3 | 11 (8.5) | 5 (5.2) | 6 (18.2) | |
| Relapse-risk group, no. (%) | <.0001 | |||
| High | 45 (34.9) | 23 (24) | 22 (66.7) | |
| Intermediate | 61 (47.3) | 51 (53.1) | 10 (30.3) | |
| Low | 23 (17.8) | 22 (22.9) | 1 (3) | |
| Morphologic subtype, no. (%) | .37 | |||
| Hypergranular | 122 (94.6) | 92 (95.8) | 30 (90.9) | |
| Microgranular | 7 (5.4) | 4 (4.2) | 3 (9.1) | |
| PML breakpoint, no. (%) | .24 | |||
| BCR1 | 70 (54.3) | 54 (56.3) | 16 (48.5) | |
| BCR2 | 3 (2.3) | 3 (3.1) | — | |
| BCR3 | 44 (34.1) | 29 (30.2) | 15 (45.5) | |
| Unknown | 12 (9.3) | 10 (10.4) | 2 (6.1) | |
| Fever, no. (%) | .31 | |||
| No | 66 (51.2) | 52 (54.2) | 14 (42.4) | |
| Yes | 63 (48.8) | 44 (45.8) | 19 (57.6) | |
| WBC counts, no. (%), ×109/L | .0002 | |||
| ≤5 | 68 (52.7) | 59 (61.5) | 9 (27.3) | |
| 5-10 | 16 (12.4) | 14 (14.6) | 2 (6.1) | |
| 10-50 | 32 (24.8) | 17 (17.7) | 15 (45.5) | |
| >50 | 13 (1.1) | 6 (6.3) | 7 (21.2) | |
| Median (range) | 4.3 (0.2, 13.2) | 2.9 (0.2, 102.7) | 23.7 (0.5, 132.5) | |
| Platelet counts, no. (%), ×109/L | .49 | |||
| ≤40 | 96 (74.4) | 73 (76) | 23 (69.7) | |
| >40 | 33 (25.6) | 23 (24) | 10 (30.3) | |
| Median (range) | 27 (3, 128) | 27 (4, 128) | 30 (3, 99) | |
| Hemoglobin, no. (%), g/dL | .15 | |||
| ≤10 | 99 (76.7) | 77 (80.2) | 22 (66.7) | |
| >10 | 30 (23.3) | 19 (19.8) | 11 (33.3) | |
| Median (range) | 8.7 (3.4, 14.1) | 8.7 (4.4, 14.1) | 9.5 (3.4, 12.5) | |
| Creatinine, no. (%), mg/dL | .65 | |||
| ≤1.4 | 123 (95.3) | 92 (95.8) | 31 (93.9) | |
| >1.4 | 6 (4.7) | 4 (4.2) | 2 (6.1) | |
| Median (range) | 0.8 (0.3, 4.3) | 0.8 (0.3, 4.3) | 0.9 (0.4, 1.9) | |
| Uric acid, no. (%), mg/dL | .32 | |||
| ≤7 | 114 (88.4) | 82 (85.4) | 32 (97) | |
| >7 | 5 (3.9) | 5 (5.2) | — | |
| Unknown | 10 (7.8) | 9 (9.4) | 1 (3) | |
| Median (range) | 3.7 (0.8, 9.2) | 3.6 (0.8, 9.2) | 4.2 (2.1, 6.6) | |
| Fibrinogen, no. (%), mg/dL | .68 | |||
| ≤170 | 70 (54.3) | 51 (53.1) | 19 (57.6) | |
| >170 | 54 (41.9) | 42 (43.8) | 12 (36.4) | |
| Unknown | 5 (3.9) | 3 (3.1) | 2 (6.1) | |
| Median (range) | 159.5 (10, 605) | 160 (10, 605) | 159 (52.0, 373.0) | |
| Albumin, no. (%), g/dL | .39 | |||
| ≤3.5 | 19 (14.7) | 12 (12.5) | 7 (21.2) | |
| >3.5 | 81 (62.8) | 61 (63.5) | 20 (60.6) | |
| Unknown | 29 (22.5) | 23 (24) | 6 (18.2) | |
| Median (range) | 4 (2.2, 5.4) | 4.1 (2.2, 5.4) | 3.9 (2.3, 5.2) | |
| Coagulopathy, no. (%) | .70 | |||
| No | 47 (36.4) | 35 (36.5) | 12 (36.4) | |
| Yes | 80 (62) | 59 (61.5) | 21 (63.6) | |
| CR, % | 85 | 89 | 76 | .09 |
| 3-y OS, % (95% CI) | 84 (77, 90) | 91 (83, 95) | 67 (48, 80) | .0005 |
| 3-y DFS, % (95% CI) | 87 (78, 92) | 95 (88, 98) | 62 (39, 78) | <.0001 |
| 3-y CIR, % (95% CI) | 9 (4, 16) | 1.2 (0.1, 6) | 30 (13, 49) | <.0001 |
| 3-y CI of NRM, % (95% CI) | 4.6 (2, 10) | 3.5 (1, 9) | 8 (1.3, 23) | .36 |
CI, cumulative incidence; CIR, cumulative incidence of relapse; CR, complete remission; NRM, nonrelapse mortality.
Of the 129 subjects included in the study, 3 patients were lost to follow-up prior to the assessment of remission status and thus were not counted in the induction outcome analysis. Overall, 110 of 126 patients (87%) achieved complete hematologic remission. Although patients with a high ΔNp73/TAp73 ratio had a lower complete remission rate (76%) compared with those with a low ΔNp73/TAp73 ratio (89%), this difference did not reach significance (P = .09). Of 16 patients (13%) who failed to achieve complete remission, 12 (9%) died within 30 days after diagnosis (early mortality). Patients with a high ΔNp73/TAp73 ratio had an almost fivefold higher risk of early mortality (odds ratio, 4.8; 95% confidence interval [CI], 1.52-15.1; P = .008).
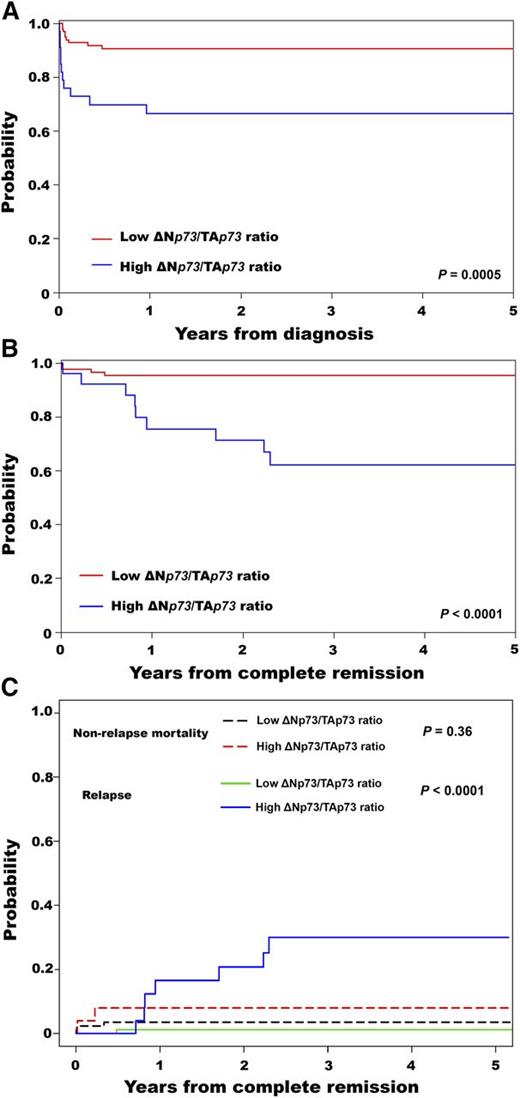
The median follow-up was 33 months (range, 1-72 months), with estimated 3-year overall survival (OS) and disease-free survival (DFS) of 84% (95% CI, 77%-90%) and 87% (95% CI, 78%-92%), respectively. Patients with a high ΔNp73/TAp73 ratio had a lower 3-year OS (67%; 95% CI, 48%-80%) compared with patients with a low ΔNp73/TAp73 ratio (91%; 95% CI, 83%-95%) (P = .0005) (Figure 1A). This result was consistent with the multivariable proportional hazards analysis (supplemental Table 2). Patients with a high ΔNp73/TAp73 ratio had significantly lower 3-year DFS (62%; 95% CI, 39%-78%) compared with patients with a low ratio (95%; 95% CI, 88%-98%) (P < .0001) (Figure 1B). In agreement, the 3-year CIR was higher in patients with a high ratio (P < .0001) (Figure 1C). The CINRM was similar between groups (P = .36; Figure 1C). Details for patient outcomes are summarized in Table 1.
Survival results for patients with APL according to the ΔNp73/TAp73 ratio. Probability of (A) OS, (B) DFS, and (C) cumulative incidence of relapse and nonrelapse mortality.
Survival results for patients with APL according to the ΔNp73/TAp73 ratio. Probability of (A) OS, (B) DFS, and (C) cumulative incidence of relapse and nonrelapse mortality.
It is important to highlight that prognostic factors that may predict inferior outcome in APL have been investigated widely. The most well-known risk factors are WBC and platelet counts at diagnosis,11 although internal tandem duplications of the FLT3 gene,12 aberrant expression of the CD56 antigen,13 deregulated expression of the MLL5 gene,14 and a polymorphic variant involving the promoter of the gene encoding the CD95 cell death receptor15 are potential predictors of poor prognosis in APL. Here, we provide the first evidence that higher ΔNp73/TAp73 ratio is associated with a lower remission rate, shorter survival, and higher risk of relapse in a relatively large series of patients with APL homogeneously treated with all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy. Importantly, we may not rule out the potential prognostic impact of the imbalance between ΔNp73 and TAp73 transcripts in other acute myeloid leukemia subtypes nor the role of other AML-associated fusion proteins than the promyelocytic leukemia/retinoic acid receptor alpha in TP73 gene expression.
Our findings are in accordance with previous studies, which also associated the ΔNp73 overexpression (alone or in association with TAp73, ie, ΔN/TA ratio) with treatment outcome in primary human tumors.16-18 Of interest, several alternative splice variants at the C termini (α-θ) and N terminus (p73Δex2, p73Δex2/3, collectively called ΔTAp73) of p73 have been described in human hematologic malignancies,19,20 and these isoforms may directly affect the ΔNp73/TAp73 ratio. Here, using specific probes for transcriptionally active (ie, TA) and inactive (ie, ΔN) isoforms, we evaluated the “pool” of TA and ΔNp73 transcripts, not distinguishing among the different C-termini or N-termini variants. At the protein level, multiple TAp73 isoforms were expressed in primary APL blasts, although no association between gene and protein expression has been demonstrated (supplemental Figure 1). This result must be regarded with caution because protein analysis was possible in only a few cases (6 patients). The underlying cause of the lack of association between messenger RNA and protein levels is not clear, but some evidences suggests that alternative pathways for selective degradation of p73 isoforms might be involved. Sayan et al showed that the ring-finger protein (PIR2) is transcriptionally induced by TAp73 and specifically degrades ΔNp73.21 In addition, Dulloo et al showed that the nonclassical polyamine-induced antizyme pathway degrades the ΔNp73 protein and stabilizes TAp73.22 Under “stress” conditions, c-Jun induces antizyme expression, which then directly interacts with ΔNp73 to induce degradation. Importantly, the decision about which isoform will be stabilized or degraded will depend on cell type and their environment, along with the need of the cell to make the decision to die or to repair the DNA damage.19,20,23,24
Particularly in APL, TP53 mutations are uncommon,25 which further highlights the relevance of the ΔNp73/TAp73 ratio to both survival advantage and failure to respond to chemotherapy. It is important to note that an intricate functional relationship between TP53 family members has been described26 and, although speculative, it is conceivable that in patients with wild-type TP53, the overexpression of ΔNp73 protein would impair p53 tumor-suppressor functions. Thus, survival advantage would be the result of a high ΔNp73/TAp73 ratio, rather than TP53 mutations. In line with this hypothesis, Concin et al showed that, in ovarian cancer patients, higher expression levels of ΔNp73 and ΔN′p73 isoforms were more frequently detected in TP53 wild-type tumors than in TP53 mutated ones.27 Moreover, patients harboring TP53 mutations and overexpressing ΔNp73 isoforms had a poorer response to chemotherapy.27
Considering that the combination of ATRA and arsenic trioxide probably will become the new standard of care for APL,28 it will be important to test the prognostic relevance of our findings in this context. It must be stressed that arsenic treatment is not available as first-line therapy in most countries in Latin America; therefore, we could not evaluate the impact of ΔNp73/TAp73 ratio in arsenic-treated patients. Future studies of the functional role of ΔNp73 could provide insight that might be useful in establishing new strategies and more effective therapies in APL, including the importance of APL treatment based on ATRA in combination with arsenic trioxide.28
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge all members of the International Consortium on Acute Leukemia of the American Society of Hematology.
This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP; grant #2013/08135-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNpQ; grant #573754/2008-0). A.R.L.-A. received a fellowship from FAPESP (grant #2007/55067-1).
Authorship
Contribution: A.R.L.-A. performed experiments, analyzed and interpreted data, and drafted the article; H.T.K. performed the statistical analyses and reviewed the paper; C.T. and J.L.C.-S. performed experiments, collected data, and reviewed the paper; R.H.J., R.A.M., R. Bittencourt, R.P., K.P., A.B.F.G., M.d.L.C., M.A., C.S.C., I.M., R. Bendlin, C.S., and C.B. provided the samples, updated the clinical data, and reviewed the paper; and S.L.S., M.S.T., D.G., A.G., N.B., R.C.R., F.L.-C., B.L., M.A.S., and E.M.R. designed the treatment protocol, reviewed the paper, and gave final approval of the submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eduardo M. Rego, Medical School of Ribeirao Preto, University of Sao Paulo. Av. Bandeirantes, 3900, Ribeirao Preto, SP 14048-900, Brazil; e-mail: edumrego@hotmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal