Key Points
Polyphosphate-activated coagulation factor XII drives prostate cancer-associated venous thrombosis.
Targeting the polyphosphate/factor XII pathway reduces procoagulant activity in prostate cancer patient plasma and may permit safe anticoagulation.
Abstract
Cancer is a leading cause of thrombosis. We identify a new procoagulant mechanism that contributes to thromboembolism in prostate cancer and allows for safe anticoagulation therapy development. Prostate cancer-mediated procoagulant activity was reduced in plasma in the absence of factor XII or its substrate of the intrinsic coagulation pathway factor XI. Prostate cancer cells and secreted prostasomes expose long chain polyphosphate on their surface that colocalized with active factor XII and initiated coagulation in a factor XII-dependent manner. Polyphosphate content correlated with the procoagulant activity of prostasomes. Inherited deficiency in factor XI or XII or high-molecular-weight kininogen, but not plasma kallikrein, protected mice from prostasome-induced lethal pulmonary embolism. Targeting polyphosphate or factor XII conferred resistance to prostate cancer-driven thrombosis in mice, without increasing bleeding. Inhibition of factor XII with recombinant 3F7 antibody reduced the increased prostasome-mediated procoagulant activity in patient plasma. The data illustrate a critical role for polyphosphate/factor XII-triggered coagulation in prostate cancer-associated thrombosis with implications for anticoagulation without therapy-associated bleeding in malignancies.
Introduction
Cancer is an independent and major risk factor for venous thromboembolism (VTE),1,2 comprising deep vein thrombosis (DVT) and pulmonary embolism (PE). Of all first VTE events, 20% to 30% are malignancy-associated, and VTE is the second leading cause of death in patients with malignancy.3,4 Anticoagulation therapy in cancer patients remains challenging with high recurrence rates of VTE and increased rates of anticoagulant-related bleeding. Currently used anticoagulants, such as low-molecular-weight heparin (LMWH) and vitamin K antagonists (VKAs), target enzymes of the coagulation cascade that are critical for fibrin formation. As a result, treatment of VTE carries an inherent risk of potentially life-threatening bleeding.5 Prostate cancer (PC) is the second most common cancer in men and ranks sixth in malignancy-related mortality.6,7 Although the incidence of ∼13 malignancy-associated VTE cases per 1000 person-year is not particularly high in PC patients,7 due to the high prevalence of the disease, concurrence of VTE and PC presents a major medical burden.
Fibrin formation is initiated in plasma by 2 distinct mechanisms, termed the extrinsic and intrinsic coagulation pathways. The extrinsic coagulation pathway is initiated by binding of circulating coagulation factor VII/VIIa to the transmembrane protein tissue factor (TF).8 In contrast, the intrinsic pathway of coagulation is triggered by contact-induced autoactivation of zymogen factor XII (FXII), resulting in the active protease FXIIa. FXIIa leads to fibrin formation via its substrate factor XI (FXI).9 Ablation of F12 and F11 genes protects mice from DVT10 and PE,11 and inherited deficiency in FXI reduces the incidence of DVT in patients.12 Although targeting FXII interferes with thrombus formation in nonhuman primates,13 there is a lack in epidemiologic studies that analyzed protection from thromboembolic disease in individuals with severe FXII deficiency.9 Despite its crucial importance for thrombosis in animal models, FXII is dispensable in hemostasis (cessation of bleeding at sites of injury), and FXII-deficient humans and mice have a normal hemostatic capacity.9 Procoagulant platelet-released polyphosphate (polyP) activates FXII in vitro14 with implications for thrombosis in vivo.15 PolyP is a linear, unbranched polymer of orthophosphate residues linked by phosphoanhydride bonds. The polymer is ubiquitously found in nature and varies in chain length from a few phosphate units to several thousands.16
The principle fibrin-forming mechanism underlying cancer-associated thrombosis is considered to be upregulation of TF expression on cancer cells and cancer cell-derived membrane vesicles. Indeed, clinical and experimental studies revealed largely increased TF antigen on PC cells and secreted exosomes (prostasomes)17 in tumor tissue and in plasma samples of PC patients, which was associated with excess activity of the extrinsic coagulation pathway.18 Prostasomes released from large intracellular storage vesicles of prostate epithelial cells were originally described in seminal fluid19 and are procoagulant in plasma.17 Prostasomes share cholesterol- and sphingomyelin-rich plasma membranes20 with other exosomes secreted by pancreatic, breast, or colon adenocarcinoma cells.21,22
Here, we identify a novel and unexpected role of the polyP/FXII-driven intrinsic pathway of coagulation in PC-associated thrombosis. Coagulation analyses of patient plasma and PE models in genetically altered mice show that PC cells and prostasomes expose long-chain polyP on their surface. The polymer activates FXII, triggers clotting in PC patient plasma, and causes thrombosis in mice. Interference with the polyP/FXII pathway provides protection from thrombosis while not increasing bleeding risk. These data identify a new coagulation mechanism that contributes to PC-driven thrombosis and suggest that interference with the polyP/FXII axis constitutes a novel target for anticoagulant drug development in PC-related thrombosis without impairing hemostasis.
Methods
Prostasome-induced pulmonary thromboembolism
Mice were anesthetized by intraperitoneal injection of 2,2,2-tribromoethanol and 2-methyl-2-butanol. PC3 cell- (American Type Culture Collection [ATCC]: CRL-1435; 0.8 µg/g body weight [bw]), seminal- (10 µg/g bw), or patient-derived prostasomes (150 µg/g bw) were mixed with epinephrine (0.06 µg/g bw) and slowly injected into the inferior vena cava. In some experiments, mice were injected intravenously with active site inhibited factor VII (ASIS; 2.5 µg/g bw), 3F7 (4.5 µg/g bw), polyP-binding domain (PPBD) of Escherichia coli exophosphatase (EC number 3.6.1.11; 150 µg/g bw), or saline 10 minutes before challenge. None of these inhibitors increased bleeding. Lung perfusion,23 bleeding time, and loss of hemoglobin assays were previously described.24 Systemic blood pressure was measured by volume pressure recording of the tail, using the CODA noninvasive blood pressure system (Kent Scientific Corp., Torrington, CT).
Supplemental methods
Detailed methods describing study design, blood collection, animals, cell culture, prostasome/exosome isolation, extraction of polyP, electron microscopy, expression of PPBD, enzyme-linked immunosorbent assay (ELISA) for polyP detection, staining of polyP, immunofluorescence, real-time thrombin generation analysis, recalcification time and activation of FXII, histologic analysis, and data analysis are in the supplemental Methods available on the Blood Web site.
Results
Prostasomes initiate pulmonary embolism via the intrinsic pathway of coagulation
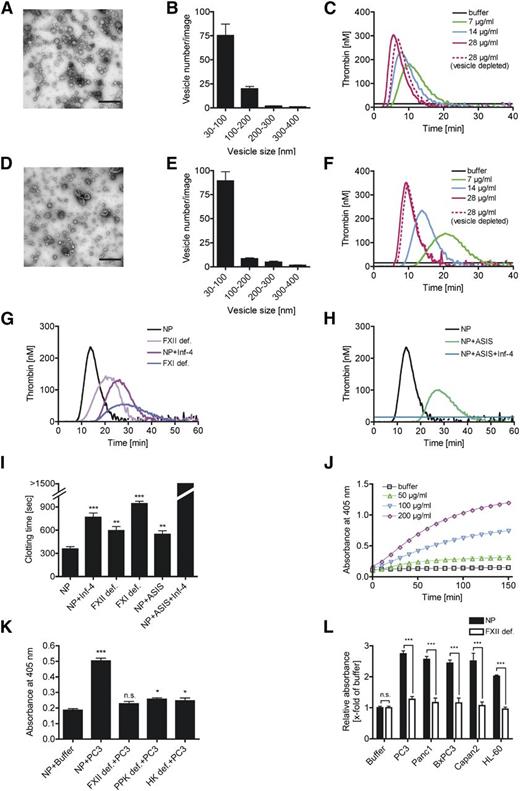
To analyze the functions of prostasomes in venous thrombosis, we challenged mice in a model of lethal PE by infusion of human PC cell (PC3)-derived prostasomes into the inferior vena cava. Mice that survived the challenge >30 minutes were considered survivors. A single animal out of 15 wild-type (WT) mice survived prostasome injection (Figure 1A). In contrast, FXII-deficient (F12−/−) mice were significantly protected from prostasome-induced PE, and 5 of 6 survived (P < .001 for F12−/− vs WT). Mice deficient in FXI (F11−/−) were also protected (6 of 6 survived, P < .001 for F11−/− vs WT, P > .05 for F11−/− vs F12−/−). In addition to FXI, FXIIa cleaves plasma prekallikrein (PPK) to generate plasma kallikrein (PK) that liberates the inflammatory mediator bradykinin (BK) from its precursor high-molecular-weight kininogen (HK). We analyzed HK- (Kng1−/−) and PPK- (Klkb1−/−) deficient mice in our PE model. Kng1−/− mice were protected from prostasome-induced challenge (5 of 5 survived, P < .01 for Kng1−/− vs WT), and the survival rate was similar to that observed in F12−/− and F11−/− mice (P > .05 for Kng1−/− vs F12−/−, P > .05 for Kng1−/− vs F11−/−). In contrast, Klkb1−/− mice were as susceptible to prostasome-driven PE as WT animals (1 of 5 survived, P > .05 for Klkb1−/− vs WT). Kinin B2 receptor-deficient (Bdkrb2−/−) mice are resistant to BK signaling. Five of 6 Bdkrb2−/− mice died on prostasome injection (P > .05 for Bdkrb2−/− vs WT), indicating that low BK is not contributing to the protection from thrombosis observed in F12−/− mice. Consistent with increased TF-dependent procoagulant activity in plasma of PC patients,25 targeting TF activity with ASIS (2.5 µg/g bw) protected WT mice from prostasome-induced PE (5 of 6 survived, P < .01 for WT + ASIS vs WT). We determined lung perfusion from prostasome-treated mice by intravenous administration of Evans blue dye (Figure 1B). Perfused lung areas turned blue, whereas occluded parts remained a natural reddish color. Consistent with prostasome-induced lethal PE, prostasome challenge in WT mice resulted in lung vessel occlusion visualized by disturbed perfusion of Evans blue. In contrast, lungs of F12−/−, F11−/−, or WT mice pretreated with ASIS presented with uniform distribution of Evans blue. Histologic sections of lungs from prostasome-challenged mice probed with the fibrin-specific antibody 59D8 (that does not cross-react with fibrinogen), and quantification of formed thrombi supported a role for FXII in prostasome-driven venous thrombosis (Figure 1C-D). Although fibrin deposition was seen throughout the pulmonary vasculature of WT mice (dead and survivors), virtually no thrombi were found in F12−/−, F11−/−, and WT mice treated with ASIS. Fibrin detection in lung tissue by immunoblot analysis confirmed reduced fibrin accumulation in lungs of F12−/− mice, F11−/− mice, and ASIS-treated WT mice compared with untreated WT animals (Figure 1E). As cell culture-derived prostasomes might differ from those generated in vivo, we isolated prostasomes from human seminal fluid19 and analyzed them in our PE model. Infusion of seminal prostasomes induced lethal PE in 5 of 6 WT mice (Figure 1F). In contrast, F12−/− (4 of 6 survived), F11−/− (4 of 6 survived), and ASIS-treated WT (3 of 6 survived) mice were largely protected from seminal prostasome-triggered thrombotic challenge (P < .05 vs WT, each). Consistently, prostasomes isolated from PC patient plasma also induced lethal PE in WT mice (0 of 5 survived, P < .01; Figure 1G). In contrast, all WT mice (5 of 5) survived challenge with exosomes isolated from healthy men.
Prostasomes initiate lethal PE in mice via the intrinsic coagulation pathway. (A) PE was induced by intravenous infusion of PC3 cell-derived prostasomes (0.8 µg/g body weight [bw]) in WT, F12−/−, F11−/−, Kng1−/−, Klkb1−/−, and Bdkrb2−/− mice or WT animals pretreated with ASIS (WT + ASIS; 2.5 µg/g bw). Mortality was assessed in each group of mice (n = 5-15). Animals alive 30 minutes after challenge were considered survivors. Horizontal bars represent mean values. ***P < .001 and **P < .01 vs untreated WT. (B) Prostasome challenged mice were intravenously infused with Evans blue shortly after the onset of respiratory arrest while the heart was still beating or after 30 minutes for those animals that survived. Lungs were excised, and perfusion defects were analyzed. (C) Immunohistochemical localization of fibrin deposition on sections from lungs of WT, F12−/−, F11−/−, and WT + ASIS-treated mice using the fibrin-specific antibody 59D8 (high magnification, lower right). Sections were counterstained with Mayer's hematoxylin (bar, 100 µm). (D) Thrombi per visual field were counted at ×10 magnification from sections such as those in C. Columns are mean ± standard error of the mean (SEM) for 35 fields. (E) Accumulation of fibrin in lungs of prostasome-challenged WT, F12−/−, F11−/−, and WT + ASIS mice was analyzed by immunoblotting. (F-G) Pulmonary thromboembolism model as in A induced by injection of 10 µg/g bw seminal prostasomes (F; n = 6) or 150 µg/g bw healthy male or patient prostasomes/exosomes (G; n = 5), *P < .05 vs untreated WT (F) and **P < .01 vs healthy males (G). P values were determined using 1-way analysis of variance (ANOVA); n.s., nonsignificant.
Prostasomes initiate lethal PE in mice via the intrinsic coagulation pathway. (A) PE was induced by intravenous infusion of PC3 cell-derived prostasomes (0.8 µg/g body weight [bw]) in WT, F12−/−, F11−/−, Kng1−/−, Klkb1−/−, and Bdkrb2−/− mice or WT animals pretreated with ASIS (WT + ASIS; 2.5 µg/g bw). Mortality was assessed in each group of mice (n = 5-15). Animals alive 30 minutes after challenge were considered survivors. Horizontal bars represent mean values. ***P < .001 and **P < .01 vs untreated WT. (B) Prostasome challenged mice were intravenously infused with Evans blue shortly after the onset of respiratory arrest while the heart was still beating or after 30 minutes for those animals that survived. Lungs were excised, and perfusion defects were analyzed. (C) Immunohistochemical localization of fibrin deposition on sections from lungs of WT, F12−/−, F11−/−, and WT + ASIS-treated mice using the fibrin-specific antibody 59D8 (high magnification, lower right). Sections were counterstained with Mayer's hematoxylin (bar, 100 µm). (D) Thrombi per visual field were counted at ×10 magnification from sections such as those in C. Columns are mean ± standard error of the mean (SEM) for 35 fields. (E) Accumulation of fibrin in lungs of prostasome-challenged WT, F12−/−, F11−/−, and WT + ASIS mice was analyzed by immunoblotting. (F-G) Pulmonary thromboembolism model as in A induced by injection of 10 µg/g bw seminal prostasomes (F; n = 6) or 150 µg/g bw healthy male or patient prostasomes/exosomes (G; n = 5), *P < .05 vs untreated WT (F) and **P < .01 vs healthy males (G). P values were determined using 1-way analysis of variance (ANOVA); n.s., nonsignificant.
Prostasomes trigger FXII-mediated thrombin and fibrin formation in vitro
We characterized human seminal- and PC3 cell culture-derived prostasomes using transmission electron microscopy (TEM). Both seminal (Figure 2A) and cell culture (Figure 2D) prostasomes appeared as cup-shaped vesicles due to dehydration. The particles shared a similar size distribution with minimal and maximal diameter of 30 and 400 nm (Figure 2B [seminal] and E [cell culture derived]). Consistent with earlier findings,19 the majority of prostasomes were within a size range of 30 to 200 nm. Less than 3% (seminal) and 6% (cell) of the analyzed prostasomes had a diameter >200 nm. Real-time thrombin generation in plasma using the calibrated automated thrombography method analyzed the procoagulant activity of seminal and cell culture prostasomes. Seminal (Figure 2C) and cell culture (Figure 2F) prostasomes induced thrombin generation in a dose-dependent manner. Prostasomes shortened lag time and time to peak and increased maximal and total thrombin (endogenous thrombin potential [ETP]). Prostasome-triggered thrombin formation was similar in normal platelet-free and vesicle-depleted plasma. Deficiency in factors XII and XI and addition of the recombinant FXIIa-inhibitor rHA-Infestin-4 (Inf-4, 500 µg/mL)15 to normal plasma significantly prolonged lag time and time to peak and reduced peak and total thrombin activated with prostasomes (Figure 2G; Table 1). Inhibition of TF activity with ASIS reduced peak and total thrombin and prolonged time to peak and lag time more than Inf-4 addition. Prostasome-induced thrombin formation was more reduced in FXI- than in FXII-deficient plasma (ETP: 568 ± 176 vs 1098 ± 186 nM⋅minute). In the absence of FXI, TF-mediated thrombin generation is unable to amplify coagulation via the FXI feedback activation loop.26 Combined application of Inf-4 and ASIS completely blunted prostasome-induced procoagulant activity (Figure 2H; Table 1). Consistent with thrombin formation assays, prostasomes initiated plasma clotting in a FXII-dependent manner (Figure 2I). Addition of Inf-4 prolonged prostasome-stimulated recalcification clotting time vs buffer in ultracentrifuged normal plasma (NP; 766 ± 55 vs 357 ± 35 seconds, P < .001). In line, prostasome-initiated clotting was prolonged in plasma deficient in factors XII (594 ± 53 seconds, P < .01 vs NP) or XI (943 ± 31 seconds, P < .001 vs NP). Addition of ASIS to NP also interfered with prostasome-induced clotting (546 ± 46 seconds, P < .01 vs NP). Combined application of Inf-4 and ASIS completely abolished prostasome-initiated fibrin formation (>1500 seconds). The FXIIa/PK chromogenic substrate S-2302 confirmed that prostasomes dose dependently initiated the contact system in NP (Figure 2J). Prostasome-induced S-2302 conversion was reduced to buffer levels in FXII-deficient plasma (P > .05) and was largely diminished in the absence of PPK or HK (P < .05 vs NP + buffer; Figure 2K). Similarly to PC3 prostasomes, exosomes derived from pancreatic cancer (Panc1, BxPC3, and Capan2) and promyelocytic leukemia cells (HL-60) triggered S-2302 cleavage in a FXII-dependent manner (Figure 2L).
Prostasomes trigger coagulation in a FXII-dependent manner. Comparison of (A-C, upper) seminal and (D-F, lower) PC3 cell prostasomes: TEM images show morphologies of isolated (A) seminal and (D) PC3 prostasomes (bar, 500 nm). Size distributions of (B) seminal and (E) PC3 prostasomes assessed from TEM images as shown in A and D, 6 fields each. Real-time thrombin generation stimulated with increasing concentrations (0-28 µg/mL) of (C) seminal and (F) PC3 prostasomes in platelet-free (solid lanes) and ultracentrifuged (dashed line) plasma. Representative curves of a series of n = 6 are shown. (G-H) Mechanism of prostasome-driven coagulation: real-time thrombin generation initiated by 14 µg/mL PC3 prostasomes in NP, NP supplemented with rHA-Infestin-4 (Inf-4; 500 µg/mL), ASIS (30 nM), or a combination of ASIS and Inf-4 (30 nM and 500 µg/mL) and plasma deficient in FXII (FXII def.) or FXI (FXI def.); n = 3 to 11. (I) PC3 prostasome (1.75 µg/mL)-stimulated recalcification clotting time in ultracentrifuged NP in the presence of inhibitors as in G and H or buffer or in FXII- or FXI-deficient plasma. **P < .01 and ***P < .001 vs NP, n = 5. (J-L) Contact activation was analyzed by conversion of the FXIIa/PK chromogenic substrate S-2302. Buffer stimulated plasma is shown as control. (J) Plasma was incubated with increasing concentrations of PC3 prostasomes (0-200 µg/mL). (K) PC3 prostasome (100 µg/mL)-induced S-2302 conversion in NP or plasma deficient in FXII (FXII def.), prekallikrein (PPK def.), or high-molecular-weight kininogen (HK def.). *P < .05 and ***P < .001 vs NP + buffer. Bars represent the absorbance at λ = 405 nm at 60 minutes; n = 6. (L) Normal (dark columns) or FXII-deficient (light columns) plasma was incubated with PC3 prostasomes and exosomes from various cancer cells (Panc1, BxPC3, Capan2, and HL-60; 100 µg/mL each). Absorbance of cleaved S-2302 in prostasome-treated plasma is given relative to buffer signal at 60 minutes. ***P < .001 NP vs FXII def., n = 3 to 6. P values were determined using 1-way ANOVA (I,K) or Student t test (L). Data are presented as mean ± SEM; n.s., nonsignificant.
Prostasomes trigger coagulation in a FXII-dependent manner. Comparison of (A-C, upper) seminal and (D-F, lower) PC3 cell prostasomes: TEM images show morphologies of isolated (A) seminal and (D) PC3 prostasomes (bar, 500 nm). Size distributions of (B) seminal and (E) PC3 prostasomes assessed from TEM images as shown in A and D, 6 fields each. Real-time thrombin generation stimulated with increasing concentrations (0-28 µg/mL) of (C) seminal and (F) PC3 prostasomes in platelet-free (solid lanes) and ultracentrifuged (dashed line) plasma. Representative curves of a series of n = 6 are shown. (G-H) Mechanism of prostasome-driven coagulation: real-time thrombin generation initiated by 14 µg/mL PC3 prostasomes in NP, NP supplemented with rHA-Infestin-4 (Inf-4; 500 µg/mL), ASIS (30 nM), or a combination of ASIS and Inf-4 (30 nM and 500 µg/mL) and plasma deficient in FXII (FXII def.) or FXI (FXI def.); n = 3 to 11. (I) PC3 prostasome (1.75 µg/mL)-stimulated recalcification clotting time in ultracentrifuged NP in the presence of inhibitors as in G and H or buffer or in FXII- or FXI-deficient plasma. **P < .01 and ***P < .001 vs NP, n = 5. (J-L) Contact activation was analyzed by conversion of the FXIIa/PK chromogenic substrate S-2302. Buffer stimulated plasma is shown as control. (J) Plasma was incubated with increasing concentrations of PC3 prostasomes (0-200 µg/mL). (K) PC3 prostasome (100 µg/mL)-induced S-2302 conversion in NP or plasma deficient in FXII (FXII def.), prekallikrein (PPK def.), or high-molecular-weight kininogen (HK def.). *P < .05 and ***P < .001 vs NP + buffer. Bars represent the absorbance at λ = 405 nm at 60 minutes; n = 6. (L) Normal (dark columns) or FXII-deficient (light columns) plasma was incubated with PC3 prostasomes and exosomes from various cancer cells (Panc1, BxPC3, Capan2, and HL-60; 100 µg/mL each). Absorbance of cleaved S-2302 in prostasome-treated plasma is given relative to buffer signal at 60 minutes. ***P < .001 NP vs FXII def., n = 3 to 6. P values were determined using 1-way ANOVA (I,K) or Student t test (L). Data are presented as mean ± SEM; n.s., nonsignificant.
Prostasome-driven thrombin generation
| . | NP . | NP + Inf-4 . | FXII def. . | FXI def. . | NP + ASIS . | NP + Inf-4 + ASIS . |
|---|---|---|---|---|---|---|
| Lag time (min) | 10.7 ± 1.0 | 22.0 ± 3.7* | 16.0 ± 2.5† | 21.0 ± 2.0* | 20.6 ± 1.9‡ | NA |
| Time to peak (min) | 15.2 ± 1.1 | 30.1 ± 5.0‡ | 25.3 ± 2.7‡ | 27.9 ± 2.1‡ | 33.3 ± 4.1‡ | NA |
| Peak thrombin (nM) | 191.1 ± 16.0 | 109.4 ± 31.3* | 94.3 ± 20.1‡ | 41.6 ± 14.5‡ | 51.9 ± 12.8‡ | NA |
| ETP (nM⋅min) | 1622 ± 59 | 998 ± 267† | 1098 ± 186† | 568 ± 176‡ | 686 ± 157‡ | NA |
| . | NP . | NP + Inf-4 . | FXII def. . | FXI def. . | NP + ASIS . | NP + Inf-4 + ASIS . |
|---|---|---|---|---|---|---|
| Lag time (min) | 10.7 ± 1.0 | 22.0 ± 3.7* | 16.0 ± 2.5† | 21.0 ± 2.0* | 20.6 ± 1.9‡ | NA |
| Time to peak (min) | 15.2 ± 1.1 | 30.1 ± 5.0‡ | 25.3 ± 2.7‡ | 27.9 ± 2.1‡ | 33.3 ± 4.1‡ | NA |
| Peak thrombin (nM) | 191.1 ± 16.0 | 109.4 ± 31.3* | 94.3 ± 20.1‡ | 41.6 ± 14.5‡ | 51.9 ± 12.8‡ | NA |
| ETP (nM⋅min) | 1622 ± 59 | 998 ± 267† | 1098 ± 186† | 568 ± 176‡ | 686 ± 157‡ | NA |
Comparison of PC3 prostasome-induced thrombin formation in platelet free normal plasma (NP), NP supplemented with rHA-Infestin-4 (Inf-4; 500 µg/mL), ASIS (30 nM), or a combination of ASIS and Inf-4 (30 nM and 500 µg/mL) and plasma deficient in FXII (FXII def.) or FXI (FXI def.) as done in Figure 2G-H. Values are expressed as mean ± SEM, n = 3 to 11. ETP, endogenous thrombin potential; NA, below the detection limit.
P < .01 vs buffer-treated NP, ANOVA.
P < .05 vs buffer-treated NP, ANOVA.
P < .001 vs buffer-treated NP, ANOVA.
Prostasome- and PC cell-derived polyP initiates fibrin formation via FXII activation
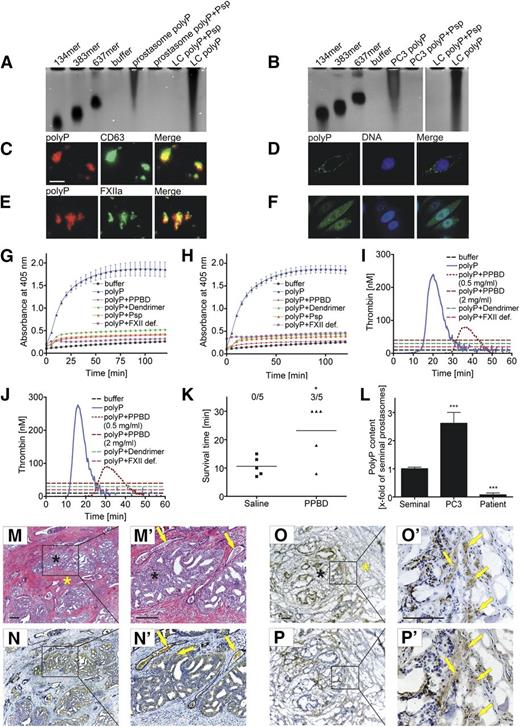
How do prostasomes and PC cells generate FXIIa? One possible candidate is the FXII contact activator polyP.14,15 We purified polyP from PC3 prostasomes (Figure 3A) and PC3 cells (Figure 3B) by anion affinity chromatography. Eluted material was separated by urea/acrylamide-gel electrophoresis and visualized by the polyP-sensitive 4,6 diamidino-2-phenylindole (DAPI)-negative staining.27 Prostasome and PC3 cell polyP migrated with similar heterodispersity and polymer chain length ranging from ∼200 to >1000-mers. Treatment with phosphatase (Psp), which hydrolyzes polyP, completely abolished the signal. We used recombinant PPBD as a probe for polyP. PPBD specifically binds to polyP of chain length >35.28 PolyP localized to the surface of prostasomes and colocalized with the prostasome cell surface marker CD6329 (Figure 3C). Treatment of prostasomes with Psp blunted PPBD binding (data not shown). Similarly to prostasomes, polyP enriched at the plasma membranes of nonpermeabilized PC3 cells (Figure 3D). PPBD detected cytoplasmic polyP in a spotty vesicular pattern in permeabilized PC3 cells (Figure 3F). We incubated prostasomes with human plasma and stained for FXIIa using Alexa 488-conjugated 3F7 antibody that specifically cross-reacts with the active enzyme but not with the zymogen form.24 Plasma incubation led to FXIIa formation and the protease colocalized with polyP at the plasma membrane (Figure 3E). PolyP isolated from prostasomes (Figure 3G) and PC3 cells (Figure 3H) stimulated S-2302 conversion in plasma. The polyP inhibitors PPBD (1 mg/mL), generation 1.0 cationic poly(amido amine) dendrimer (25 µg/mL), and degradation of polyP with Psp (1 U/mL) abolished polyP-induced amidolytic activity. PolyP was inactive in FXII-deficient plasma. Consistent with PC polyP-stimulated FXII formation, prostasome (Figure 3I) and PC3 cell (Figure 3J) polyP triggered thrombin formation in a FXII-dependent manner, and PPBD and generation 1.0 cationic poly(amido amine) dendrimer blocked the procoagulant activity conferred by PC-derived polyP. To analyze the functions of PC-derived polyP in thrombosis, we injected WT mice intravenously with saline or PPBD (150 µg/g bw) 10 minutes before prostasome challenge (Figure 3K). PPBD significantly protected mice from lethal PE, whereas all saline-infused animals died within 30 minutes after challenge (3 of 5 vs 0 of 5 mice survived, P < .05). We analyzed polyP on prostasome plasma membranes using a PPBD-based ELISA (Figure 3L). PolyP content decreased from PC3- over seminal- to patient plasma-derived prostasomes. PolyP content correlated with the procoagulant activity of prostasomes. We analyzed histologic sections of human prostate adenocarcinoma for the procoagulant polyP/FXII pathway (Figure 3M-P′). Immunohistochemistry showed FXII antigen in the lumen of occluded blood vessels. Consistent with ectopic FXII expression by ovarian cancer cells,30 the blood coagulation factor was detected in PC tissue (Figure 3N-N′). We probed for polyP and fibrin in PC tissue frozen sections as the water-soluble polyP gets lost during the deparaffinization procedure. PPBD showed polyP enriched in cancer glands and in stromal smooth muscle fibers (Figure 3O-O′), and 59D8 antibody visualized extravascular fibrin deposition at these sites (Figure 3P-P′).
Prostasomes, PC cells, and PC tissue expose procoagulant polyP. Isolation of polyP from prostasomes and PC cells: (A-B) PolyP was extracted from PC3 (A) prostasomes and (B) cells by an anion exchanger chromatography, separated by electrophoresis on polyacrylamide/urea gel and visualized by DAPI-negative staining. Synthetic polyP with mean chain lengths of 134, 383, and 637 serves as molecular size standard. Purified polyP and synthetic LC polyP were loaded before and after incubation with phosphatase (Psp, 10 U/mL for 1 hour). (C,E) Epifluorescence images of polyP on PC3 prostasomes using Alexa 594-labeled PPBD. The polymer colocalizes with (C) prostasome surface membrane marker CD63 (anti-CD63) and (E) FXIIa (3F7). (D,F) Confocal laser scanning microscopy of polyP in (D) nonpermeabilized and (F) permeabilized PC3 cells. PolyP is stained with Alexa 488-conjugated PPBD, and DNA was visualized with DAPI. Bar is 500 nm in C and E and 10 µm in D and F. (G-H) PC polyP activates FXII. Platelet-free plasma was incubated with 1.5 µg/mL PC3 (G) prostasome or (H) cell polyP in the absence or presence of PPBD (1 mg/mL), generation 1.0 dendrimer (25 µg/mL), or Psp (1 U/mL). PolyP-treated FXII-deficient plasma (FXII def.) and buffer-stimulated normal plasma (buffer) is shown as control. Hydrolysis of the chromogenic substrate S-2302 measures formed FXIIa and PK. Mean ± SEM, n = 6. (I-J) Real-time thrombin formation generated in platelet-free plasma stimulated with 1.5 µg/mL (I) prostasome or (J) PC3 cell polyP in the absence or presence of PPBD (0.5 and 2 mg/mL) or generation 1.0 dendrimer (25 µg/mL). Thrombin formation in polyP-activated FXII-deficient plasma (FXII def.) and buffer-stimulated normal plasma is blotted for comparison. A representative thrombin generation curve of a series of n = 6 is shown. (K) Mortality associated with intravenous injection of PC3 prostasomes (0.8 µg/g bw) in WT mice pretreated with saline or PPBD (150 µg/g bw) was assessed in each group (n = 5); animals alive at 30 minutes after challenge were considered survivors. *P < .05 vs saline, unpaired Student t test. (L) PolyP detection on seminal, PC3 cell, and patient prostasomes using PPBD binding in an ELISA. Bars represent polyP content relative to seminal prostasomes, mean ± SEM, n = 3. ***P < .001 vs seminal prostasomes, 1-way ANOVA. (M-P′) Immunohistochemistry of FXII, polyP, and fibrin in human PC tissue sections. (M-M′) Hematoxylin/eosin-staining of the adenocarcinoma (arrows denote occluded vessels). Immunohistochemical localization of FXII using (N-N′) anti-FXII antibodies (arrows indicate FXII-positive occluded vessels and cancer tissue, respectively), (O-O′) polyP using recombinant PPBD as a probe (arrows), and (P-P′) fibrin deposits using 59D8 antibody (arrows) in (M-N′) paraffin and (O-P′) cryo sections. Black and yellow asterisks denote tissue areas containing cancer cells and nonmalignant tissue, respectively. Sections were counterstained with Mayer’s hematoxylin. Scale bars, 100 µm.
Prostasomes, PC cells, and PC tissue expose procoagulant polyP. Isolation of polyP from prostasomes and PC cells: (A-B) PolyP was extracted from PC3 (A) prostasomes and (B) cells by an anion exchanger chromatography, separated by electrophoresis on polyacrylamide/urea gel and visualized by DAPI-negative staining. Synthetic polyP with mean chain lengths of 134, 383, and 637 serves as molecular size standard. Purified polyP and synthetic LC polyP were loaded before and after incubation with phosphatase (Psp, 10 U/mL for 1 hour). (C,E) Epifluorescence images of polyP on PC3 prostasomes using Alexa 594-labeled PPBD. The polymer colocalizes with (C) prostasome surface membrane marker CD63 (anti-CD63) and (E) FXIIa (3F7). (D,F) Confocal laser scanning microscopy of polyP in (D) nonpermeabilized and (F) permeabilized PC3 cells. PolyP is stained with Alexa 488-conjugated PPBD, and DNA was visualized with DAPI. Bar is 500 nm in C and E and 10 µm in D and F. (G-H) PC polyP activates FXII. Platelet-free plasma was incubated with 1.5 µg/mL PC3 (G) prostasome or (H) cell polyP in the absence or presence of PPBD (1 mg/mL), generation 1.0 dendrimer (25 µg/mL), or Psp (1 U/mL). PolyP-treated FXII-deficient plasma (FXII def.) and buffer-stimulated normal plasma (buffer) is shown as control. Hydrolysis of the chromogenic substrate S-2302 measures formed FXIIa and PK. Mean ± SEM, n = 6. (I-J) Real-time thrombin formation generated in platelet-free plasma stimulated with 1.5 µg/mL (I) prostasome or (J) PC3 cell polyP in the absence or presence of PPBD (0.5 and 2 mg/mL) or generation 1.0 dendrimer (25 µg/mL). Thrombin formation in polyP-activated FXII-deficient plasma (FXII def.) and buffer-stimulated normal plasma is blotted for comparison. A representative thrombin generation curve of a series of n = 6 is shown. (K) Mortality associated with intravenous injection of PC3 prostasomes (0.8 µg/g bw) in WT mice pretreated with saline or PPBD (150 µg/g bw) was assessed in each group (n = 5); animals alive at 30 minutes after challenge were considered survivors. *P < .05 vs saline, unpaired Student t test. (L) PolyP detection on seminal, PC3 cell, and patient prostasomes using PPBD binding in an ELISA. Bars represent polyP content relative to seminal prostasomes, mean ± SEM, n = 3. ***P < .001 vs seminal prostasomes, 1-way ANOVA. (M-P′) Immunohistochemistry of FXII, polyP, and fibrin in human PC tissue sections. (M-M′) Hematoxylin/eosin-staining of the adenocarcinoma (arrows denote occluded vessels). Immunohistochemical localization of FXII using (N-N′) anti-FXII antibodies (arrows indicate FXII-positive occluded vessels and cancer tissue, respectively), (O-O′) polyP using recombinant PPBD as a probe (arrows), and (P-P′) fibrin deposits using 59D8 antibody (arrows) in (M-N′) paraffin and (O-P′) cryo sections. Black and yellow asterisks denote tissue areas containing cancer cells and nonmalignant tissue, respectively. Sections were counterstained with Mayer’s hematoxylin. Scale bars, 100 µm.
FXIIa inhibition provides safe protection from prostasome-induced lethal pulmonary embolism
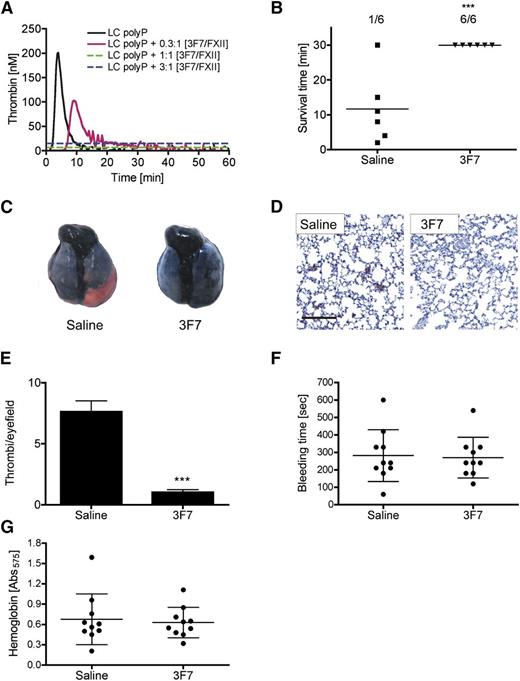
The fully humanized anti-FXIIa antibody 3F7 is a candidate future therapeutic agent, binds into the FXIIa enzymatic pocket, and inhibits the amidolytic activity of the protease.24 3F7 dose dependently interfered with synthetic long chain polyP (LC polyP)-stimulated thrombin generation, and antibody concentrations ≥375 nM completely abolished polyP-triggered coagulation in murine plasma (Figure 4A). To analyze the prophylactic application of 3F7 for interference with PC-mediated PE, we injected WT mice intravenously with saline or 3F7 (4.5 µg/g bw) 10 minutes before prostasome challenge (Figure 4B). 3F7 conferred significant protection (P < .001 vs saline) from prostasome-induced PE (6 of 6 mice survived), whereas all saline-infused animals, with the exception of a single mouse, died within 30 minutes after challenge. Increased survival rate in 3F7-treated mice correlated with increased lung perfusion and less vascular occlusion compared with lungs of saline-treated animals (Figure 4C). Fibrin staining in histologic sections of lungs from saline-treated mice confirmed diffuse thrombotic occlusion, whereas fibrin deposition was significantly less (P < .001 vs saline) in 3F7-infused animals (Figure 4D-E). We determined the tail bleeding time and extent of bleeding (blood loss) by quantification of lost hemoglobin until cessation of bleeding as a measure of hemostatic capacity. 3F7 neither prolonged the bleeding time (270 ± 117 vs 282 ± 148 seconds, P > .05; Figure 4F) nor increased hemoglobin loss (A575 of 0.63 ± 0.22 vs 0.68 ± 0.38, P > .05; Figure 4G) compared with saline-treated mice.
FXIIa inhibition protects mice from prostasome-induced PE. (A) Real-time thrombin formation in murine platelet-free plasma stimulated with PC size synthetic polyP (LC polyP, 100 µg/mL) in the absence or presence of increasing concentrations of anti-FXIIa antibody 3F7 (n = 3). Molar antibody concentrations are relative to plasma FXII (375 nM). (B) Mortality associated with intravenous injection of PC3 cell prostasomes (0.8 µg/g bw) in WT mice pretreated with saline or 3F7 (4.5 µg/g bw) was assessed in each group (n = 6); animals still alive at 30 minutes after challenge were considered survivors. ***P < .001 vs saline, unpaired Student t test. (C) Mice challenged with prostasomes were intravenously infused with Evans blue shortly after respiratory arrest while lungs were still perfused. Excised lungs show perfusion defects in red. (D) Sections from lungs of saline- and 3F7-treated WT mice were analyzed for fibrin by immunohistochemistry with 59D8 antibody and counterstained with Mayer’s hematoxylin; bar, 100 µm. (E) Thrombi per visual field were counted at ×10 magnification from sections such as those in D. ***P < .001 vs saline, unpaired Student t test. Columns are mean ± SEM for 35 fields. (F-G) 3F7 treatment does not impair hemostatic capacity. (F) Tail bleeding times and (G) total hemoglobin loss assessed by hemoglobin absorbance at λ = 575 nm was determined in saline- and 3F7-infused mice. Mean ± standard deviation, n = 10.
FXIIa inhibition protects mice from prostasome-induced PE. (A) Real-time thrombin formation in murine platelet-free plasma stimulated with PC size synthetic polyP (LC polyP, 100 µg/mL) in the absence or presence of increasing concentrations of anti-FXIIa antibody 3F7 (n = 3). Molar antibody concentrations are relative to plasma FXII (375 nM). (B) Mortality associated with intravenous injection of PC3 cell prostasomes (0.8 µg/g bw) in WT mice pretreated with saline or 3F7 (4.5 µg/g bw) was assessed in each group (n = 6); animals still alive at 30 minutes after challenge were considered survivors. ***P < .001 vs saline, unpaired Student t test. (C) Mice challenged with prostasomes were intravenously infused with Evans blue shortly after respiratory arrest while lungs were still perfused. Excised lungs show perfusion defects in red. (D) Sections from lungs of saline- and 3F7-treated WT mice were analyzed for fibrin by immunohistochemistry with 59D8 antibody and counterstained with Mayer’s hematoxylin; bar, 100 µm. (E) Thrombi per visual field were counted at ×10 magnification from sections such as those in D. ***P < .001 vs saline, unpaired Student t test. Columns are mean ± SEM for 35 fields. (F-G) 3F7 treatment does not impair hemostatic capacity. (F) Tail bleeding times and (G) total hemoglobin loss assessed by hemoglobin absorbance at λ = 575 nm was determined in saline- and 3F7-infused mice. Mean ± standard deviation, n = 10.
Activation of FXII contributes to increased procoagulant activity of PC-derived plasma prostasomes
Thrombin generation was increased in plasma samples from PC patients (supplemental Table 1) over levels in healthy men, confirming previous studies.25 Lag time (26.7 ± 5.1 vs 43.7 ± 4.7 minutes, P < .05; Figure 5A) and time to peak (30.2 ± 4.7 vs 47.6 ± 3.7 minutes, P < .05; Figure 5B) were shortened, and maximum (122 ± 27.4 vs 29.9 ± 10.7 nM, P < .05; Figure 5C) and total thrombin (859.7 ± 183.9 vs 355.2 ± 136.8 nM⋅minute, P < .05; Figure 5D) were significantly increased in patients over controls. Depletion of vesicles abolished the procoagulant activity in patient plasma and reconstitution of the ultracentrifuged plasma samples with purified prostasomes restored their procoagulant activity. We analyzed the contribution of prostasome polyP-generated FXIIa for procoagulant activity in PC patients. Prostasomes/exosomes isolated from plasma of 20 PC patients and 10 healthy male and 10 healthy female controls (supplemental Table 2) triggered thrombin formation in normal plasma. Exosomes derived from female plasma exhibited procoagulant activity similar to that of healthy males (ETP: 938 ± 114 vs 683 ± 121 nM⋅minute, P > .05). For comparison, we supplemented ultracentrifuged plasma with synthetic phospholipids. Procoagulant activity conferred by phospholipids alone was similar to those induced by healthy individuals (ETP: 1073 ± 106 nM⋅minute, P > .05 vs healthy males and females). The procoagulant activity of prostasomes from PC patients was significantly higher than those from healthy male controls. Lag time (25.1 ± 2.1 vs 14.5 ± 1.1 minutes, P < .001; Figure 5E) and time to peak (32.0 ± 1.9 vs 20.8 ± 1.3 minutes, P < .001; Figure 5F) were shortened by 42% and 35%, respectively, and maximum (39 ± 7 vs 145 ± 14 nM, P < .001; Figure 5G) and total thrombin (683 ± 121 vs 1763 ± 39 nM⋅minute, P < .001; Figure 5H) were increased by 3.7- and 2.6-fold, respectively. Inhibition of FXIIa activity with 3F7 (100 µg/mL) significantly reduced the increased procoagulant activity of patient prostasomes. 3F7 prolonged lag time (22.0 ± 2.2 minutes, P < .01 vs without 3F7) and time to peak (28.2 ± 2.2 minutes, P < .01 vs without 3F7). Consistently, 3F7 reduced peak (95 ± 16 nM, P < .05 vs without 3F7) and total thrombin (1270 ± 125 nM⋅minute, P < .001 vs without 3F7) in normal plasma samples supplemented with prostasomes from PC patients. FXIIa inhibition similarly reduced procoagulant activity of patient- and cell culture-derived prostasomes (Figure 2G; Table 1; 38% ETP reduction each). Procoagulant activity of prostasomes isolated from PC patients with a history of thromboembolic disease was increased over samples from patients without previous vascular occlusive events (supplemental Figure 1). Taken together, these data show a role of prostasome-generated FXIIa in the increased procoagulant activity seen in PC patients.
Prostasome/FXIIa-driven increased procoagulant activity in PC patient plasma. (A-D) Real-time thrombin generation in plasma of healthy individuals (Controls) and patients (Patients). Thrombin formation in prostasome-depleted [Patients(−)] and prostasome-reconstituted [Patients(+)] patient plasma is blotted for control; n = 9 each. (E-H) Real-time thrombin generation in normal plasma stimulated by addition of prostasomes/exosomes (250 µg/mL) from healthy female and male controls (n = 10 each) or PC patients (n = 20). Patient prostasome-triggered coagulation was measured in the absence or presence of 3F7 (100 µg/mL). (A,E) Lag time until thrombin formation starts, (B,F) time to peak thrombin, (C,G) maximum thrombin (peak thrombin), and (D,H) total thrombin (ETP). Each symbol represents an individual. Horizontal bars indicate mean values. *P < .05, **P < .01, and ***P < .001, unpaired Student t test; n.s., nonsignificant.
Prostasome/FXIIa-driven increased procoagulant activity in PC patient plasma. (A-D) Real-time thrombin generation in plasma of healthy individuals (Controls) and patients (Patients). Thrombin formation in prostasome-depleted [Patients(−)] and prostasome-reconstituted [Patients(+)] patient plasma is blotted for control; n = 9 each. (E-H) Real-time thrombin generation in normal plasma stimulated by addition of prostasomes/exosomes (250 µg/mL) from healthy female and male controls (n = 10 each) or PC patients (n = 20). Patient prostasome-triggered coagulation was measured in the absence or presence of 3F7 (100 µg/mL). (A,E) Lag time until thrombin formation starts, (B,F) time to peak thrombin, (C,G) maximum thrombin (peak thrombin), and (D,H) total thrombin (ETP). Each symbol represents an individual. Horizontal bars indicate mean values. *P < .05, **P < .01, and ***P < .001, unpaired Student t test; n.s., nonsignificant.
Discussion
Malignancy has been associated with increased risk of thrombosis for more than a century.31 The TF/factor VIIa pathway is believed to be the principal initiator of fibrin formation in cancer patients.32 Cancer cells express TF on their plasma membrane33 and release TF-bearing microparticles (MPs) into the circulation.34 MPs are procoagulant as they promote thrombus propagation in mouse models and trigger clotting in human plasma.35 In various types of cancer,36-38 including PC,39 plasma MP number and procoagulant activity are increased, supporting a role of TF-bearing MP in malignancy-associated thrombosis. Indeed, some studies show that MP-associated TF activity is increased in cancer patients with VTE over asymptomatic cancer patients,36,40 and impedance-based flow cytometry established TF-bearing MP as a prognostic risk factor for VTE.38 However, other studies that analyzed plasma MP counts indirectly via prothrombinase assays37 or MP TF activity41,42 could not establish an association between MP levels/TF activity and risk for VTE. Furthermore, plasma TF activity in cancer patients does not correlate with the MP count,25,36 and plasma TF activity in cancer patients does not correlate with other biomarkers of thrombosis, including d-dimer40 or thrombin-antithrombin complexes.43 Collectively, clinical data suggest that mechanisms other than the TF pathway operate and contribute to thrombosis in cancer patients.
Here, we show that particles derived from PC, pancreatic cancer, and promyelocytic leukemia cells initiate coagulation in a FXII-dependent manner (Figure 2), triggered by exposure of polyP on their plasma membranes (Figure 3). These findings extend earlier studies showing that MPs derived from platelets of healthy individuals initiate coagulation via FXII activation.44 Vice versa platelet-stimulated fibrin formation is defective in the absence of FXII.45-47 Supporting a function for the polyP/FXII coagulation pathway in cancer-associated thrombosis, zymogen FXII plasma levels are low in samples from patients with advanced gastrointestinal48 and nonmetastatic colorectal cancer49 compared with healthy controls. Furthermore, diminished plasma FXII is associated with reduced PPK levels and an increase in PK bound to its endogenous protease inhibitors in lung cancer patient plasma samples.50 These observations suggest that polyP/FXII-initiated coagulation has implications in patients with PC and also in patients with other malignancies.
PolyP procoagulant activity increases with chain length,51 and polymers of <45 units do not support FXII contact activation.15 Consistent with synthetic long chain polyP,51 natural PC-derived polyP (200 to >1000-mers) is a potent FXII contact activator (Figure 3). PolyP content exposed on the plasma membrane of various prostasomes varied and correlated with the procoagulant activity of these particles. There was a trend for higher polyP expression on prostasomes derived from PC patients with a history of thromboembolic disease; however, further studies are warranted to confirm this observation. In addition to initiating the FXIIa-driven intrinsic pathway of coagulation, polyP accelerates multiple downstream procoagulant mechanisms including factor V and factor XI feedback activation and blocks the anticoagulant activity of TF pathway inhibitor.16 The polyP/FXII pathway operates independently of TF-mediated coagulation52 and also results in PK-mediated release of the proinflammatory and vasodilatory peptide BK.15,53 Therefore, targeting polyP using cationic proteins, polymers, or small molecules54 may represent a promising strategy for interfering with cancer-driven coagulation and potentially, inflammatory reactions.55 PolyP/FXII inhibition and deficiency in factors XI, XII, or HK interfered with prostasome-induced lethal PE (Figure 1). Similar to infusion of pure polyP56 and platelet stimulation,57 injection of prostasomes induced microvascular PE. In humans, microvascular PE represents a complication of tumor cell embolism.58-60 Klkb1−/− mice died on intravenous pure polyP infusion.56 The animals were not protected from prostasome challenge either (Figure 1), supporting the presence of polyP on prostasomes. Infusion of polyP into Bdkrb2−/− animals induced lethal PE,15 and consistently, the mice were also susceptible to prostasome-triggered PE. Although polyP initiates BK formation, intravenous prostasome infusion did not reduce systemic blood pressure. A plausible explanation is that formed BK is rapidly inactivated in the pulmonary microvasculature by kinin-degrading enzymes before the mediator reaches pressure-regulating vessels.61-64
Our animal models and patient material data show that both TF/FVIIa and polyP/FXIIa pathways contribute to PC-associated hypercoagulable states. Thrombin formation in vitro was more reduced in TF- than in FXIIa-inhibited plasma (Figure 2); however, targeting each of the pathways was sufficient to protect mice from lethal PE in vivo (Figure 1). TF/FVIIa and polyP/FXIIa have distinct and differential functions in thrombus initiation and propagation. TF appears as the initiator of thrombosis on ruptured plaques, whereas FXIIa drives coagulation distant from the vessel wall within the propagating thrombus.65 Indeed, inhibition of FXII activity interferes with mechanical thrombus stability in whole blood15 and increases embolization rate in treated mice.65 Emphasizing a role for FXII in thrombus growth, inherited deficiency or pharmacologic neutralization of the factor is sufficient to protect mice from TF-triggered lethal PE.66
In cancer-related thrombosis, LMWH is the preferred mode of anticoagulation over VKAs (warfarin).67 This recommendation is based on data showing decreased VTE recurrence with use of LMWH compared with VKAs.68 In addition, VKA dosing in cancer patients is challenging as clinical studies indicated that the incidence of bleeding remains high even when international normalized ratio (INR) values being close to or below 2.0.69,70 Bleeding complications are not restricted to VKA therapy as prospective randomized controlled trials comparing LMWH and VKA therapy in cancer-associated VTE have reported similar bleeding rates.68 Data are insufficient to recommend the routine use of new oral anticoagulants as therapy for VTE in cancer patients.71 Due to the potentially serious and life-threatening bleeding, all patients require an individualized assessment of their bleeding risk prior to the initiation of anticoagulation. Thus, there is need for effective anticoagulation strategies for patients with cancer. Targeting polyP/FXII interfered with the increased procoagulant activity in patient plasma (Figure 5) and prevented PC-driven PE in mice without treatment-related risk of bleeding (Figures 3 and 4). Importantly, the decisive role of the intrinsic pathway of coagulation in thrombosis is not restricted to murine models. We recently showed that the FXIIa-inhibitory antibody 3F7 offers a safe thromboprotective strategy in a preclinical setting.24 3F7 blocks thrombosis as effectively as heparin in a cardiopulmonary bypass system in rabbits (extracorporeal membrane oxygenation [ECMO] system). However, in sharp contrast to heparin, 3F7 did not impair the hemostatic capacity of treated animals. Fully humanized 3F7 antibody has an immediate anticoagulant effect and is thus suitable for management of VTE in an acute setting.24 Humanized antibodies expressed in mammalian cells are an important class of human therapeutic products and are expected to display minimal immunogenic potential in humans.72,73 An alternative method to interfere with FXII function is the use of antisense oligonucleotides that knock down FXII expression and confers protection from thrombosis without an increase in therapy-associated bleeding.74,75 Furthermore, lowering FXI levels with FXI-antisense oligonucleotides reduced the rate of postoperative thrombosis and was safe with respect to bleeding risk in patients.76 A disadvantage of this approach, however, is the long lag time in achieving therapeutic levels of anticoagulation (injections required 1-3 times weekly for 5 weeks). Currently, small-molecule FXIIa inhibitors are under development with potential anticoagulant and additional anti-inflammatory activities.
In summary, the current study identifies a novel procoagulant mechanism driven by polyP on PC cells and prostasomes that contributes to clotting in patient plasma in vitro and PE in mouse models in vivo. Interference with the polyP/FXII pathway provides a new approach for anticoagulation in PC-associated thrombosis that lacks the bleeding risk of currently used anticoagulants.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr James H. Morrissey and Dr Stephanie A. Smith (University of Illinois at Urbana-Champaign, Urbana, IL) for the kind gift of polyP size standards, Dr Con Panousis (CSL Limited, Parkville, Australia) for providing us with 3F7, and Dr Matthias Löhr (Karolinska Institutet, Stockholm, Sweden) for support with human pancreatic cancer cells. The authors also thank Drs David Gailani (Vanderbilt University, Nashville, TN) and Keith McCrae (Cleveland Clinic, Cleveland, OH) for providing FXI and HK deficient mice, respectively, and Dr Kjell Hultenby (Karolinska Institutet, Stockholm, Sweden) for elegant TEM analyses.
This work was supported in part by grants from Cancerfonden (100615), Vetenskapsrådet (K2013-65X-21462-014-5), Hjärt Lungfonden (20140741), Stockholms läns landsting (ALF, 20140464), the German Research Society (SFB877, TP A11 and SFB841, TP B8), and a European Research Council grant (ERC-StG-2012-311575_F-12) to T.R.
Authorship
Contribution: K.F.N. and L.L. performed most of the experiments; F.L., C.B., G.S., and M.G. provided clinical material and analyzed patient samples; Göran Ronquist and Gunnar Ronquist provided tools and materials to isolate and characterize prostasomes; N.M. and T.A.F. discussed content and contributed to the experimental design; T.R. provided grant support, designed the experiments, and wrote the manuscript with E.X.S. and K.F.N; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Renné, Clinical Chemistry, Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital Solna (L1:00), SE-171 76 Stockholm, Sweden, and Institute of Clinical Chemistry and Laboratory Medicine (O26), University Medical Center Hamburg-Eppendorf, D-20246 Hamburg, Germany; e-mail: thomas@renne.net.

![Figure 1. Prostasomes initiate lethal PE in mice via the intrinsic coagulation pathway. (A) PE was induced by intravenous infusion of PC3 cell-derived prostasomes (0.8 µg/g body weight [bw]) in WT, F12−/−, F11−/−, Kng1−/−, Klkb1−/−, and Bdkrb2−/− mice or WT animals pretreated with ASIS (WT + ASIS; 2.5 µg/g bw). Mortality was assessed in each group of mice (n = 5-15). Animals alive 30 minutes after challenge were considered survivors. Horizontal bars represent mean values. ***P < .001 and **P < .01 vs untreated WT. (B) Prostasome challenged mice were intravenously infused with Evans blue shortly after the onset of respiratory arrest while the heart was still beating or after 30 minutes for those animals that survived. Lungs were excised, and perfusion defects were analyzed. (C) Immunohistochemical localization of fibrin deposition on sections from lungs of WT, F12−/−, F11−/−, and WT + ASIS-treated mice using the fibrin-specific antibody 59D8 (high magnification, lower right). Sections were counterstained with Mayer's hematoxylin (bar, 100 µm). (D) Thrombi per visual field were counted at ×10 magnification from sections such as those in C. Columns are mean ± standard error of the mean (SEM) for 35 fields. (E) Accumulation of fibrin in lungs of prostasome-challenged WT, F12−/−, F11−/−, and WT + ASIS mice was analyzed by immunoblotting. (F-G) Pulmonary thromboembolism model as in A induced by injection of 10 µg/g bw seminal prostasomes (F; n = 6) or 150 µg/g bw healthy male or patient prostasomes/exosomes (G; n = 5), *P < .05 vs untreated WT (F) and **P < .01 vs healthy males (G). P values were determined using 1-way analysis of variance (ANOVA); n.s., nonsignificant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/11/10.1182_blood-2015-01-622811/4/m_1379f1.jpeg?Expires=1765911946&Signature=JExRYcVLQWxb0TuDh-ysk~F0RhLG~fFV8KKR8skCfmd8ujy1jXbmC2CjhcAHZQ2cns3qqZDQSD1YDavWm1ptV~zHemLdTa5Nn68l1dOUYT~qL2XsgtXuExVcjXxrysYlpGng8aFrUixd1Xm-gTVJDtqb9zD6~hxgn70xot8IEnEgXDWA1HMQanjlfOtHGoGJbcZRMiVw0uCNdp7vxz-0j0~FNgeEtcH1sN2YI2HkxiZvQFx-B9wFdIqqKW-QTfnj5p6NkDCWua9fL6kZz3CbgSc8mZHsCma~keLXcPwLHGaeRKlwcXkphVigUubpz1C2WxKR-sBocbcn4NoOGFx4xQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Prostasome/FXIIa-driven increased procoagulant activity in PC patient plasma. (A-D) Real-time thrombin generation in plasma of healthy individuals (Controls) and patients (Patients). Thrombin formation in prostasome-depleted [Patients(−)] and prostasome-reconstituted [Patients(+)] patient plasma is blotted for control; n = 9 each. (E-H) Real-time thrombin generation in normal plasma stimulated by addition of prostasomes/exosomes (250 µg/mL) from healthy female and male controls (n = 10 each) or PC patients (n = 20). Patient prostasome-triggered coagulation was measured in the absence or presence of 3F7 (100 µg/mL). (A,E) Lag time until thrombin formation starts, (B,F) time to peak thrombin, (C,G) maximum thrombin (peak thrombin), and (D,H) total thrombin (ETP). Each symbol represents an individual. Horizontal bars indicate mean values. *P < .05, **P < .01, and ***P < .001, unpaired Student t test; n.s., nonsignificant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/11/10.1182_blood-2015-01-622811/4/m_1379f5.jpeg?Expires=1765911946&Signature=i4bJ74Ezc5tVETZ5bwrbPvSj6PCXYOXrRN04LhQiGhI0-Jx3PtPXumH1emy8EsTzbFdAaXkkh0uhT8UC65AVbkfmIDWBSNMelueci-mgVI6BLz4v8dWN7y~5FE2mUMRoEFDwO02e6L8zTus~3V1LepNdp3~RhRmRnObXVuxXjcI~9E5RvFXCGAbdwcvEAiijbc4jbJgmfeEafpX7W1ZX3EPeWdJRMlKb8-ObgQiLkYTLIN80rOZ4quz3l7MFT0XfAtTonmFP7GBUrI8q~XY95TsFxKz02kUYoVZ4ixezRFRRk42hULHYgn4Ys8-NOSURbk4ciKydUkYkt1YLfZ22XQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)