Key Points
RhoA GTPase activates pMRLC and localizes to the site of midbody formation to regulate erythroblast cytokinesis.
Cytokinesis failure in erythroblasts caused by RhoA deficiency triggers p53-mediated DNA-damage response, cell-cycle arrest, and apoptosis.
Abstract
RhoA GTPase has been shown in vitro in cell lines and in vivo in nonmammalian organisms to regulate cell division, particularly during cytokinesis and abscission, when 2 daughter cells partition through coordinated actomyosin and microtubule machineries. To investigate the role of this GTPase in the rapidly proliferating mammalian erythroid lineage, we developed a mouse model with erythroid-specific deletion of RhoA. This model was proved embryonic lethal as a result of severe anemia by embryonic day 16.5 (E16.5). The primitive red blood cells were enlarged, poikilocytic, and frequently multinucleated, but were able to sustain life despite experiencing cytokinesis failure. In contrast, definitive erythropoiesis failed and the mice died by E16.5, with profound reduction of maturing erythroblast populations within the fetal liver. RhoA was required to activate myosin-regulatory light chain and localized at the site of the midbody formation in dividing wild-type erythroblasts. Cytokinesis failure caused by RhoA deficiency resulted in p53 activation and p21-transcriptional upregulation with associated cell-cycle arrest, increased DNA damage, and cell death. Our findings demonstrate the role of RhoA as a critical regulator for efficient erythroblast proliferation and the p53 pathway as a powerful quality control mechanism in erythropoiesis.
Introduction
The first circulating “primitive” erythroid cells in the mouse embryo emerge in blood islands of the yolk sac at around embryonic day 7.5 (E7.5) and remain the only circulating erythroid cells until E12.5, transporting oxygen to all tissues of the rapidly growing embryo.1 They are characterized by their large size, the presence of a nucleus, and the expression of embryonic hemoglobins.2 Primitive erythroblasts continue to mature and divide in circulation and enucleate between E12.5 and E16.5 after interactions with the macrophages of the fetal liver.1,3 As the embryo increases in size, growth and life cannot be sustained by the limited potential of primitive erythropoiesis; the vastly more numerous definitive red blood cells (RBCs) begin to be released from the fetal liver at ∼E12.5, enucleated and containing adult hemoglobin.4 When primitive erythropoiesis fails, embryos do not survive beyond E9.5 to 10.5, whereas disruption of genes necessary for definitive erythropoiesis causes fetal demise after ∼E15.5.5 No other normal mammalian tissue proliferates as fast as the erythroid lineage, which produces in the adult human at steady-state 2 million new RBCs per second. The erythroid proliferation rate is even faster during embryonic development in which a 70-fold increase in the red cell mass has been estimated to occur in fetal mice in the period E12.5 to E16.5 of gestation.6 It is clear that any disruption of the cell division mechanism would have a detrimental effect on the efficiency of erythropoiesis.
RhoA, a member of the Rho GTPase family of proteins, is a major regulator of actomyosin contractility and vesicular trafficking,7,8 processes that play a significant role in cytokinesis, the final stage of cell division.9 Studies in urchin and frog cells have shown that microtubules creating the mitotic spindle determine the position of the cleavage furrow via localization of active RhoA to this zone.10 After actomyosin ring contraction and cleavage furrow ingression, the 2 daughter cells remain connected via the midbody, a minute cytoplasmic bridge that contains microtubules.11 Abscission, the separation of the 2 daughter cells, requires new membrane formation, likely through vesicular trafficking.11,12 Our understanding of the role of RhoA in cytokinesis in mammalian cells has come mainly from work in cell lines using dominant-negative and constitutively active mutants of RhoA and its effectors to inhibit or overstimulate RhoA-related signaling. Evaluation of these pathways in vivo has been hampered by the fact that mice with constitutional deletion of RhoA could not be created because of very early embryonic lethality.13,14
In this study, we investigate the role of RhoA in vivo in the erythroid lineage using a Cre-lox recombination system in which Cre-recombinase expression is controlled by the erythropoietin receptor (EpoR) promoter, thereby resulting in erythroid-specific deletion of the floxed RhoA gene.15 We found that RhoA is essential for cytokinesis in both primitive and definitive erythroid lineages. Defective cytokinesis in RhoA-deficient erythroids manifested as polyploidy and maturation delay and was accompanied by increased phosphorylation of p53 and transcriptional upregulation of p21, leading to cell-cycle arrest and increased cell death. Although frequently multinucleated and dysplastic, RhoA-deficient primitive erythroid cells were able to support survival of the embryo, whereas failure of definitive erythropoiesis led to in utero demise by E16.5. These data reveal the important role of RhoA during maturation and expansion of the rapidly proliferating erythroid lineages, the associated quality control mechanisms that manifest in RhoA-deficient cells, and their differential effects in definitive vs primitive erythropoiesis.
Methods
Mice
All mouse protocols were approved by the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital Medical Center. Our experimental mouse colony was established by crossing mice with conditional RhoA alleles (RhoAflox/flox),16 where exon 3 of the RhoA gene is flanked by loxP sites (supplemental Figure 1, available on the Blood Web site), with EpoR-CreTg/+ mice,15 where Cre recombinase expression is controlled by the promoter of the erythropoietin receptor. The resulting EpoRCreTg/+;RhoAWT/flox and EpoRCre−/−; RhoAWT/flox siblings were crossed together and their offspring were backcrossed for at least 8 generations on a C57/BL6 background. To have an easily detectable Cre-reporter, the EpoR-CreTg/+ mice were bred with B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, where a loxP-flanked STOP cassette prevents transcription of the downstream red fluorescent protein variant tdTomato, in the cells not expressing Cre.
Timed pregnancies and embryo harvest
After pairing EpoRCreTg/+;RhoAWT/f with EpoRCre−/−;RhoAf/f mice for 24 hours, females were pulled and housed in separate cages. On embryonic days E11.5 to 16.5 (based on the date of pairing), pregnant females were euthanized and embryos were collected, as detailed in supplemental Methods.
Fixation, permeabilization, and staining for imaging flow cytometry
Fixation, permeabilization, and staining of dividing erythroblasts for imaging flow cytometry was performed as previously described.17 In brief, wild-type (WT) mice subjected to phlebotomy (removal of 300 μL blood for 3 consecutive days to induce stress erythropoiesis) were euthanized 3 days after last phlebotomy and the spleens collected. Splenocytes were pelleted at 1600 rpm/3 min in a bench-top centrifuge, fixed in phosphate-buffered saline containing 4% formaldehyde for 15 minutes at room temperature (RT), and permeabilized by consecutive suspensions in ice-cold 50% acetone, 100% acetone, and again 50% acetone solution. Cells were then incubated with anti-RhoA (1:50; Santa Cruz Biotechnology), washed in fluorescence-activated cell sorting buffer, and incubated with anti–β-tubulin-AF488 (1:200; Cell Signaling), anti-mouse-AF594 (1:100; Life Technologies), and the nuclear stain 4′,6 diamidino-2-phenylindole (DAPI) (Life Technologies). Samples were counted on the ImageStreamX (Amnis) using a 60×/0.9NA objective. At least 10 000 events per experimental sample were collected and analyzed with the associated Image Data Exploration and Analysis Software (IDEAS; Amnis).
DNA damage detection through γH2AX
Fetal liver cells were fixed with 3.7% formaldehyde in PBS for 10 minutes at 37°C and then put on ice for 1 minute. Permeabilization was attained by adding (without removing the formaldehyde) 900 μL of ice-cold 100% methanol to a final concentration of 90% methanol. Samples were immediately vortexed and kept on ice for 30 minutes. Cells were then stained with anti-γH2AX antibody (Millipore; 1:300) 30 minutes at RT, followed by staining with anti-mouse-AF488 (1:200), anti-Ter119-APC (BD Biosciences), and DAPI (5 μg/mL final concentration) and visualized on ImageStreamX (60×/0.9NA objective). Erythroblast populations were gated as previously described,18 and multinucleated cells were identified by direct visual observation of all events within the Ter119+DAPI+ population with large nuclear size (increased area_DAPI) and elongated nuclear shape (low aspect ratio_DAPI). Total and multinucleated Ter119+DAPI+ cells were analyzed for staining intensity for γH2AX.
Statistics
Statistical analysis was performed using KaleidaGraph software, v.4.1 (Synergy Software). Comparison between 2 groups with values assuming normal distribution was performed using Student t test for unpaired data with equal variance (results presented as mean ± standard error of the mean [SEM]). The ImageStreamX data of γH2AX intensity in the experiments shown in Figure 6B-C were exported for statistical evaluation. Because the distribution of the values for γH2AX intensity appears asymmetric indicating non-normality, we used the Wilcoxon rank-sum test, which makes no assumptions about the underlying probability distributions and is thus appropriate for non-normal data. For each set of measurements, we report the mean and median for each group and the 2-tailed P value according to the Wilcoxon rank-sum test. Significance was set at P < .05.
The remaining methods for this article are detailed in the supplemental Materials.
Results
Erythroid-specific RhoA deficiency results in embryonic lethality caused by failure of definitive erythropoiesis
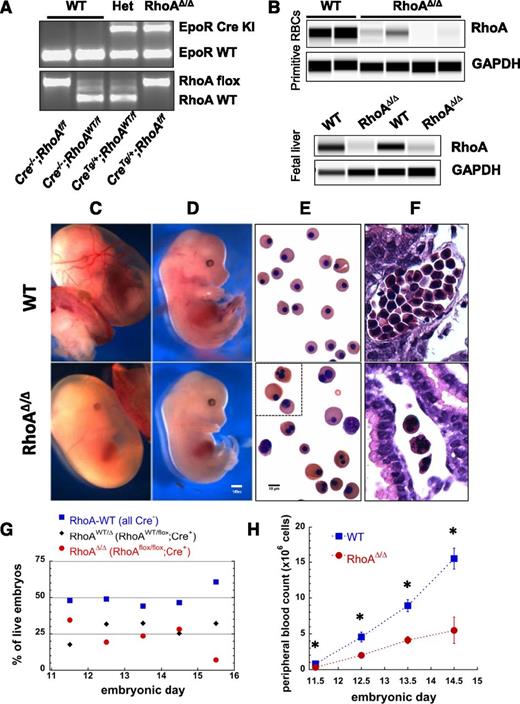
To investigate the role of RhoA in erythropoiesis, we generated mice with conditional RhoA alleles16 and Cre-recombinase expression controlled by the EpoR promoter,15 resulting in erythroid-specific RhoA deficiency (Figure 1A-B). Although primitive erythropoiesis is not completely dependent upon EpoR,19 EpoR mRNA expression is detected in yolk sac blood islands in E8.5 embryos,20 and EpoR-null embryos generate only 10% normal numbers of late-stage primitive erythroblasts.21 The associated Cre recombinase appears to be active at variable levels, as is frequently the case with the Cre system,22 and causes deletion of the floxed RhoA gene in both primitive and definitive erythroblasts, as demonstrated by significant decrease of the RhoA protein in E11.5 peripheral blood cells and E13.5 fetal liver erythroblasts (Figure 1B). Mice not expressing Cre (EpoR-Creneg;RhoAflox/flox or EpoR-Creneg;RhoAWT/flox, hereafter called WTs) or heterozygotes for RhoA deletion (EpoR-CreTg/+;RhoAWT/flox or RhoAWT/∆) were born healthy and fertile, whereas mice with erythroid-specific RhoA deficiency (EpoR-CreTg/+;RhoAflox/flox, hereafter called RhoAΔ/Δ) died in utero by E16.5. Timed pregnancies between EpoR-Cre−/−;RhoAflox/flox and EpoR-CreTg/+;RhoAWT/flox parents were used to investigate the causes of the lethality of erythroid-specific RhoA deficiency. Anemia was pronounced in the RhoAΔ/Δ embryos with the yolk sac blood vessels being barely visible as a result of the paucity of RBCs and the embryos being pale with small livers (Figure 1C-D). The primitive erythroid cells in their circulation were generally larger than the WT, heterogeneous in size and shape, and frequently binucleated or multinucleated (Figure 1E-F). Genotypes of live embryos roughly followed the expected Mendelian ratio with 25% EpoR-CreTg/+;RhoAflox/flox embryos up to E14.5, but that ratio dropped to zero by E16.5 (Figure 1G). Peripheral blood counts of RhoAΔ/Δ embryos demonstrated anemia already at E11.5, indicating effects on primitive erythropoiesis, which worsened precipitously by E14.5 (Figure 1H), leading to fetal death. To pinpoint the stage of erythroid differentiation in which Cre recombinase starts being expressed and hence RhoAflox/flox becomes deleted, we bred EpoR-CreTg/+ mice with Gt(ROSA)26Sortm9(CAG-tdTomato)Hze mice to use tdTomato as a Cre-reporter.23 Analysis of the erythroid progenitors and precursors in fetal livers from EpoR-CreTg/+;Gt(ROSA)26Sortm9(CAG-tdTomato)Hze embryos using the CD71-Ter119 flow cytometric assay24 showed that EpoR expression drives tdTomato expression already in a fraction of the S0 subpopulation and in 100% of the S1 subpopulation containing erythroid colony-forming cells (CFU-E),25 as well as in the erythroblasts throughout the further stages of erythroid differentiation in population E1 and all Ter119+ cells (supplemental Figure 2).
EpoR-CreTg/+; RhoAflox/flox (RhoAΔ/Δ) mice with erythroid-specific RhoA deficiency die in utero by E16.5 because of failure of definitive erythropoiesis. (A) Representative results of embryo genotyping by PCR (somatic DNA) show bands for EpoR-Cre (top, WT:431bp, Cre+:679bp) and RhoA (bottom, WT:482bp, flox:633bp) for all possible genotypes of the offspring of an EpoR-CreTg/+;RhoAWT/flox × EpoR-Cre−/−;RhoAflox/flox timed pregnancy. (B) Immunodetection of RhoA protein in peripheral WT and RhoAΔ/Δ blood cells from E11.5 embryos (top panel) and in CD71+ cells from WT and RhoAΔ/Δ E13.5 fetal livers (bottom panel), using a size-based capillary electrophoresis instrument, shows significant reduction of RhoA in the RhoAΔ/Δ embryos. (C-D) Stereoscopic images of E13.5 embryos showing severe anemia in RhoAΔ/Δ yolk sac (C), as well as pallor and small fetal liver in RhoAΔ/Δ embryos (D). The scale bar represents 1 mm. (E-F) Light microscopy images of peripheral blood cytospins showing poikilocytosis with large and frequently multinucleated primitive RhoAΔ/Δ erythroid cells in contrast to the homogeneous population of WT primitive red cells (E); hematoxylin and eosin–stained yolk sacs show the paucity of primitive erythroid cells in the RhoAΔ/Δ yolk sac vessels (F). The scale bar represents 10 µm. (G) The offspring of EpoR-CreTg/+;RhoAWT/flox × EpoR-Cre−/−;RhoAflox/flox follow Mendelian ratios up to E14.5. By E15.5, only 6% of live embryos are RhoAΔ/Δ, with none being alive by E16.5. Data based on genotyping of >60 embryos per each time point. (H) RhoAΔ/Δ embryos exhibit anemia already by E11.5, worsening significantly by E14.5. Data are represented as mean ± SEM of blood counts for at least 3 embryos per genotype per each time point. *P < .05 of RhoAΔ/Δ vs WT.
EpoR-CreTg/+; RhoAflox/flox (RhoAΔ/Δ) mice with erythroid-specific RhoA deficiency die in utero by E16.5 because of failure of definitive erythropoiesis. (A) Representative results of embryo genotyping by PCR (somatic DNA) show bands for EpoR-Cre (top, WT:431bp, Cre+:679bp) and RhoA (bottom, WT:482bp, flox:633bp) for all possible genotypes of the offspring of an EpoR-CreTg/+;RhoAWT/flox × EpoR-Cre−/−;RhoAflox/flox timed pregnancy. (B) Immunodetection of RhoA protein in peripheral WT and RhoAΔ/Δ blood cells from E11.5 embryos (top panel) and in CD71+ cells from WT and RhoAΔ/Δ E13.5 fetal livers (bottom panel), using a size-based capillary electrophoresis instrument, shows significant reduction of RhoA in the RhoAΔ/Δ embryos. (C-D) Stereoscopic images of E13.5 embryos showing severe anemia in RhoAΔ/Δ yolk sac (C), as well as pallor and small fetal liver in RhoAΔ/Δ embryos (D). The scale bar represents 1 mm. (E-F) Light microscopy images of peripheral blood cytospins showing poikilocytosis with large and frequently multinucleated primitive RhoAΔ/Δ erythroid cells in contrast to the homogeneous population of WT primitive red cells (E); hematoxylin and eosin–stained yolk sacs show the paucity of primitive erythroid cells in the RhoAΔ/Δ yolk sac vessels (F). The scale bar represents 10 µm. (G) The offspring of EpoR-CreTg/+;RhoAWT/flox × EpoR-Cre−/−;RhoAflox/flox follow Mendelian ratios up to E14.5. By E15.5, only 6% of live embryos are RhoAΔ/Δ, with none being alive by E16.5. Data based on genotyping of >60 embryos per each time point. (H) RhoAΔ/Δ embryos exhibit anemia already by E11.5, worsening significantly by E14.5. Data are represented as mean ± SEM of blood counts for at least 3 embryos per genotype per each time point. *P < .05 of RhoAΔ/Δ vs WT.
Circulating primitive RhoA-deficient erythroid cells exhibit polyploidy
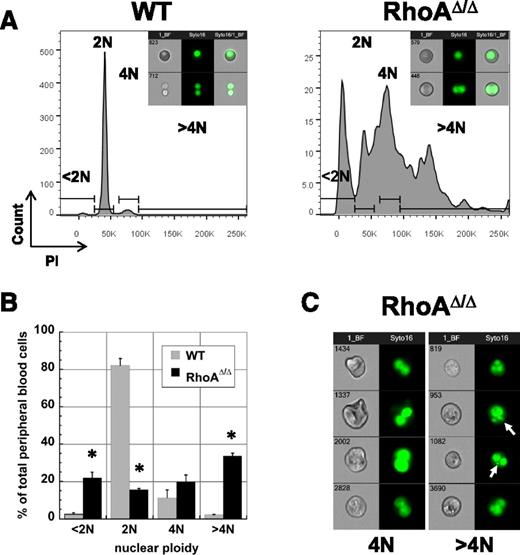
A striking characteristic of the RhoAΔ/Δ embryos was that the primitive erythroids frequently exhibit polyploidy with ≥2 nuclei, indicating a failure to complete cytokinesis. At E12.5, the majority of circulating WT primitive erythroid cells are nucleated and predominantly diploid (2N), with a few tetraploid (4N) primitive erythroblasts and the rest being either enucleated primitive or definitive RBCs (<2N). In contrast, the majority of the RhoAΔ/Δ circulating primitive erythroblasts were tetraploid and hypertetraploid; cells with <2N DNA content were also increased, likely because of the presence of cells with nuclear fragmentation as well as cytoplasmic fragments (Figure 2A-B). Analysis by imaging flow cytometry confirmed that WT cells within the 4N gate are undergoing mitosis, whereas the tetraploid RhoAΔ/Δ cells did not appear to undergo cytokinesis (bottom panels in Figure 2A, insets). RhoAΔ/Δ multinucleated cells frequently contained additional micronuclei (Figure 2C), which may result from nonsegregation of chromosomes in mitosis associated with cytokinesis failure or formation of a multipolar spindle and unbalanced chromosome segregation in tetraploid cells that fail to arrest in G1.26,27
RhoAΔ/Δ primitive erythroid cells are often multinuclear. (A) PI staining of E12.5 primitive circulating erythroid cells reveals a significant number of 4N, >4N, as well as <2N cells in the RhoAΔ/Δ population compared with the almost exclusively 2N cells of the WT population. The graphs are representative of 3 biological repeats. Insets: representative imaging flow cytometry pictures of brightfield and Syto-16–stained WT and RhoAΔ/Δ primitive erythroid cells within the 2N (top) and 4N gate (bottom). (B) Graphic representation as mean ± SEM from the data of 3 experiments (3 different biological repeats) of PI staining of E12.5 primitive erythroid cells. *P < .005 of RhoAΔ/Δ vs WT. (C) Imaging flow cytometry reveals that tetraploid (4N) and >4N RhoAΔ/Δ primitive erythroid cells do not appear to be undergoing mitosis. Cells were stained with the nuclear stain Syto16. Micronuclei or nuclear fragments (arrows) can be observed in the >4N population.
RhoAΔ/Δ primitive erythroid cells are often multinuclear. (A) PI staining of E12.5 primitive circulating erythroid cells reveals a significant number of 4N, >4N, as well as <2N cells in the RhoAΔ/Δ population compared with the almost exclusively 2N cells of the WT population. The graphs are representative of 3 biological repeats. Insets: representative imaging flow cytometry pictures of brightfield and Syto-16–stained WT and RhoAΔ/Δ primitive erythroid cells within the 2N (top) and 4N gate (bottom). (B) Graphic representation as mean ± SEM from the data of 3 experiments (3 different biological repeats) of PI staining of E12.5 primitive erythroid cells. *P < .005 of RhoAΔ/Δ vs WT. (C) Imaging flow cytometry reveals that tetraploid (4N) and >4N RhoAΔ/Δ primitive erythroid cells do not appear to be undergoing mitosis. Cells were stained with the nuclear stain Syto16. Micronuclei or nuclear fragments (arrows) can be observed in the >4N population.
RhoA is necessary for efficient definitive erythropoiesis in the fetal liver
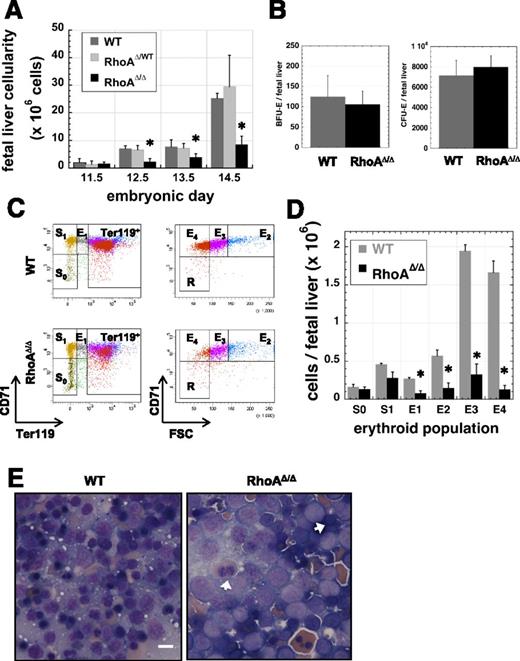
Despite the abnormal phenotype of the circulating primitive erythroid cells, RhoAΔ/Δ embryos survived the period supported exclusively by primitive erythropoiesis, indicating that primitive RBCs are able to perform their function even with abnormal nuclear material, but died by E16.5 when survival depends on fetal liver–derived definitive erythropoiesis. Fetal livers were small and pale in RhoAΔ/Δ embryos compared with those of their WT littermates. From E12.5 on, fetal liver cellularity of RhoAΔ/Δ embryos was significantly reduced (Figure 3A). To investigate the stage at which definitive erythropoiesis fails, we first performed colony assays using fetal liver cells (Figure 3B). Erythroid burst-forming unit (BFU-E) colony numbers were normal as expected, because EpoR-Cre expression is not established until the CFU-E stage (supplemental Figure 2). CFU-E numbers were also similar between the RhoAΔ/Δ and WT embryos despite the fact that excision of the floxed RhoA gene is attained at this stage. CD71-Ter119 analysis of fetal liver cells24,28 demonstrated that RhoAΔ/Δ erythroblasts diminish in number as they mature (Figure 3C-D), resulting in a severely decreased number of reticulocytes (population E4).29 Cell sorting of cells after similar analysis revealed an increased incidence of dysplastic, binucleated RhoAΔ/Δ fetal liver cells at stages E1-E3, indicating pathologic cytokinesis of definitive erythroblasts (supplemental Figure 3). RhoAΔ/Δ fetal liver erythroblasts were larger and had fewer condensed nuclei than their WT counterparts, as demonstrated in touch-preps of the tissue. Binucleated erythroblasts were also more frequent in RhoAΔ/Δ fetal livers, though not as prominent as within the primitive erythroid lineage in circulation (Figure 3E). The increased ratio of early erythroblasts and the paucity of mature erythroblasts suggested a differentiation defect (ie, reduced cell-cycle and proliferation capacity) and/or increased loss of the RhoA-deficient erythroid precursors.
Fetal liver cellularity is severely reduced and erythroblast maturation delayed in RhoAΔ/Δ embryos. (A) Beginning at E12.5, RhoAΔ/Δ fetal livers have reduced cellularity compared with the WTs. Cellularity is unaffected in the heterozygous (RhoAWT/Δ) fetal livers. Data are represented as mean ± SEM of the cell count of at least 5 fetal livers for each embryonic date. *P < .05 of RhoAΔ/Δ vs WT. (B) The number of cells per fetal liver with BFU-E (left panel) and CFU-E (right panel) activity is unaffected in E12.5 RhoAΔ/Δ embryos compared with the WT. Data are represented as mean ± SEM of 3 fetal liver samples per each genotype, each sample evaluated by colony assay in triplicate plates. (C-D) Flow cytometry analysis of fetal liver cells from E12.5 embryos shows that RhoAΔ/Δ erythroblasts diminish in number as they move through the stages of erythroid maturation. Flow cytometry dot plots are representative of 3 different biological repeats (C). Bar graph of mean ± SEM of the cell count per each erythroid population as defined by CD71-Ter119 analysis of 3 fetal livers per each genotype (D). *P < .05 of RhoAΔ/Δ vs WT. (E) Touch preps prepared from E13.0 fetal livers show an abundance of larger, more immature cells in RhoAΔ/Δ fetal livers compared with the WT. Binucleated erythroblasts (arrows) are evident in RhoAΔ/Δ fetal livers, though they are less prominent than among the primitive erythroid cells in circulation. The scale bar represents 10 µm.
Fetal liver cellularity is severely reduced and erythroblast maturation delayed in RhoAΔ/Δ embryos. (A) Beginning at E12.5, RhoAΔ/Δ fetal livers have reduced cellularity compared with the WTs. Cellularity is unaffected in the heterozygous (RhoAWT/Δ) fetal livers. Data are represented as mean ± SEM of the cell count of at least 5 fetal livers for each embryonic date. *P < .05 of RhoAΔ/Δ vs WT. (B) The number of cells per fetal liver with BFU-E (left panel) and CFU-E (right panel) activity is unaffected in E12.5 RhoAΔ/Δ embryos compared with the WT. Data are represented as mean ± SEM of 3 fetal liver samples per each genotype, each sample evaluated by colony assay in triplicate plates. (C-D) Flow cytometry analysis of fetal liver cells from E12.5 embryos shows that RhoAΔ/Δ erythroblasts diminish in number as they move through the stages of erythroid maturation. Flow cytometry dot plots are representative of 3 different biological repeats (C). Bar graph of mean ± SEM of the cell count per each erythroid population as defined by CD71-Ter119 analysis of 3 fetal livers per each genotype (D). *P < .05 of RhoAΔ/Δ vs WT. (E) Touch preps prepared from E13.0 fetal livers show an abundance of larger, more immature cells in RhoAΔ/Δ fetal livers compared with the WT. Binucleated erythroblasts (arrows) are evident in RhoAΔ/Δ fetal livers, though they are less prominent than among the primitive erythroid cells in circulation. The scale bar represents 10 µm.
RhoA-deficient fetal liver erythroblasts exhibit disrupted cell cycle and increased cell death
Based on the increased incidence of binucleated cells in RhoAΔ/Δ fetal livers, we evaluated the proliferation status of these cells. Immunohistochemistry for the cell-proliferation marker Ki67 revealed that in contrast to binucleated WT fetal liver cells that were positive for Ki67, binucleated RhoAΔ/Δ fetal liver cells did not stain for Ki67, indicating that they are not actively proliferating and thus implying cell-cycle arrest (Figure 4A). Quantitative evaluation in vivo with BrdU assay demonstrated that the cell cycle was similar in RhoAΔ/Δ and WT erythroid progenitor S0-S1 populations and early erythroblasts (E1).25,29 However, the RhoA-deficient terminal erythroblasts in the E2 and E3 populations (basophilic to orthochromatic stages) exhibited decreased S and increased G2/M phase compared with WT (Figure 4B-D). Moreover, there was a marked increase in the percentage of hypertetraploid cells in the RhoAΔ/Δ cells, accumulating to ∼15% in the E2 population. Of note, even more mature CD71high;Ter119high erythroblasts, if hypertetraploid, may still appear to belong in the E2 population because of their large size and corresponding increase in forward scatter (Figure 4C-D and supplemental Figure 3). Although no difference was found in annexin V–only positive cells between RhoAΔ/Δ and WT erythroblasts, an increased rate of late apoptosis (annexin V+;7AAD+) and necrosis (annexin V–;7AAD+) was observed in RhoA-deficient Ter119high cells (Figure 4E).
RhoAΔ/Δ fetal liver cells exhibit increased cell-cycle arrest and cell death. (A) Fetal liver sections stained for the cell-proliferation marker Ki67 show that the RhoAΔ/Δ binucleated cells in the fetal liver (arrows) are not actively proliferating, implying an abnormal arrest in the binucleated state and failure to complete cytokinesis. The scale bar represents 10 µm. (B) Graphic representation of the gating used to separate fetal liver cell populations in Figure 4C-E, defining the erythroid progenitors S0 (CD71–;Ter119– cells) and S1 (CD71+;Ter119– cells) based on CD71 level, and the erythroid precursors E1-E4 based on CD71 and Ter119 levels and size (forward scatter), as detailed in supplemental Figure 2. (C-D) Cell-cycle analysis of WT and RhoAΔ/Δ fetal liver cells from E14.5 embryos using in vivo BrdU assay shows a significant increase of the polyploid cells (>4N) and the G2/M population in the RhoAΔ/Δ late erythroblasts (E2 and E3 populations) along with a decrease in the S phase. Flow cytometry dot plots are representative of 5 different biological repeats (C). Bar graphs of mean ± SEM of the percentage of each cell-cycle stage per erythroid population of 5 fetal livers for each genotype (D). *P < .05 of RhoAΔ/Δ vs WT. (E) No significant difference was found in early apoptosis of the RhoAΔ/Δ erythroblasts (left panel); however, late apoptosis was increased in E2, E3, and E4 RhoAΔ/Δ fetal liver cells, as evidenced by an increased number of cells in those populations positive for both annexin V and 7-AAD (middle panel). Clearly necrotic cells (negative for annexin V but positive for 7AAD) were also found to be increased in RhoAΔ/Δ fetal liver erythroblasts (right panel). Data are shown as mean ± SEM from 6 WT and 4 RhoAΔ/Δ fetal livers. *P < .05 of RhoAΔ/Δ vs WT.
RhoAΔ/Δ fetal liver cells exhibit increased cell-cycle arrest and cell death. (A) Fetal liver sections stained for the cell-proliferation marker Ki67 show that the RhoAΔ/Δ binucleated cells in the fetal liver (arrows) are not actively proliferating, implying an abnormal arrest in the binucleated state and failure to complete cytokinesis. The scale bar represents 10 µm. (B) Graphic representation of the gating used to separate fetal liver cell populations in Figure 4C-E, defining the erythroid progenitors S0 (CD71–;Ter119– cells) and S1 (CD71+;Ter119– cells) based on CD71 level, and the erythroid precursors E1-E4 based on CD71 and Ter119 levels and size (forward scatter), as detailed in supplemental Figure 2. (C-D) Cell-cycle analysis of WT and RhoAΔ/Δ fetal liver cells from E14.5 embryos using in vivo BrdU assay shows a significant increase of the polyploid cells (>4N) and the G2/M population in the RhoAΔ/Δ late erythroblasts (E2 and E3 populations) along with a decrease in the S phase. Flow cytometry dot plots are representative of 5 different biological repeats (C). Bar graphs of mean ± SEM of the percentage of each cell-cycle stage per erythroid population of 5 fetal livers for each genotype (D). *P < .05 of RhoAΔ/Δ vs WT. (E) No significant difference was found in early apoptosis of the RhoAΔ/Δ erythroblasts (left panel); however, late apoptosis was increased in E2, E3, and E4 RhoAΔ/Δ fetal liver cells, as evidenced by an increased number of cells in those populations positive for both annexin V and 7-AAD (middle panel). Clearly necrotic cells (negative for annexin V but positive for 7AAD) were also found to be increased in RhoAΔ/Δ fetal liver erythroblasts (right panel). Data are shown as mean ± SEM from 6 WT and 4 RhoAΔ/Δ fetal livers. *P < .05 of RhoAΔ/Δ vs WT.
RhoA organizes myosin and microtubules during erythroblast cytokinesis
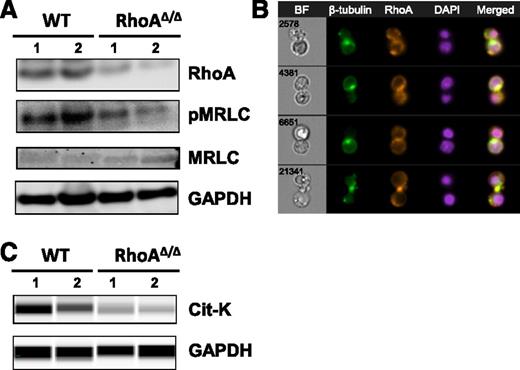
To further investigate the binucleated phenotype observed in RhoAΔ/Δ fetal liver erythroblasts, we examined the effect of RhoA on the phosphorylation of the myosin regulatory light chain (MRLC), which is necessary for effective assembly of the actomyosin contractile ring at the cleavage furrow during cytokinesis.30 RhoAΔ/Δ fetal liver cells had decreased phosphorylation of MRLC (Figure 5A) in agreement with the action of RhoA in smooth muscle cell contraction,31 as well as in cytokinesis of cultured cells in vitro32 and of hematopoietic progenitors in vivo.33
RhoA regulates myosin regulatory light-chain phosphorylation and microtubule organization during erythroblast cytokinesis. (A) Myosin regulatory light-chain phosphorylation is decreased in E14.5 RhoAΔ/Δ fetal liver cells as shown by western blotting. (B) RhoA colocalizes with β-tubulin in WT erythroblasts undergoing mitosis, possibly participating in the formation of the microtubule-derived midbody structure observed in cytokinesis. The nuclear stain DAPI was used to indicate the nucleus. Four representative cells are shown of at least 30 dividing cells with similar morphology. Images were obtained with a 60× objective lens by ImageStreamX. (C) Citron kinase was significantly decreased in E13 RhoAΔ/Δ Ter119+ fetal liver cells, as shown by capillary electrophoresis and immunodetection.
RhoA regulates myosin regulatory light-chain phosphorylation and microtubule organization during erythroblast cytokinesis. (A) Myosin regulatory light-chain phosphorylation is decreased in E14.5 RhoAΔ/Δ fetal liver cells as shown by western blotting. (B) RhoA colocalizes with β-tubulin in WT erythroblasts undergoing mitosis, possibly participating in the formation of the microtubule-derived midbody structure observed in cytokinesis. The nuclear stain DAPI was used to indicate the nucleus. Four representative cells are shown of at least 30 dividing cells with similar morphology. Images were obtained with a 60× objective lens by ImageStreamX. (C) Citron kinase was significantly decreased in E13 RhoAΔ/Δ Ter119+ fetal liver cells, as shown by capillary electrophoresis and immunodetection.
RhoA has also been shown to regulate microtubules during cytokinesis, taking part in the formation of the midbody, a transient structure composed of microtubules connecting the 2 daughter cells just before abscission.34 In WT fetal liver cultures, inhibition of microtubules with taxol resulted in a binucleated phenotype similar to that observed in the RhoAΔ/Δ fetal liver cells (supplemental Figure 4). Therefore, we explored the localization of RhoA in dividing WT splenic erythroblasts by imaging flow cytometry. RhoA stained strongly at the site where β-tubulin marked the midbody formation before abscission (Figure 5B). Citron kinase (Cit-K) has been shown before as the major downstream RhoA effector that localizes to the midbody of Drosophila and HeLa cells during cytokinesis, mediating the regulation of microtubule organization by RhoA during these final steps of cytokinesis.35-37 Cit-K protein levels were significantly decreased in the RhoA∆/∆ erythroid (Ter119+) fetal liver cells (Figure 5C), indicating a likely association of this RhoA effector with the abscission failure in RhoA-deficient erythroblasts.
Cytokinesis failure caused by RhoA deficiency results in DNA damage and p53 phosphorylation
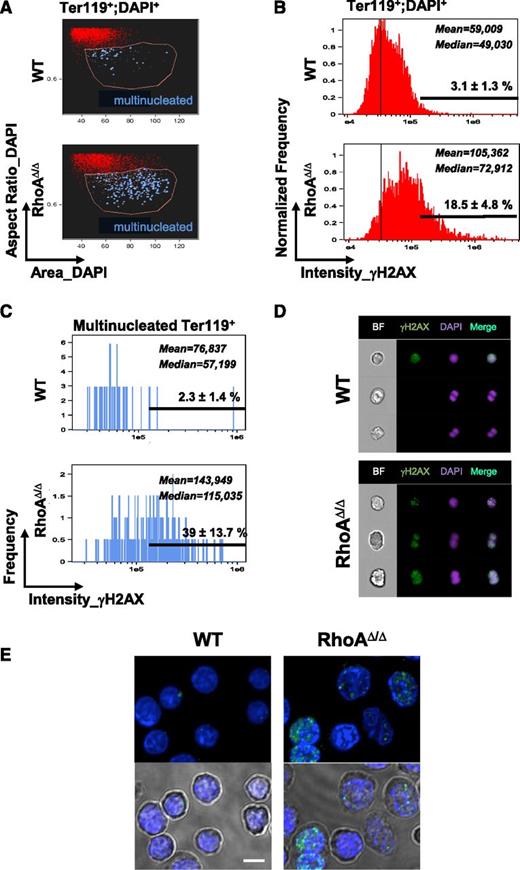
Because cytokinesis failure is known to be associated with DNA damage,38 we next investigated the RhoAΔ/Δ fetal liver cells for evidence of DNA damage. DNA damage was significantly increased in RhoAΔ/Δ fetal liver erythroid precursors (Ter119+DAPI+ cells) compared with WT (Figure 6A-B), as demonstrated by staining for phosphorylated histone H2AX (γH2AX) in imaging flow cytometry. Moreover, we explored the multinucleated erythroblasts for γH2AX positivity. Multinucleated erythroblasts were enriched within the Ter119+DAPI+ population with large nuclear size (“increased area_DAPI”) and elongated nuclear shape (“aspect ratio_DAPI,” calculated by ratio of the minor axis/major axis of nuclear stain; a low value indicates an elongated area of nuclear stain, whereas a high value of approximately 1 indicates a round nucleus). Truly binucleated or multinucleated cells were identified by direct visual observation within this population (blue dots in Figure 6A). Bi- and multinucleated RhoA-deficient Ter119+ cells had significantly increased staining for γH2AX (Figure 6C). Although the binucleated WT cells appeared to be normal mitotic cells and were negative for γH2AX, the binucleated RhoAΔ/Δ cells were strongly positive, indicative of DNA damage (Figure 6D-E).
Cytokinesis failure results in DNA damage in RhoAΔ/Δ fetal liver erythroblasts, as evidenced by staining for the DNA damage marker γH2AX. (A) Fetal liver cells from E14.0 WT and RhoAΔ/Δ embryos were analyzed by imaging flow cytometry as shown before,18 up to the gating of erythroblasts (Ter119+DAPI+ cells). Further analysis based on the size of the nucleus (area_DAPI) and the shape of the nucleus (aspect ratio_DAPI=ratio of the minor axis/major axis) gave a population (gated by the pink line) enriched in binucleated cells. Truly binucleated or multinucleated cells were then identified by visual observation and marked blue in the dot plot. (B-C) Concurrent staining for the DNA damage marker γH2AX revealed that γH2AX, as measured in arbitrary units of fluorescence, was significantly higher in the RhoAΔ/Δ erythroblasts compared with WT. Considering a threshold for H2AX positivity at 1.5 × 105, the percentage of γH2AX-positive cells was 18.5 ± 4.8% for RhoA∆/∆ Ter119+ nucleated fetal liver cells vs 3.1 ± 1.3% for the WT counterparts (B). The difference was more significant in the multinucleated erythroblasts: 39 ± 13.7% in RhoAΔ/Δ vs 2.3 ± 1.4% in the WT (C). Mean and median values of the fluorescence intensity of γH2AX in the WT and RhoAΔ/Δ erythroid precursors are shown (P < .0001 of RhoAΔ/Δ vs WT). At least 5000 Ter119+;DAPI+ cells were analyzed in each experiment and results are representative of 3 biological repeats for each genotype. (D) Binucleated RhoAΔ/Δ cells were strongly positive for γH2AX, indicative of DNA damage. In contrast, binucleated WT cells were negative for γH2AX and likely normal mitotic cells. Three representative cells are shown for each genotype of at least 250 RhoAΔ/Δ and 40 WT hyperdiploid cells with similar morphology. Images were obtained with a 60× objective lens by ImageStreamX. DAPI was used as a nuclear stain. (E) Fetal liver cells from E14 embryos were stained for γH2AX foci and imaged by confocal microscopy. Cells with >4 foci per nucleus were counted as positive, and at least 50 cells were evaluated per sample. The percentage of γH2AX-positive cells was 38.5% for RhoA∆/∆ vs 2% for the WT. Bottom panels show the nuclear stain merged with the corresponding brightfield image to demonstrate the binucleated and dysplastic RhoA∆/∆ cells. The scale bar represents 10 µm.
Cytokinesis failure results in DNA damage in RhoAΔ/Δ fetal liver erythroblasts, as evidenced by staining for the DNA damage marker γH2AX. (A) Fetal liver cells from E14.0 WT and RhoAΔ/Δ embryos were analyzed by imaging flow cytometry as shown before,18 up to the gating of erythroblasts (Ter119+DAPI+ cells). Further analysis based on the size of the nucleus (area_DAPI) and the shape of the nucleus (aspect ratio_DAPI=ratio of the minor axis/major axis) gave a population (gated by the pink line) enriched in binucleated cells. Truly binucleated or multinucleated cells were then identified by visual observation and marked blue in the dot plot. (B-C) Concurrent staining for the DNA damage marker γH2AX revealed that γH2AX, as measured in arbitrary units of fluorescence, was significantly higher in the RhoAΔ/Δ erythroblasts compared with WT. Considering a threshold for H2AX positivity at 1.5 × 105, the percentage of γH2AX-positive cells was 18.5 ± 4.8% for RhoA∆/∆ Ter119+ nucleated fetal liver cells vs 3.1 ± 1.3% for the WT counterparts (B). The difference was more significant in the multinucleated erythroblasts: 39 ± 13.7% in RhoAΔ/Δ vs 2.3 ± 1.4% in the WT (C). Mean and median values of the fluorescence intensity of γH2AX in the WT and RhoAΔ/Δ erythroid precursors are shown (P < .0001 of RhoAΔ/Δ vs WT). At least 5000 Ter119+;DAPI+ cells were analyzed in each experiment and results are representative of 3 biological repeats for each genotype. (D) Binucleated RhoAΔ/Δ cells were strongly positive for γH2AX, indicative of DNA damage. In contrast, binucleated WT cells were negative for γH2AX and likely normal mitotic cells. Three representative cells are shown for each genotype of at least 250 RhoAΔ/Δ and 40 WT hyperdiploid cells with similar morphology. Images were obtained with a 60× objective lens by ImageStreamX. DAPI was used as a nuclear stain. (E) Fetal liver cells from E14 embryos were stained for γH2AX foci and imaged by confocal microscopy. Cells with >4 foci per nucleus were counted as positive, and at least 50 cells were evaluated per sample. The percentage of γH2AX-positive cells was 38.5% for RhoA∆/∆ vs 2% for the WT. Bottom panels show the nuclear stain merged with the corresponding brightfield image to demonstrate the binucleated and dysplastic RhoA∆/∆ cells. The scale bar represents 10 µm.
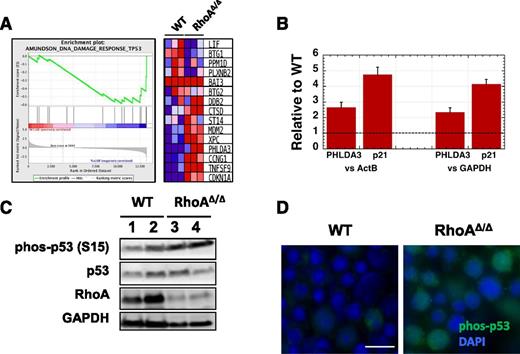
RNA-Seq analysis of magnetically sorted CD71+ fetal liver cells confirmed a DNA-damaging stress response in the RhoAΔ/Δ fetal liver erythroblasts with upregulation of p53-related DNA damage-response genes39 (Figure 7A). Follow-up real-time polymerase chain reaction (RT-PCR) verified the increased expression of PHLDA3 and CDKN1A that encodes p21, a cyclin-dependent kinase inhibitor (Figure 7B). Both PHLDA3 and p21 are downstream transcriptional targets of p53; the first contains a PH domain that competes with the PH domain of AKT and promotes apoptosis40 and the second mediates cell-cycle arrest.41 These data pointed to a p53-mediated pathway leading to cell-cycle arrest and/or programmed cell death in RhoAΔ/Δ fetal liver erythroblasts. Stabilization of p53 by increased phosphorylation at Ser-15 was indeed found by western blotting and immunofluorescence (Figure 7C-D) in RhoAΔ/Δ fetal liver erythroblasts compared with WT. Increased early and late apoptosis was also found in the primitive circulating RhoAΔ/Δ erythroids (supplemental Figure 5). The fact that early apoptosis (annexin V–only positivity) was easily observed in primitive but not definitive erythroid cells may indicate faster progression to cell death for the definitive erythroblasts because of their proximity to macrophages in the fetal liver.
DNA damage in RhoAΔ/Δ fetal liver erythroblasts leads to p53 and caspase activation. (A) Gene Set Enrichment Analysis (GSEA) from RNA-Seq data of CD71+ cells isolated from E13.0 WT and RhoAΔ/Δ fetal livers (n = 3 for each genotype) demonstrating upregulation of p53-related DNA damage response genes39 in the RhoA-deficient erythroblasts. (B) The upregulation of PHLDA3 and p21 (CDKN1A) mRNA found by RNA-Seq was confirmed using RT-PCR on RNA isolated from CD71+ cells from E13.0 WT and RhoAΔ/Δ fetal livers (n = 3 for each genotype). Data are represented as mean ± SEM of fold-expression relative to WT; expression normalized vs Actin-B and vs glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown. (C) Increased p53 phosphorylation at Ser-15 is demonstrated by western blotting in CD71+ cells isolated from E13.0 RhoAΔ/Δ fetal livers compared with WT. (D) Immunostaining on fetal liver touch-preps further confirmed increased phosphorylation of p53 phosphorylated at Ser-15 in E13.0 RhoAΔ/Δ fetal liver cells. The scale bar represents 10 µm.
DNA damage in RhoAΔ/Δ fetal liver erythroblasts leads to p53 and caspase activation. (A) Gene Set Enrichment Analysis (GSEA) from RNA-Seq data of CD71+ cells isolated from E13.0 WT and RhoAΔ/Δ fetal livers (n = 3 for each genotype) demonstrating upregulation of p53-related DNA damage response genes39 in the RhoA-deficient erythroblasts. (B) The upregulation of PHLDA3 and p21 (CDKN1A) mRNA found by RNA-Seq was confirmed using RT-PCR on RNA isolated from CD71+ cells from E13.0 WT and RhoAΔ/Δ fetal livers (n = 3 for each genotype). Data are represented as mean ± SEM of fold-expression relative to WT; expression normalized vs Actin-B and vs glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown. (C) Increased p53 phosphorylation at Ser-15 is demonstrated by western blotting in CD71+ cells isolated from E13.0 RhoAΔ/Δ fetal livers compared with WT. (D) Immunostaining on fetal liver touch-preps further confirmed increased phosphorylation of p53 phosphorylated at Ser-15 in E13.0 RhoAΔ/Δ fetal liver cells. The scale bar represents 10 µm.
Discussion
In this study, we identify a critical role for RhoA during cytokinesis of mouse embryonic erythroid cells both in primitive and in definitive erythropoiesis. We show that erythroid cells deficient in RhoA often exhibit a multinuclear phenotype indicating failure of cytokinesis. Cytokinesis failure in erythroblasts was also reported recently in a mouse model with germline deletion of mDia2,42 a formin, which catalyzes the nucleation and polymerization of unbranched actin filaments downstream of Rho GTPases.43 mDia2-deficient mice die in utero by E12.5, showing anemia at E11.5 with about 15% multinucleated cells in peripheral blood and a decreased number of BFU-E and CFU-E colonies in colony assays from fetal liver cells,42 therefore exhibiting a more severe phenotype than the phenotype of our mice with EpoR-Cre–driven RhoA deficiency. This may be caused by germline deletion of mDia2 likely contributing to cytokinesis failure very early in hematopoietic and other fast-growing embryonal tissues. Moreover, a deletion of mDia2 may display effects downstream of multiple Rho GTPases (RhoA and B, Rac1 and 2, Cdc42, and Rif) with which this formin has been shown to have direct and/or indirect interactions.43-48 Cytokinesis failure in mDia2−/− cells was attributed to either impaired F-actin accumulation at the cleavage furrow or to F-actin localization at aberrant sites.42 We found decreased phosphorylation of MRLC in RhoAΔ/Δ fetal liver erythroblasts compared with WT in agreement with previous in vitro work with cell-free systems and pharmacologic and genetic studies in Drosophila and C. elegans cells, which have shown RhoA-downstream kinases (ROCK1 or ROCK2, MLCK, and Cit-K) to phosphorylate MRLC on Ser19.9,49 In addition, we demonstrated in WT-dividing erythroblasts that RhoA marked the presumptive abscission site along with the microtubule bundle that forms the midbody, the transient structure that connects 2 daughter cells at the end of cytokinesis, as it has been observed before in HeLa cells.12,34 These 2 molecular mechanisms used by RhoA in cytokinesis may contribute to the impaired contractility or aberrant localization of the actomyosin contractile ring observed in mDia2−/− erythroblasts.
Germline deletion of ROCK1 was found to have no effect on baseline erythropoiesis and provided a survival advantage with increased response to stress erythropoiesis, associated with downregulation of p53 phosphorylation, decreased reactive oxygen species production, and reduced caspase-3 activation in erythroid cells.50 In contrast, in our model, RhoA-deficient fetal liver erythroblasts exhibited higher levels of cell death compared with WT, with an increased rate of late apoptosis (annexin V+;7AAD+) and necrosis (annexin V–;7AAD+). This difference may be caused by Cit-K and not ROCK mediating the effect of RhoA to the midbody during the final steps of cytokinesis. Failure of cytokinesis and development of binuclear and multinuclear cells has been previously shown in Drosophila and HeLa cells upon Cit-K ablation or mutation,35-37 similar to what we observe with RhoA deficiency. Indeed, we found that Cit-K protein levels were significantly decreased in RhoA∆/∆ fetal liver erythroblasts.
The RhoA-deficient fetal liver cells had increased p53 phosphorylation at S15, and thus increased p53 activity within the cell,51 and increased p21 expression, which has been shown as a transcriptional target of p53 to regulate cell-cycle arrest, apoptosis, or DNA repair.52 It is unclear how cytokinesis failure leads to p53 activation. p53 has been named a “guardian of ploidy” and has been shown, as a tumor suppressor, to either induce cell-cycle arrest or apoptosis of tetraploid cells, which, without p53, proceed to develop DNA damage including chromosomal instability or aneuploidy.25,53,54 An initial hypothesis of a “tetraploidy checkpoint” causing p53 stabilization in response to tetraploidy and centrosome amplification with subsequent arrest of binucleated cells was found not to be universally true when it was demonstrated that mammalian binucleated cells after inhibition of cytokinesis by low-dose cytochalasin were able to start again DNA synthesis (ie, re-enter mitosis).55 It has been suggested that the connection of cytokinesis failure to cell-cycle arrest noted in higher doses of cytochalasin may be caused by a more persistent disorganization of the actin cytoskeleton, even after washing out the drug.53,55 Genetic RhoA deficiency would cause a similar disorganization of the cellular actin cytoskeleton. Recently, the Hippo pathway was proposed to mediate cell-cycle arrest after cytokinesis failure by activation of LATS2 kinase, which stabilizes p53 and inhibits the transcriptional regulators YAP and TAZ.56 Further investigation will be required to evaluate whether this pathway is active and exercises quality control during erythropoiesis.
It is of interest that despite defective cytokinesis and pronounced multinuclearity, primitive erythropoiesis is able to sustain the embryo through the time definitive erythropoiesis begins to take over at E11.5 to E12.5.57 In contrast, the cytokinesis defect during definitive erythropoiesis proves to be lethal with severe progressive anemia leading to death by E16.5. This difference might be caused by the increasing erythropoietic demands of the mouse embryo in the period E12.5 to E16.5 of gestation,6 whereas an additional factor contributing to the earlier demise and engulfment/consumption of multinucleated definitive erythroblasts might be the presence of the macrophages in close proximity to the abnormal cells in the fetal liver posing an external quality control mechanism.
Based on data from in vivo and in vitro models of Diamond-Blackfan anemia and 5q- syndrome, it has been suggested that the p53 pathway plays an important role in regulating erythropoiesis and preventing malignant transformations in the rapidly proliferating erythroid lineage.58 Loss or silencing of specific genes coding for ribosomal proteins triggers increased activation of p53, which in turn results in cell-cycle arrest via p21 and in apoptosis.59,60 Cell-cycle arrest mediated by the p53-p21 pathway was also observed in the RhoA-deficient erythroblasts, offering another example of p53 applying quality control in erythropoiesis. Binucleated and multinucleated erythroid cells are often observed in certain types of congenital dyserythropoietic anemias (CDAs), a group of human genetic disorders with heterogeneous and, for many of them, not yet fully clarified causality.61 CDA type III, characterized by large, multinucleated erythroblasts in the patients’ bone marrow, is caused by mutations in the KIF23 gene, which codes for a mitotic protein essential for cytokinesis.62 It is intriguing to speculate whether any of the as yet nonelucidated CDAs may be related to RhoA-associated pathways.
In conclusion, by using an erythroid-specific model of RhoA-deficiency, we identify an essential role of the RhoA GTPase in erythroblast cytokinesis both in primitive and definitive erythropoiesis. Understanding the unique and overlapping roles of RhoA and associated signaling molecules in primitive vs definitive erythropoiesis will provide valuable insights into erythroid lineage development and may reveal potential targets for improving RBC production in vivo in patients with hypoproductive anemias and in vitro as a transfusion resource.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Research Flow Cytometry Core and the Sequencing Core at Cincinnati Children’s Hospital for excellent technical support.
This work was supported by the National Institutes of Health grants R01HL116352 (National Heart, Lung, and Blood Institute; to T.A.K.) and P30 DK090971 (National Institute of Diabetes and Digestive and Kidney Diseases; to Y.Z.).
Authorship
Contribution: D.G.K., K.M.G., and T.A.K. designed and performed research, analyzed data, and wrote the manuscript; M.R., S.P., P.Z., P.D., and S.Y. performed research and analyzed data; and U.K., P.A., J.P., and Y.Z. contributed valuable reagents and instrumental suggestions on research design, data analysis, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Theodosia A. Kalfa, Division of Hematology/Oncology, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 7015, Cincinnati, OH 45229-3039; e-mail: theodosia.kalfa@cchmc.org.
References
Author notes
D.G.K. and K.M.G. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal