Key Points
Anti-Xa, aPTT, and ACT discriminate well between different heparin dose protocols but the assays are poorly correlated with each other.
The heparin effect was lower in younger children. This influence of age was dose-dependent and more pronounced at low- vs high-dose heparin.
Abstract
Monitoring unfractionated heparin (UFH) is crucial to prevent over- or under-anticoagulation. However, the optimal parameters for monitoring UFH in children are not well established. The study objectives were to investigate (1) the relationship between UFH dose and its anticoagulant effect as assessed by anti-Xa, activated partial thromboplastin time (aPTT) and activated clotting time (ACT); (2) other factors influencing UFH effect; (3) the agreement between the assays; and (4) the association between UFH effect and clinical outcome. HEARTCAT was a parallel-cohort randomized controlled trial comparing high-dose (100 U/kg bolus followed by age-based continuous infusion in randomized children) vs low-dose UFH (50 U/kg bolus) during cardiac catheterization in children. Blood samples were drawn before and after UFH administration at 30, 60, and 90 minutes. Four-hundred and two samples of 149 patients were evaluable. Anti-Xa, aPTT, and ACT all showed good discrimination between UFH doses. Regression models demonstrated the following determinants of UFH effect: UFH dose, age, baseline antithrombin (for anti-Xa), and baseline levels of aPTT and ACT, respectively. UFH effects were lower in infants compared with older children, which was more pronounced at low-dose than at high-dose UFH. Agreement between the 3 assays was poor. Most aPTT values were above therapeutic range or beyond measuring limit and thus of limited value for UFH monitoring. No association of UFH dose or effect with clinical outcome could be observed. In conclusion, all assays reflected a significant UFH dose-effect relationship, however, with poor agreement between the respective tests. The age-dependency of UFH effect was confirmed. Notably, the influence of age on UFH effect was dose-dependent.
Introduction
Unfractionated heparin (UFH) is the most commonly used anticoagulant administered for primary prophylaxis of thrombotic events (TE) in children.1 As much as 15% of inpatients at tertiary care pediatric centers are regularly exposed to UFH.2 The pharmacodynamic and pharmacokinetic properties of UFH are complex, leading to significant interindividual variation of the anticoagulant response to the same weight-adapted UFH dose.3 This variation is especially profound in infants and children whose hemostatic system is still developing.4-7 Therefore, laboratory monitoring of the UFH effect is crucial to prevent both under- and overanticoagulation. For children receiving therapeutic UFH, an activated partial thromboplastin time (aPTT) range correlating to an anti-Xa level of 0.35 to 0.7 U/mL is recommended.1 However, these recommendations are extrapolated from studies in adults, and their appropriateness for children is increasingly questioned.1,8-11
Several pediatric studies demonstrated poor correlation between UFH dose and its anticoagulant effect as assessed by various laboratory methods and poor agreement between the respective tests.12-24 However, only 3 studies prospectively investigated the effect of a defined IV single bolus of UFH in children, and none of them compared different UFH dosage protocols or evaluated the clinical outcome regarding thrombotic or bleeding events.21-23,25 Because UFH, given its short half-life and reversibility, will continue to be a preferred anticoagulant for children, systematic studies on monitoring UFH in children are needed.
HEARTCAT (Heparin Anticoagulation Randomized Trial in Cardiac Catheterization) was a parallel-cohort randomized controlled trial comparing 2 UFH dose protocols for primary prevention of TE in children undergoing cardiac catheterization.26 The present study reports the results of the laboratory substudy investigating the monitoring of UFH in children. The specific study objectives were (1) to investigate the relationship between UFH dose and UFH anticoagulant effect as assessed by various assays, (2) to assess other factors influencing the UFH effect, (3) to determine the agreement between these assays, and (4) to describe the association between UFH effect and clinical outcome.
Methods
Study design
The study design was a single-center, double-blinded, parallel-cohort randomized controlled trial (RCT) of consecutive children undergoing cardiac catheterization comparing a high-dose (100 U/kg body weight bolus followed by continuous infusion of 20 U/kg per hour for children >1 year or 28 U/kg per hour for infants) vs a low-dose (50 U/kg bolus) UFH protocol.26 Patients with no consent for randomization received UFH as per standard-of care and were followed in a parallel cohort (50 U/kg for venous diagnostic catheterization, 100 U/kg for arterial diagnostic and any interventional catheterization). Patients in the cohort study consented to clinical outcome assessment and laboratory testing. All patients were treated with UFH (UFH “Immuno”, 1000 IU/mL; EBEWE Pharma, Austria) IV during cardiac catheterization. The study protocol was approved by the ethics committee of the Medical University of Vienna and registered as a clinical trial in EudraCT, registration #2005-004150-27 (https://clinicaltrialsregister.eu). The study design has previously been described in detail.26
Study population
The study population consisted of patients, 0 to 18 years of age, requiring diagnostic or interventional cardiac catheterization at the Division of Pediatric Cardiology, Medical University of Vienna. Written informed consent was obtained from parents and patients of appropriate age, in accordance with the Declaration of Helsinki. Exclusion criteria were preexisting anticoagulation or antiplatelet therapy.
Study outcomes
To monitor UFH during cardiac catheterization, blood samples were taken to measure anti–factor-Xa (anti-Xa), aPTT, ACT, anti–factor-IIa (anti-IIa), protamine titration, and antithrombin levels. The results of anti-IIa, protamine titration, and thrombin generation will be reported in a separate paper.
Clinical efficacy outcome was a thromboembolic event at the puncture site diagnosed by vascular ultrasonography, and safety outcome was bleeding at the puncture site or other locations as previously described in detail.26
Blood sample acquisition
Blood samples were drawn directly from cardiac catheters or vascular sheaths. To avoid UFH contamination, UFH was strictly administered via an additional peripheral venous line. A baseline sample was obtained immediately after the insertion of the femoral venous or arterial sheath and before the administration of UFH. Thereafter, blood samples were taken during the procedure at predefined time points (30, 60, and 90 minutes after UFH administration, and at the end of the procedure). The ACT was measured immediately, and the rest of the blood samples were collected in tubes containing 3.8% sodium citrate in a proportion of 9:1. Platelet-poor plasma was prepared by centrifugation at 1300g for 10 minutes, aliquoted, and frozen at −80°C for batch analysis.
Anti–factor-Xa assay
The anti-Xa assay was performed on the STA Compact Coagulation Analyzer (Diagnostica STAGO) according to the manufacturer’s instructions and using the commercially available test kit STA Rotachrom Heparin, Diagnostica STAGO, which does not contain antithrombin supplementation. The therapeutic range used for anti-Xa was 0.35 to 0.7 U/mL.
aPTT
APTT values were measured using the commercially available test kit STA APTT, Diagnostica Stago (STA Compact Coagulation Analyzer, Diagnostica STAGO) according to the manufacturer’s instructions. For the purpose of this study, the upper limit of time measurement was set at 300 seconds, and unrecordable high values were set at 301 seconds. The therapeutic range used for the aPTT was 60 to 85 seconds.
Activated clotting time
The ACT was measured using the ACTester (QUEST Medical Inc., Allentown Parkway, Allen, TX). Immediately after acquiring each blood sample, 0.6 mL of whole blood was added into a cartridge containing a coagulation activator (6-20 mg bruised diatomaceous earth) and swung 20 times. Then the cartridge was put into the ACTester and the time measurement was started. Once a clot was detected by photometric methods, the time measurement stopped and the ACT was shown on the screen of the ACTester. For the purpose of this study, unrecordable high results were set at 500 seconds. The therapeutic range used for the ACT was 150 to 250 seconds, representing the therapeutic range used for children on extracorporeal membrane oxygenation at our institution.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 for Windows (Chicago, IL). Continuous variables are presented as median, minimum, and maximum values; categorical variables as absolute frequencies; and percentages. Figures display medians with 95% confidence intervals (CI). Linear mixed-regression modeling was performed to assess the influence of UFH dose, body weight, age, sex, and baseline antithrombin level on the UFH anticoagulant response assessed by anti-Xa, aPTT, and ACT, respectively. Various models with UFH dose or age either as continuous variable or as categorical variable (low-dose vs high-dose group; infants vs children) were calculated, and the best fitting model according to Akaike information criterion values was chosen as the final model, which is presented in the Results section. No significant differences in UFH levels over time could be observed between the high-dose group of the RCT (receiving a UFH bolus followed by continuous infusion) and the high-dose group of the cohort study (high-dose UFH bolus only). Therefore, the results of the RCT and the cohort study were combined. To quantify the agreement between the results of different UFH monitoring assays, Cohen κ was calculated. A κ value of <0.4 was considered a poor agreement.27 To test whether UFH effects were associated with clinical outcomes, logistic regression modeling was performed with TE or bleeding complications as dependent variables and anti-Xa, aPTT, and ACT results (30 minutes post-UFH samples), respectively, as independent variables, without and with inclusion of covariables such as dose and age.
Results
Study population
The flow of participants in the HEARTCAT study has previously been described in detail in the publication reporting clinical outcomes.26 Of 227 children enrolled in the overall study, 163 had blood samples taken for UFH monitoring. The blood samples of 14 patients could not be evaluated for the study for several, mostly technical reasons (eg, clotted sample, no heparin administered). Therefore, the final cohort of the laboratory study consisted of 149 patients (402 blood samples including baseline samples before UFH administration).
Patient demographics
The median age was 5.5 years (minimum 0.01, maximum 18) and the median body weight was 17 kg (3.1; 90.5). There were 29 (19%) infants, 50 (34%) children 1 to 5 years old, 30 (20%) children 6 to 10 years old, and 40 (27%) children 11 to 18 years old. Eighty-five (57%) patients were female. Overall, 78 (52%) patients were in the low-dose and 71 (48%) were in the high-dose UFH group; 94 (63%) patients were enrolled in the RCT (47 [50%] low-dose, 47 [50%] high-dose UFH), and 55 (37%) patients in the parallel cohort receiving UFH as per standard-of-care (31 [56%] low-dose, 24 [44%] high-dose UFH).
Timing of blood samples
Samples obtained during cardiac catheterization were taken at the predefined time points of 30, 60, and 90 minutes after UFH administration. However, the final samples at completion of catheterization were taken at various times because the duration of the procedure varied considerably, sometimes ending even before 30 minutes. As a result, the actual timing of sampling was somewhat heterogeneous. For the purpose of analysis, samples obtained within defined time bands were assigned to the predefined time points (20-44 = 30 min, n = 149; 45-74 = 60 min, n = 42; 75-104 = 90 min, n = 41). Because only a few (n = 21) samples were collected beyond 104 minutes after UFH administration, these were not used for the analysis of UFH time course and age effect but were evaluated for the comparison of UFH assays.
Time course of UFH effect
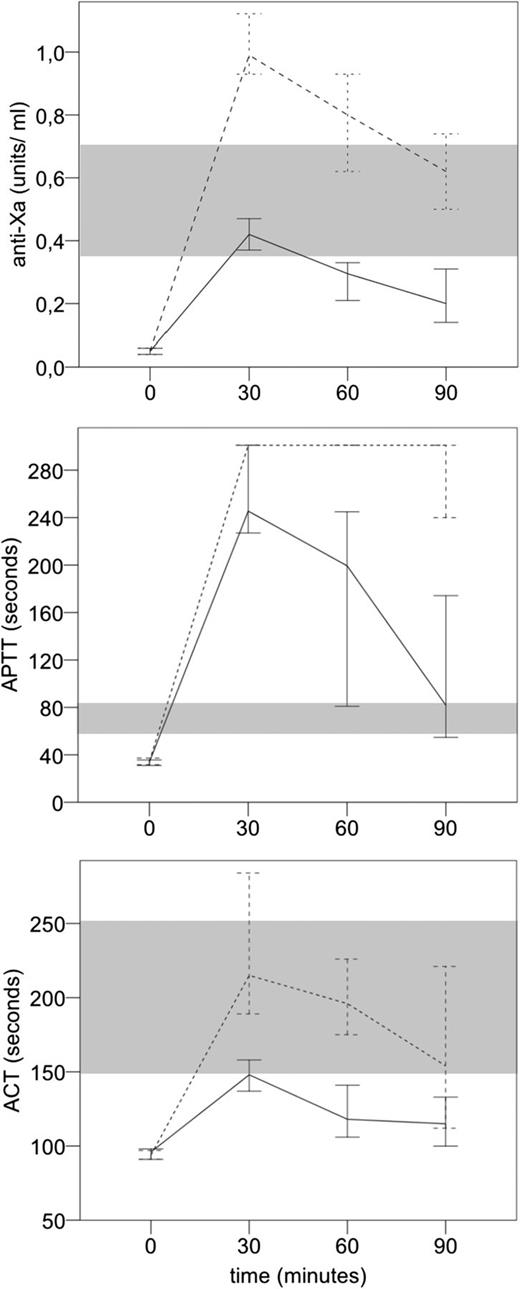
Figure 1 shows the time course of median anti-Xa, aPTT, and ACT values after administration of UFH. All parameters showed peak levels at 30 minutes and then decreased quite rapidly, except for aPTT values in the high-dose group, which remained above the measurement limit. For all parameters, there was a clear discrimination between high and low dose over all times points. Anti-Xa levels in the high-dose group were above therapeutic range until 60 minutes after UFH administration, whereas in the low-dose group, anti-Xa levels were within therapeutic range only at 30 minutes and dropped below the lower limit at 60 minutes. aPTT values were above therapeutic range at all time points in both UFH dose groups. ACT values in the high-dose group were within therapeutic range at all time points, whereas in the low-dose group, they were below therapeutic range at all times.
Time course of anti-Xa, aPTT, and ACT values (median, 95% CI) comparing the high-dose vs the low-dose UFH groups. Shaded areas represent the therapeutic ranges for the respective test.
Time course of anti-Xa, aPTT, and ACT values (median, 95% CI) comparing the high-dose vs the low-dose UFH groups. Shaded areas represent the therapeutic ranges for the respective test.
Factors influencing UFH effect
Table 1 shows the results of the linear mixed models analyzing the influence of various parameters on anti-Xa, aPTT, or ACT levels over time after UFH administration. The best fitting models are presented including only significant determinants (P < .05).
Linear mixed regression models of factors influencing the effect of UFH (anti-Xa, aPTT, ACT)
| UFH assay . | Determinant . | b . | 95% CI . | P . |
|---|---|---|---|---|
| Anti-Xa (U/mL) | UFH dose group (high vs low) | 0.66 | 0.42; 0.89 | <.001 |
| Age (per year) | 0.02 | 0.01; 0.04 | .001 | |
| Baseline antithrombin (per 0.1 U/mL) | 0.06 | 0.02; 0.10 | .003 | |
| Sample time point (30, 60, 90 min) | −0.16 | −0.21; −0.10 | <.001 | |
| Interaction UFH dose group × sample time point | −0.08 | −0.01; −0.15 | .019 | |
| aPTT (s) | Interaction UFH dose group × sample time point | 42 | 17; 68 | .001 |
| Age (per year) | 3 | 1; 5 | .003 | |
| Sex (female) | 25 | 45.2; 4.9 | .015 | |
| Baseline aPTT (per second) | 2 | 1.8; 4 | .004 | |
| ACT (s) | UFH dose group (high vs low) | 190 | 117; 263 | <.001 |
| Baseline ACT (per second) | 1 | 0; 2 | .009 | |
| Sample time point (30, 60, 90 min) | −55 | −70; −39 | <.001 | |
| Interaction UFH dose group × sample time point | −36 | −57; −16 | .001 |
| UFH assay . | Determinant . | b . | 95% CI . | P . |
|---|---|---|---|---|
| Anti-Xa (U/mL) | UFH dose group (high vs low) | 0.66 | 0.42; 0.89 | <.001 |
| Age (per year) | 0.02 | 0.01; 0.04 | .001 | |
| Baseline antithrombin (per 0.1 U/mL) | 0.06 | 0.02; 0.10 | .003 | |
| Sample time point (30, 60, 90 min) | −0.16 | −0.21; −0.10 | <.001 | |
| Interaction UFH dose group × sample time point | −0.08 | −0.01; −0.15 | .019 | |
| aPTT (s) | Interaction UFH dose group × sample time point | 42 | 17; 68 | .001 |
| Age (per year) | 3 | 1; 5 | .003 | |
| Sex (female) | 25 | 45.2; 4.9 | .015 | |
| Baseline aPTT (per second) | 2 | 1.8; 4 | .004 | |
| ACT (s) | UFH dose group (high vs low) | 190 | 117; 263 | <.001 |
| Baseline ACT (per second) | 1 | 0; 2 | .009 | |
| Sample time point (30, 60, 90 min) | −55 | −70; −39 | <.001 | |
| Interaction UFH dose group × sample time point | −36 | −57; −16 | .001 |
b, unstandardized partial correlation coefficient; CI, confidence interval.
Anti-Xa levels were significantly associated with UFH dose group, age, baseline antithrombin level, and sample time point, and there was interaction between dose group and sample time point. For example, anti-Xa levels increased by an average of 0.02 U/mL for every year of age, and by 0.06 U/mL per 0.1 U/mL higher baseline antithrombin levels. In the overall study cohort, median antithrombin at baseline was 0.9 U/mL (minimum 52, maximum 116), and 24% of patients had antithrombin levels below respective age-specific reference values.7 Anti-Xa levels decreased over time after UFH administration and decreased more rapidly in the high-dose group (Figure 1).
For aPTT levels, there was a significant interaction between UFH-dose group and sample time point since (Table 1), an artificial phenomenon because levels in the high-dose dose group remained beyond measurement limits, whereas the low-dose group declined steeply over time (Figure 1). In addition, post-UFH APTT levels were positively associated with age, female sex, and baseline aPTT level.
ACT levels significantly increased with UFH dose group and baseline ACT level, and decreased over sample time points, with an interaction between dose group and sample time point (ie, a more rapid decrease in the high-dose group) (Figure 1). Age was not significantly associated with the UFH effect on ACT levels.
Influence of age on UFH effect
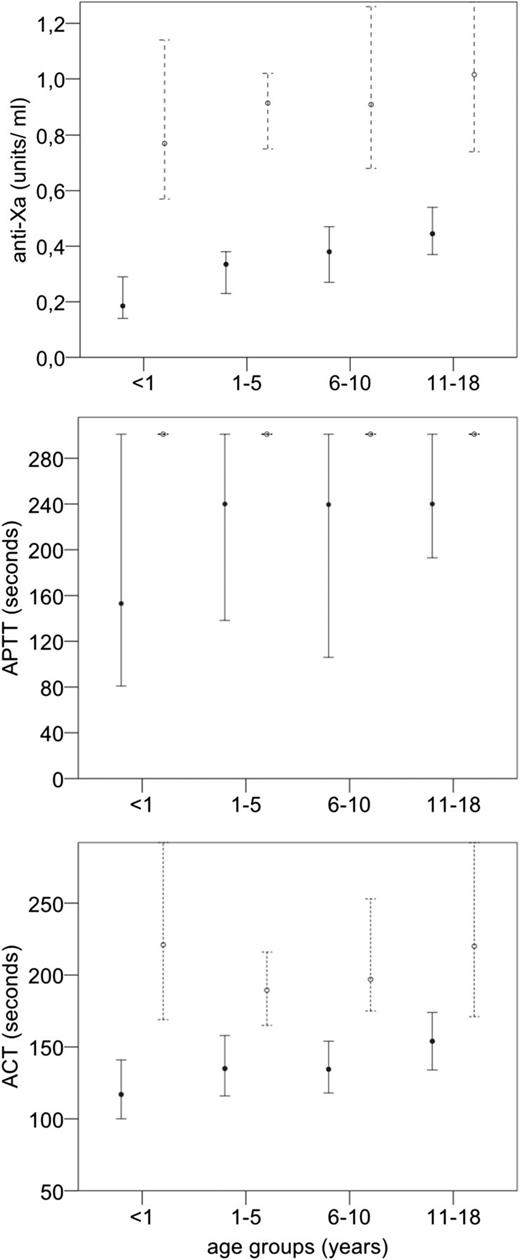
Figure 2 shows median anti-Xa, aPTT, and ACT values after UFH administration by age groups, separate for dose groups but independent of time point (30, 60, and 90 minutes). All parameters were lowest in infants and, in most instances, steadily increased over age groups 1 to 5 years, 6 to 10 years, and 11 to 18 years. Exceptions were aPTT levels in the high-UFH dose group that were all beyond measurement limits, and ACT values that did not increase with age in the high-dose group and only slightly increased over age groups in the low-dose group.
Stratification by age groups of anti-Xa, aPTT, and ACT values (median, 95% CI) in samples after UFH administration comparing dose groups (high-dose vs low-dose).
Stratification by age groups of anti-Xa, aPTT, and ACT values (median, 95% CI) in samples after UFH administration comparing dose groups (high-dose vs low-dose).
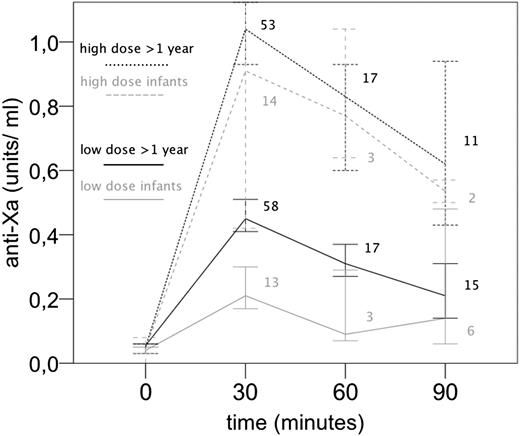
In the overall regression models including all time points, there was no significant interaction of age and dose (ie, the differences between age groups was not dependent on dose). However, when evaluating anti-Xa levels at peak (30 minutes after UFH administration), infants had significantly lower levels compared with older children in the low-dose group but not in the high-dose group (Figure 3; interaction dose group × age group, P = .001). Thus, although infants had a significantly decreased response to low-dose UFH compared with older children, this influence of age on the UFH effect diminished at high-dose UFH. Similar trends were seen for the aPTT and ACT but were not statistically significant.
Time course of anti-Xa values (median, 95% CI) comparing age groups (infants vs older children) and dose groups (high-dose vs low-dose). Numbers indicate the number of samples available per time point. Significant interaction of age group × dose group at time point 30 minutes (P = .001).
Time course of anti-Xa values (median, 95% CI) comparing age groups (infants vs older children) and dose groups (high-dose vs low-dose). Numbers indicate the number of samples available per time point. Significant interaction of age group × dose group at time point 30 minutes (P = .001).
Agreement between assays
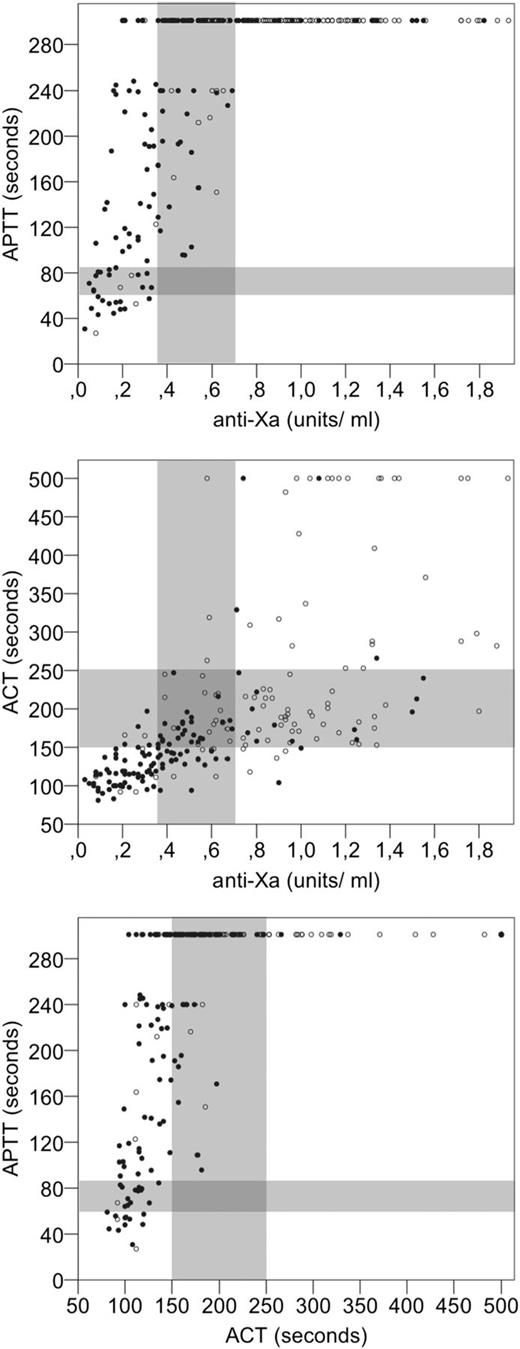
Figure 4 shows the results of anti-Xa, aPTT, and ACT plotted against each other for all samples after UFH administration. Shaded areas represent the respective target therapeutic ranges as defined. Table 2 shows agreement between the respective assays, the bold font indicates concordant cells for results below, within, and above therapeutic ranges. aPTT results showed very poor agreement with both anti-Xa and ACT results. There was zero concordance of results within therapeutic range. Values within the therapeutic range for anti-Xa and ACT were all above therapeutic range for APTT and mostly beyond measuring limit (median 301 seconds; minimum 96; maximum 301). For samples with anti-Xa values below therapeutic range, aPTT results ranged from 38 to 300 seconds. Agreement was better between anti-Xa and ACT with more concordant results below, between and above therapeutic ranges, respectively (in total 57%). However, there were relevant proportions of values within therapeutic range for anti-Xa but below for ACT (13%) and above therapeutic range for anti-Xa but within for ACT (23%). Cohen κ as a measure of agreement between assays was κ = 0.37 for ACT vs anti-Xa, κ = 0.21 for aPTT vs anti-Xa, and κ = 0.04 for aPTT vs ACT.
Agreement between anti-Xa, aPTT, and ACT results for individual samples after UFH administration plotted against each other. Black dots represent low dose and white dots the high-dose group.
Agreement between anti-Xa, aPTT, and ACT results for individual samples after UFH administration plotted against each other. Black dots represent low dose and white dots the high-dose group.
Agreement between anti-Xa, APTT and ACT assays for results below, within, and above therapeutic ranges for samples after administration of UFH
| . | anti-Xa . | ||
|---|---|---|---|
| aPTT . | <0.35 U/mL . | 0.35-0.7 U/mL . | >0.7 U/mL . |
| >85 s | 38 (16%) | 82 (33%) | 97 (39%) |
| 60-85 s | 15 (6%) | 0 (0%) | 0 (0%) |
| <60 s | 14 (6%) | 0 (0%) | 0 (0%) |
| . | anti-Xa . | ||
|---|---|---|---|
| aPTT . | <0.35 U/mL . | 0.35-0.7 U/mL . | >0.7 U/mL . |
| >85 s | 38 (16%) | 82 (33%) | 97 (39%) |
| 60-85 s | 15 (6%) | 0 (0%) | 0 (0%) |
| <60 s | 14 (6%) | 0 (0%) | 0 (0%) |
| . | anti-Xa . | ||
|---|---|---|---|
| ACT . | <0.35 U/mL . | 0.35-0.7 U/mL . | >0.7 U/mL . |
| >250 s | 0 (0%) | 3 (1%) | 34 (15%) |
| 150-250 s | 7 (3%) | 41 (18%) | 51 (23%) |
| <150 s | 53 (24%) | 29 (13%) | 6 (3%) |
| . | anti-Xa . | ||
|---|---|---|---|
| ACT . | <0.35 U/mL . | 0.35-0.7 U/mL . | >0.7 U/mL . |
| >250 s | 0 (0%) | 3 (1%) | 34 (15%) |
| 150-250 s | 7 (3%) | 41 (18%) | 51 (23%) |
| <150 s | 53 (24%) | 29 (13%) | 6 (3%) |
| . | ACT . | ||
|---|---|---|---|
| aPTT . | <150 s . | 150-250 s . | >250 s . |
| >85 s | 62 (28%) | 98 (44%) | 37 (17%) |
| 60-85 s | 14 (6%) | 0 (0%) | 0 (0%) |
| <60 s | 13 (6%) | 0 (0%) | 0 (0%) |
| . | ACT . | ||
|---|---|---|---|
| aPTT . | <150 s . | 150-250 s . | >250 s . |
| >85 s | 62 (28%) | 98 (44%) | 37 (17%) |
| 60-85 s | 14 (6%) | 0 (0%) | 0 (0%) |
| <60 s | 13 (6%) | 0 (0%) | 0 (0%) |
Cells show absolute numbers (% of total number of samples: n = 246 for anti-Xa vs APTT; n = 224 for anti-Xa vs ACT and APTT vs ACT). Concordant cells are bold.
Association of UFH effect with clinical outcome
The previous HEARTCAT paper reported that there was no significant relationship between UFH dose group and the incidence of TE and bleeding complications during and after cardiac catheterization.26 Infants had an increased incidence of TE and bleeding independent of dose. To test whether the UFH effects actually achieved in plasma were associated with clinical outcomes, logistic regression was performed, without and with inclusion of the covariables UFH dose group, age, antithrombin at baseline, and sex. The results of logistic regression analysis revealed no association of UFH effect as measured by any of the assays with TE or bleeding complications.
Discussion
HEARTCAT was a large RCT comparing high-dose vs low-dose UFH during cardiac catheterization in children that evaluated UFH effect in patients’ plasma monitored by various laboratory assays and determined clinical outcomes using objective assessment. Thus, HEARTCAT was well designed for the objectives of (1) to investigate the relationship between UFH dose and UFH effect, (2) to identify other determinants of UFH effect, (3) to compare various laboratory assays to monitor UFH, and (4) to determine whether UFH dose or effect are associated with clinical outcome. Thereby, the study aimed to critically assess the optimal UFH dose to prevent TE or bleeding during cardiac catheterization.
Previous studies on UFH monitoring in children had several limitations. Some studies were performed only in vitro using plasma from children,28,29 whereas others included only small numbers of patients,14,16,17,20 focused exclusively on older children18 or on critically ill children during intensive care who received different UFH doses for various indications.14-17,19 Only 3 studies prospectively investigated the anticoagulant response to a defined IV single UFH bolus in children, but none compared different UFH doses and none evaluated clinical outcome regarding thrombotic or bleeding complications.21-23,25
HEARTCAT investigated primary UFH prophylaxis in clinically stable children who mostly had elective catheterization. Another design feature was the clear separation of UFH administration via a peripheral vein and UFH sampling via large-bore cardiac catheters. Thus, HEARTCAT represents a homogeneous patient population studied with well-controlled methodology. The results can be taken as a model situation; however, it should be noted that considerations for dose and therapeutic range cannot fully be extrapolated to other clinical situations. A limitation of the current study is that most patients received a single UFH bolus and only a proportion of patients received a continuous UFH infusion (RCT high dose). Because there was no difference in UFH levels between those with and without continuous infusion, likely the UFH effect over the first 90 minutes was primarily affected by the UFH bolus. Therefore, the data do not reflect steady-state heparinization.
In HEARTCAT, anti-Xa, aPTT, and ACT discriminated well between the high-dose and low-dose UFH protocol studied. The clear dose-response relationship observed in the current study differs from the results of a previous study by Kuhle et al, who reported a poor correlation between UFH dose administered and the anticoagulant response as assessed by anti-Xa and aPTT.17 At least 3 facts may explain these differences. First, in this randomized comparison there was a more clear-cut separation of doses and probably less analytical variability. Second, Kuhle et al investigated the UFH response in critically ill children requiring intensive care. In clinically unstable patients, there may be more factors influencing the UFH effect (eg, variable plasma and cell binding of UFH, variable antithrombin levels, etc). Third, the UFH dose administered was lower (20-40 U/kg) than both the low and the high doses in the current study.
Patients’ ages had a significant influence on the UFH effect as measured by anti-Xa and aPTT. Infants demonstrated reduced UFH effect compared with older children. Age dependency of UFH effect was most evident when measured by the anti-Xa followed by the aPTT, despite its high variability and the artificial ceiling effect. The ACT also showed a trend to increase with age. These findings are consistent with previous reports on the age dependency of the UFH effect in children.14,15,19,25,29 An in vitro study by Ignjatovic et al showed that aPTT values corresponding to a therapeutic anti-Xa level (0.35-0.7 U/mL) were significantly higher in plasma from infants and young children than in older children.29 They also investigated the in vivo effect of UFH administered for various indications on a pediatric intensive care unit and found that younger children had lower anti-Xa compared with older children.19 Newall et al investigated the UFH effect in children undergoing cardiac catheterization and found a trend toward increasing anti-Xa levels with age, suggesting that older children were more sensitive to UFH.25 HEARTCAT confirms the age dependency of the UFH effect in a large cohort and adds important information regarding UFH dose. Interestingly, the difference in UFH effect between age groups was more pronounced at low UFH dose and almost inexistent at high-dose UFH. This age-dose interaction was significant only for anti-Xa and when comparing infants vs all older age groups, but there were similar trends for the other UFH assays and across increasing age groups. Thus, the influence of age on UFH effect appears to be dose-dependent.
Other determinants of UFH effect were baseline antithrombin levels for anti-Xa and baseline levels of aPTT and ACT for their respective post-UFH levels. The anti-Xa assay used in this study was not supplemented with excess antithrombin, hence patients’ endogenous antithrombin level did affect the UFH effect. The results support the view that nonantithrombin-supplemented anti-Xa assays better reflect the physiologic dependence of UFH on in vivo antithrombin levels.16,17,28 Antithrombin was an independent determinant of anti-Xa in addition to the influence of age in multiple regression analysis. Thus, lower antithrombin levels in infants, as well as pathologically decreased antithrombin levels, influenced the UFH effect. Conversely, the decreased response to UFH at a young age is apparently not solely mediated through lower antithrombin levels.
There was poor agreement among the 3 assays investigated. For samples within therapeutic anti-Xa range, aPTT values were all high and mostly beyond measuring limit but still showed large variability. These results are similar to previous studies that also found highly increased and partially unrecordable aPTT values at therapeutic anti-Xa range.15,22 However, in contrast to Newall et al, who found 28% of samples within anti-Xa and aPTT therapeutic range, the present study observed no concordance within the therapeutic range. There was better agreement between anti-Xa and ACT assays, but there were relevant proportions of discordant values. The therapeutic range applied for the ACT is used for children receiving extracorporeal membrane oxygenation, because there is no target range specific for cardiac catheterization reported in the literature.
The best discrimination between doses was achieved by the anti-Xa. Because of its test principle, the anti-Xa is most specific for the effect of UFH and is less sensitive to other variables in the coagulation system. Moreover, the anti-Xa test seems to have the least analytical variability. Both ACT and aPTT are influenced by other variables in the coagulation system and therefore may be better markers of the overall coagulability of blood. Interestingly, however, the anti-Xa is influenced by physiologic factors because it showed the most distinct age-dependent differences of UFH effect. In summary, the study results favor anti-Xa over aPTT for UFH monitoring in children. We conclude that the aPTT used was simply too sensitive for monitoring UFH in children in the setting of cardiac catheterization. Moreover, it is well known that the aPTT is affected by numerous preanalytic and analytic variables that cause its unspecific variability.30 The ACT proved a comparably reliable assay for UFH monitoring and is a good alternative where a bedside test is more appropriate.
Frequencies of TE and bleeding were low and not associated with UFH dose or effect. Therefore, our study did not allow validating the optimal dose and appropriate therapeutic range based on clinical outcomes. One may conclude that neither the high nor low UFH dose was associated with a relevant risk of over- or underanticoagulation in cardiac catheterization. Interestingly, the incidence of both thrombosis and bleeding was increased in infants compared with older children, but this was also not dependent on UFH dose or UFH effect. Thus, the lower UFH effects observed in infants did not translate into different risks of clinical outcomes. Rather, the higher frequency of complications in infants may be attributed to anatomical differences and the technical challenges of catheterization of smaller vessels. In a study of infants younger than 6 months of age who were treated for deep venous thrombosis with continuous UFH targeting therapeutic anti-Xa levels, many infants remained subtherapeutic despite increased UFH doses.24 The majority of infants had thrombus regression and no recurrence, but there was an 11% risk of major bleeding. Based on these data, infants may not require increased UFH doses despite decreased UFH response based on standard laboratory tests.
Clinical outcomes were not informative to establish the optimal UFH dose for children undergoing cardiac catheterization. Further, there is no established anticoagulant target level for the prevention of cardiac catheterization–associated thrombotic complications in children. As a proxy, one may speculate based on UFH effects achieved in plasma and using the anti-Xa and its therapeutic range of 0.35 to 0.7 U/mL used for treatment of TE. The 100 U/kg UFH bolus achieved mostly supratherapeutic anti-Xa levels, whereas 50 U/kg resulted in marginal/subtherapeutic levels. For infants, the UFH effect of 50 U/kg was too low, but 100 U/kg still induced supratherapeutic anti-Xa levels (Figure 4). Thus, a 75 U/kg bolus dose appears equally appropriate for children and infants during cardiac catheterization, because age differences of UFH effect were small at high dose.
In conclusion, HEARTCAT demonstrated that all 3 assays discriminated well between high-dose and low-dose UFH, but with poor agreement between assays. Infants showed lower UFH effects in plasma compared with older children. This age dependency of UFH effect was more pronounced at low UFH dose than at high dose. The aPTT showed limited value for UFH monitoring. There was no association of UFH dose or UFH effect achieved in plasma with clinical outcome. From the UFH effects observed, we conclude that a UFH bolus dose of 75 U/kg appears most appropriate for the setting of cardiac catheterization, both for older children and for infants.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Paul Monagle for his valuable advice on data analysis and interpretation, and Eva Wissmann for her contribution to data and the blood sample collection.
Authorship
Contribution: A.H. designed the study, analyzed data, and wrote the manuscript; E.K. and K.T. collected data and contributed substantially in writing the manuscript; H.K., N.P., and J.V. collected blood samples and data and performed laboratory analyses; U.S.T. performed statistical analyses and critically revised the manuscript; I.M.-B. provided valuable support for performing the study and critically revised the manuscript; F.N. contributed to data analysis and critically revised the manuscript; and C.M. designed the study, participated in data analysis, and contributed substantially in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Male, Division of Pediatric Cardiology, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Waehringer Gürtel 18-20, 1090 Vienna, Austria; e-mail: christoph.male@meduniwien.ac.at.