In this issue of Blood, Hanslik et al compare 2 dose protocols for unfractionated heparin in children undergoing cardiac catheterization and demonstrate poor agreement between the 3 assays (anti-Xa, activated partial thromboplastin time [aPTT], and activated clotting times [ACTs]) used to measure heparin’s anticoagulant effect.1
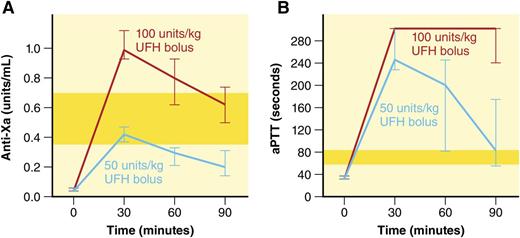
Time course of anti-Xa and aPTT values (median, 95% confidence interval) comparing high-dose (100-U/kg bolus followed by continuous infusion) vs low-dose (50-U/kg bolus without continuous infusion) UFH dosing protocols. Yellow-shaded bars indicate therapeutic ranges. This figure has been adapted from Figure 1 in the article by Hanslik et al that begins on page 2091. Professional illustration by Patrick Lane, ScEYEnce Studios.
Time course of anti-Xa and aPTT values (median, 95% confidence interval) comparing high-dose (100-U/kg bolus followed by continuous infusion) vs low-dose (50-U/kg bolus without continuous infusion) UFH dosing protocols. Yellow-shaded bars indicate therapeutic ranges. This figure has been adapted from Figure 1 in the article by Hanslik et al that begins on page 2091. Professional illustration by Patrick Lane, ScEYEnce Studios.
Unfractionated heparin (UFH) remains an important therapeutic modality for anticoagulation in children. Its short half-life and easy reversibility with protamine sulfate make it an attractive option in critically ill children. Although initially described as a screening tool for hemophilia, the aPTT is a global hemostatic assay that reflects the integrity of the intrinsic and common pathways of the in vitro coagulation cascade and is commonly used to monitor UFH.
In a landmark publication in 1972, Basu et al2 demonstrated that the risk of recurrent thrombosis in adults receiving heparin could be significantly reduced by aiming for a 1.5 to 2.5 times prolongation of the aPTT. A subsequent cohort study by Andrew et al3 documented a 73% agreement between aPTT and anti-Xa activity in 52 children receiving UFH therapy (r2 = 0.51), and the therapeutic range of 1.5 to 2.5 times baseline was extrapolated for children. Over time, however, it has become apparent that the aPTT is affected by several preanalytical, analytical, and biological variables, and current guidelines recommend that the therapeutic aPTT range corresponds to an anti-Xa of 0.35 to 0.7 U/mL.4 Recent studies have documented poor agreement between the 2 assays (r2: 0.08-0.27), with the aPTT frequently overestimating the heparin effect compared with anti-Xa.5-7
In the current article, the authors report post hoc analysis on laboratory data collected during a parallel cohort, randomized, double-blinded controlled study comparing 2 dosing protocols of UFH to prevent thromboembolism in children undergoing cardiac catheterization (HEART CAT).1 The primary clinical outcomes of the study were previously published and did not demonstrate a significant difference in thrombosis or bleeding between high-dose and low-dose UFH.8 aPTT, anti-Xa, and ACTs were obtained at baseline and 30, 60, and 90 minutes after heparin bolus; in total, 492 blood samples were collected from 149 subjects.
Although all 3 assays discriminated well between high-dose and low-dose UFH, there was poor correlation between the assays. Congruent with previous reports, the aPTT frequently overestimated the UFH effect reported by anti-Xa and was supratherapeutic for all measured time points in both protocols (see figure). In the high-dose arm (100-U/kg bolus followed by continuous infusion), the anti-Xa was supratherapeutic initially, but became therapeutic by 90 minutes. In the low-dose arm (50-U/kg bolus without continuous infusion), the anti-Xa was therapeutic initially but rapidly became subtherapeutic. The authors therefore suggest a 75-U/kg bolus in pediatric subjects undergoing cardiac catheterization procedures. They also conclude that that the aPTT was overly sensitive to heparin effect in this cohort and suggest using anti-Xa, with the ACT recommended as a comparably reliable alternative when a bedside test is more appropriate.
Clinical trials of antithrombotic therapy in children have been plagued by poor recruitment, early closure, and underpowering. The current study is an example of strategies that can be used to overcome these barriers. Adding a parallel cohort arm to the traditional randomized design allowed for inclusion of subjects who may otherwise have not been eligible for randomization due to scientific, ethical, or logistical reasons.9 These subjects constitute an important cohort of the disease population, and such a design allows for a more heterogeneous and “real-world” study population. Another challenge with patient recruitment is that thrombosis is a complication of other underlying diseases, and hematologists are often not the primary providers for these complex and often acutely ill patients. It is imperative that clinician scientists in the thrombosis arena include other disciplines such as cardiology, critical care, and surgery to enhance access to potential study patients. In this case, a team composed primarily of cardiology investigators have added substantially to our knowledge of anticoagulants in children by studying heparinized patients undergoing cardiac catheterization.
The results of this study, however, need to be interpreted with caution. UFH was used in relatively stable children undergoing diagnostic catheterization in controlled settings. As most patients received a single dose of UFH, the results may not be applicable to critically ill children receiving therapeutic UFH infusions. With regard to superiority of anti-Xa over aPTT, the decision remains unclear. A recent retrospective study did not show a difference in bleeding outcomes or time to therapeutic range after transitioning from an aPTT-based nomogram to an anti-Xa–based one.10 The anti-Xa is more specific to heparin activity and is less affected by preanalytic variables. However, it is more expensive and does not take into account heparin’s anti-IIa activity, which may contribute to its therapeutic effect. Standardized collection of clinical outcome and safety data, particularly data on clot resolution, recurrence, bleeding, and rates of postthrombotic syndrome from prospective trials, are needed to help decide if the aPTT is truly obsolete in patients receiving heparin.
Conflict-of-interest disclosure: The authors declare no competing financial interests.