To the editor:
Survivors of Hodgkin lymphoma (HL) are at an increased risk of various secondary malignancies.1 Among females, breast cancer is the most common secondary malignancy, which develops approximately 10 years after the diagnosis of HL.2 Prior studies have suggested that age at diagnosis of HL, time from initial therapy, and radiation dose and field size may affect the cumulative risks of developing secondary breast cancer.2,3 Over the past 2 decades, however, less toxic chemotherapy regimens and involved-field radiation therapy have been used to reduce toxicities.4
We used the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) 9 database (1973-2011) for the purpose of this study. SEER 9 database collects cancer incidence and follow-up data from 9 tumor registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah) representing ∼10% of the United States. We identified all women diagnosed with HL using International Classification of Diseases for Oncology, third edition codes 9650/3-9667/3. We selected women between the ages of 0 and 84 years to avoid underreporting of second cancers among older patients as a result of shorter life expectancies. We excluded cancers diagnosed by autopsy or death certificate only. Using a 10-year latency exclusion period, we determined the occurrence of secondary breast cancer among survivors of HL.
We computed standardized incidence ratios and absolute excess risk for the occurrence of secondary breast cancer. We then used Poisson regression models to calculate the adjusted incidence of secondary breast cancers by year of diagnosis. The regression model consisted of age at diagnosis of HL (in continuous 1-year increments), year of diagnosis, and time since HL diagnosis (latency in years) as the exposure variables. Similarly, Poisson regression was used to study the yearly trend in the use of radiation therapy. Flexible but smooth rates were obtained with the use of regression splines on 1 to 5 equally spaced knots and selected using Akaike’s information criteria.5 Log-linear trends in absolute rates were summarized using estimated annual percentage change (EAPC) calculated as the antilog of regression coefficient for year minus 1 times 100 (ie, EAPC = {exp(year of diagnosis) − 1} × 100). Statistical analysis was done using SEERstat 8.2.1 (released April 7, 2015) and STATA 13.0 (College Station, TX). All P values were 2 sided, and the level of significance was chosen at .05.
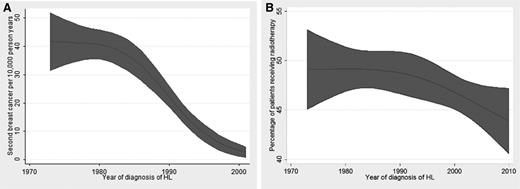
A total of 5776 women with HL were followed up for a median duration of 229 months (range, 120-467 months). The median age at diagnosis of HL was 28 years (range, 2-84 years). The majority of these patients (89%, n = 5159) were whites. Among patients with known staging (n = 4099), majority were either stage I (26%, n = 1080) or stage II (46%, n = 1904). Among 3974 patients with known staging and radiation status, the use of radiation therapy was highest in stage I (64%; n = 691) and stage II (68%; n = 1302) patients followed by stage III (38%; n = 248) and stage IV (28%; n = 122) patients. A total of 378 patients developed secondary breast cancer (6.5%), with a standardized incidence ratio of 3.51 (95% confidence interval [CI], 3.16-3.88; P < .01) and an absolute excess risk of 44.16 per 10 000 population. The median age at diagnosis of secondary breast cancer was 46 years (range, 24-95 years), and the median latency period was 231 months (range 120-433 months). The estimated age-adjusted incidence rate of second primary breast cancer was 22.02 per 10 000 person-years of follow up (95% CI, 19.24-25.4 per 10 000 person-years of follow up). From 1973 to 2011, the age-adjusted incidence rates of secondary breast cancer steadily declined at an EAPC of −6.28 (95% CI −7.66 to −4.87). (Figure 1A). During the same time period, the age-adjusted rates of radiation therapy (in percentage) steadily declined at an EAPC of −0.31 (95% CI, −0.57 to −0.04) (Figure 1B).
Radiation use and breast cancer among female survivors of HL. (A) Restricted cubic spline graph showing the relationship between the year of diagnosis of HL and the incidence of secondary breast cancer. (B) Restricted cubic spline graph showing the relationship between the year of diagnosis of HL and the rate of use of radiation therapy.
Radiation use and breast cancer among female survivors of HL. (A) Restricted cubic spline graph showing the relationship between the year of diagnosis of HL and the incidence of secondary breast cancer. (B) Restricted cubic spline graph showing the relationship between the year of diagnosis of HL and the rate of use of radiation therapy.
Our population-based study demonstrates a declining trend in the use of radiation therapy as well as a declining incidence of secondary breast cancer among female survivors of HL. De Bruin et al previously reported a reduction in the incidence of secondary breast cancer among women receiving involved-field radiation therapy as compared with extended-field radiation therapy.6 Avoidance of long-term toxicities from the use of radiation therapy is particularly important in early-stage HL, where the treatment-related mortality may exceed deaths from HL.7 One of the important limitations of our study was inability to ascertain whether the reduction in breast cancer incidence was related to reduced use of radiation therapy, reduced dose and field of radiation therapy, change in chemotherapy regimens, or a combination of these factors.
Authorship
Acknowledgments: The interpretation of this data is the sole responsibility of the authors. The authors acknowledge the efforts of Surveillance Research Program, National Cancer Institute and the SEER program tumor registries in the creation of the SEER database.
This study used the SEER 9 database. Citation: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
This work was supported in part by a 2015-2016 Physician-Scientist Training Program Grant to V.R.B. from the College of Medicine, University of Nebraska Medical Center.
Contribution: S.G., V.R.B., and M.G.M. developed the concept for the study; S.G. designed the study; S.G. and R.P. conducted the study and were involved with data analysis; S.G., V.R.B., R.P., and M.G.M. contributed to the interpretation of the results; S.G. and V.R.B. drafted the manuscript; R.P. and M.G.M. reviewed and critically revised the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vijaya Raj Bhatt, University of Nebraska Medical Center, Department of Internal Medicine, Division of Hematology-Oncology, 987680 Nebraska Medical Center, Omaha, NE 68198-7680; e-mail: vijaya.bhatt@unmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal