Key Points
Platelet function in WAS/XLT, measured by agonist-induced surface-activated GPIIb-IIIa and P-selectin, is proportional to platelet size.
Eltrombopag increased platelet counts, but did not improve platelet activation, in most WAS/XLT patients.
Abstract
Because Wiskott-Aldrich syndrome (WAS) and X-linked thrombocytopenia (XLT) patients have microthrombocytopenia, hemorrhage is a major problem. We asked whether eltrombopag, a thrombopoietic agent, would increase platelet counts, improve platelet activation, and/or reduce bleeding in WAS/XLT patients. In 9 WAS/XLT patients and 8 age-matched healthy controls, platelet activation was assessed by whole blood flow cytometry. Agonist-induced platelet surface activated glycoprotein (GP) IIb-IIIa and P-selectin in WAS/XLT patients were proportional to platelet size and therefore decreased compared with controls. In contrast, annexin V binding showed no differences between WAS/XLT and controls. Eltrombopag treatment resulted in an increased platelet count in 5 out of 8 patients. Among responders to eltrombopag, immature platelet fraction in 3 WAS/XLT patients was significantly less increased compared with 7 pediatric chronic immune thrombocytopenia (ITP) patients. Platelet activation did not improve in 3 WAS/XLT patients whose platelet count improved on eltrombopag. In conclusion: (1) the reduced platelet activation observed in WAS/XLT is primarily due to the microthrombocytopenia; and (2) although the eltrombopag-induced increase in platelet production in WAS/XLT is less than in ITP, eltrombopag has beneficial effects on platelet count but not platelet activation in the majority of WAS/XLT patients. This trial was registered at www.clinicaltrials.gov as #NCT00909363.
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked immunodeficiency and platelet disease, characterized by a combination of thrombocytopenia with small platelets, eczema, recurrent infections, and a high incidence of autoimmunity and malignancy.1 Whereas the immune defects vary in clinical severity, the thrombocytopenia is a universal and almost always profound characteristic of the disease.
WAS is caused by mutations of the WAS gene encoding the WAS protein (WASP), exclusively expressed in hematopoietic cells and involved in cell signaling and cytoskeleton reorganization.2-4 Although the gene responsible for WAS has been identified, the cause of thrombocytopenia is not fully known. WASP deficiency appears to affect both platelet production and consumption.5 Some patients develop autoimmune thrombocytopenia, resulting in a more severe degree of thrombocytopenia and bleeding, and lesser effectiveness of platelet transfusions.6 Point mutations in exons 1 and 2 of the WAS gene typically result in X-linked thrombocytopenia (XLT), a milder form of the disease usually without infections or eczema.7,8 WAS and XLT represent polar ends of the disease spectrum, although there is actually a continuum including intermediate forms. XLT patients have profound thrombocytopenia and bleeding but not the immune dysregulation as seen in patients with WAS. Treatment of the thrombocytopenia in WAS/XLT has primarily included splenectomy or stem cell transplantation.
Bleeding occurs in the majority (84%) of patients with WAS/XLT, including petechiae or purpura (78%), epistaxis (16%), and hematemesis and melena (28%).9 Other significant bleeding is present in 5% of patients, including bleeding from the umbilical stump, postsurgical bleeding, posttraumatic bleeding, testicular hemorrhage, and subconjunctival hemorrhage. Severe, life-threatening bleeding episodes, such as gastrointestinal and intracranial hemorrhage (ICH), occur in up to 30% of patients with WAS/XLT and cause death in 4% to 10% of these patients.9,10
Expression of the major platelet membrane glycoproteins (GP) in WAS patients is normal.11 However, platelet function in WAS/XLT has been very difficult to evaluate because of both the reduced platelet count and size, which affect all platelet function assays except flow cytometry.12 Attempts to measure platelet function in WAS patients have resulted in highly variable outcomes: an absence of platelet aggregation in response to multiple agonists,13 normal aggregation,14 and a slightly enhanced platelet response.15 In another study, when only WASP-deficient platelets with normal size were included in the analysis by electronic gating, flow cytometry results showed that the number of GPIIb-IIIa molecules per platelet was lower in platelets from WAS patients compared with healthy controls, and agonist-induced expression of GPIIb-IIIa, P-selectin, and CD63 was diminished in WAS platelets, suggesting that there may be a defect in activation.16 Two studies by Shcherbina et al showed increased expression of phosphatidylserine (PS) on the surface of circulating platelets and an increase in platelet-derived microparticles in the plasma of WAS/XLT patients, raising the possibility that these features explain or at least contribute to the reduced platelet lifespan and size in this disease.17,18
To better understand the pathogenesis of bleeding in WAS/XLT, the present study examined platelet activation by whole blood flow cytometry in WAS/XLT patients compared with healthy age-matched controls. Platelet activation markers were corrected for platelet size difference because of the microthrombocytopenia in WAS/XLT. In addition, the present study tested a thrombopoietin receptor agonist (eltrombopag) as a potential novel treatment of the thrombocytopenia of WAS/XLT. Platelet counts, platelet production, platelet activation, and bleeding diathesis were examined before and after eltrombopag treatment. Platelet production in WAS/XLT patients was compared with that of pediatric immune thrombocytopenia (ITP) patients treated with eltrombopag.
Methods
Subjects
Thirteen patients with WAS/XLT and 8 age-matched healthy controls, or their parents, gave written informed consent in accordance with the Declaration of Helsinki prior to participation in this Weill Cornell Medical College Institutional Review Board-approved study. Patients were classified as either WAS or XLT based on their clinical history, laboratory testing, and presentation and given a grade based on the Ochs WAS score (Table 1).19 Nine patients consented to platelet activation studies, which required only additional blood to be drawn at scheduled blood draws. Four of these 9 patients went on to treatment with eltrombopag in the study and 5 WAS/XLT patients only had their platelet activation tested: 3 patients had platelet counts >100 × 109/L (two of whom had undergone splenectomy), 1 patient was too young to take tablets, and 1 patient’s parents allowed the blood draw but not treatment with eltrombopag. Eltrombopag (Promacta/Revolade; GlaxoSmithKline) was administered to 8 patients as part of a phase 2 study (Pathobiology of Thrombocytopenia and Bleeding in Patients With Wiskott-Aldrich Syndrome, #NCT00909363). Male patients between 3 months and 80 years of age with a clinical diagnosis of WAS/XLT and baseline platelet counts <50 × 109/L were eligible to enroll in the treatment study and all eligible patients for whom consent was obtained were enrolled. Eltrombopag (9 to 75 mg) was administered orally once daily and dosing was adjusted to maintain the platelet count >50 × 109/L. Blood samples from 3 WAS/XLT patients treated with eltrombopag were studied for platelet activation before and after initiation of treatment. Blood from a fourth WAS/XLT patient treated with eltrombopag was studied for platelet activation but only prior to treatment. Of the other 4 patients who were treated with eltrombopag but did not have their platelet activation studied, 3 patients were in Israel and therefore unavailable for testing, and patient 4 could only come to clinic on Fridays and therefore his blood could not be processed and analyzed by flow cytometry within 48 hours. Bone marrow (BM) examination was performed in 1 patient who had been on eltrombopag, whereas others were being scheduled.
Description of WAS/XLT patients
| Patient . | Age (y) . | WAS mutation . | WAS grade19 . | Splenectomy (y) . | Baseline platelet count (109/L) . | Baseline MPV (fL) . | Treatment with eltrombopag . |
|---|---|---|---|---|---|---|---|
| 1* | 0.5 | c.172-176del | 4 | — | 37 | 7.9 | — |
| 2* | 8 | c.134C>T | 2 | + (2004) | 94 | 5.3 | — |
| 3* | 6 | c.206C>A | 2 | — | 102 | 6.5 | — |
| 4* | 4 | c.122_124del | 2 | — | 26 | 6.3 | — |
| 5* | 13 | c.257G>A | 2 | + (2005) | 145 | 6.7 | — |
| 6* | 26 | c.256C>T | 3 | — | 19 | 8.1 | + |
| 7* | 6 | c.593+5G>A | 3 | — | 16 | 6.2 | + |
| 8* | 2 | c.862A>T | 4 | — | 44 | 5.2 | + |
| 9* | 1.6 | c. 539+5G>A | 2 | — | 20 | 9.0 | + |
| 10 | 12 | c.1213_1214insG | 4 | + (2011) | 9 | 6.2 | + |
| 11 | 7 | c.194G>A | 2 | — | 15 | 7.7 | + |
| 12† | 5 | p.Val332Ala | 2 | — | 14 | 6.2 | + |
| 13 | 19 | p.Glu133Gln | 3 | — | 13 | 9.5 | + |
| Patient . | Age (y) . | WAS mutation . | WAS grade19 . | Splenectomy (y) . | Baseline platelet count (109/L) . | Baseline MPV (fL) . | Treatment with eltrombopag . |
|---|---|---|---|---|---|---|---|
| 1* | 0.5 | c.172-176del | 4 | — | 37 | 7.9 | — |
| 2* | 8 | c.134C>T | 2 | + (2004) | 94 | 5.3 | — |
| 3* | 6 | c.206C>A | 2 | — | 102 | 6.5 | — |
| 4* | 4 | c.122_124del | 2 | — | 26 | 6.3 | — |
| 5* | 13 | c.257G>A | 2 | + (2005) | 145 | 6.7 | — |
| 6* | 26 | c.256C>T | 3 | — | 19 | 8.1 | + |
| 7* | 6 | c.593+5G>A | 3 | — | 16 | 6.2 | + |
| 8* | 2 | c.862A>T | 4 | — | 44 | 5.2 | + |
| 9* | 1.6 | c. 539+5G>A | 2 | — | 20 | 9.0 | + |
| 10 | 12 | c.1213_1214insG | 4 | + (2011) | 9 | 6.2 | + |
| 11 | 7 | c.194G>A | 2 | — | 15 | 7.7 | + |
| 12† | 5 | p.Val332Ala | 2 | — | 14 | 6.2 | + |
| 13 | 19 | p.Glu133Gln | 3 | — | 13 | 9.5 | + |
Platelet activation measured by flow cytometry. (Patient #8 had platelet function assessed only prior to treatment with eltrombopag).
Mutation was identified in WAS gene in patient #12 and predicted to cause XLT. However, the patient’s mother was also found to be homozygous for this mutation. Both the patient and the mother were shown to have reduced WASP expression and complete exome sequencing is in progress.
Freshly drawn venous blood from WAS/XLT patients, and from healthy volunteers who had not taken any antiplatelet medications within 7 days before the study, was collected into 4.5 mL 3.2% trisodium citrate and EDTA vacutainers (BD Biosciences). The EDTA sample was used for measurements of platelet counts in a Bayer-ADVIA automated complete blood count counter immediately after the blood draw, and the absolute immature platelet fraction (AIPF) in a Sysmex XE-2100 automated analyzer.
Platelet activation
Less than 10 minutes after blood draw, aliquots of whole blood from the citrate sample were incubated with fluorescently labeled monoclonal antibodies (mAbs) and either 0.5 μM adenosine 5′-diphosphate (ADP) (low), 20 μM ADP (high), 1.5 μM thrombin receptor activating peptide (TRAP; low), 20 μM TRAP (high), or vehicle (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES] Tyrode’s buffer [10 mM HEPES, 137 mM sodium chloride, 2.8 mM potassium chloride, 1 mM magnesium chloride, 12 mM sodium hydrogen carbonate, 0.4 mM sodium phosphate dibasic, 5.5 mM glucose, 0.35% w/v bovine serum albumin, pH 7.4]) for 15 minutes. The antibodies used were: phycoerythrin (PE)-conjugated anti–P-selectin mAb (CD62P, clone AK-4; BD Pharmingen); fluorescein isothiocyanate (FITC)-conjugated mAb PAC1 (BD Biosciences), which only binds to the activated conformation of GPIIb-IIIa; and PE-Cy5–conjugated anti-CD41 (GPIIb) mAb (Beckman Coulter). PE-conjugated MIgG1 isotype (BD Pharmingen) and FITC-PAC1, together with 2.5 μg/mL of the GPIIb-IIIa antagonist eptifibatide to block specific binding served as the negative controls for platelet surface P-selectin and platelet surface-activated GPIIb-IIIa, respectively. The reaction was stopped with a 15-fold dilution in 1% formaldehyde in HEPES-saline buffer.
Platelet surface binding of annexin V was measured in platelet-rich plasma (PRP). PRP was prepared by centrifugation (10 minutes, 120 × g) and platelet count was normalized to 30 × 109/L. Aliquots of the adjusted PRP were incubated with a cocktail of mAbs, containing FITC-conjugated annexin V antibody (BD Pharmingen), PE-Cy5–conjugated CD42b, and PE-conjugated CD41 (Dako) for 10 minutes before the addition to tubes containing either 6 mM calcium chloride and 3 mM Gly-Pro-Arg-Pro in HEPES-Tyrode’s buffer, to the same buffer with 1 μg/mL or 5 μg/mL convulxin (Pentapharm) or HEPES-Tyrode’s buffer alone for exactly 5 minutes. Samples were fixed by the addition of 1% formaldehyde in HEPES-saline buffer.
Flow cytometry
Fixed samples were stored at 4°C and sent by overnight courier to the Center for Platelet Research Studies at Boston Children’s Hospital for flow cytometric analysis. This was performed in a FACSCalibur flow cytometer (Becton Dickinson) that was calibrated daily to assure proper instrument function and consistent fluorescence measurements over time. Platelet surface-activated GPIIb-IIIa, P-selectin, and annexin V binding were measured relative to the isotype control as percentage positive cells and mean fluorescence intensity (MFI) for activated GPIIb-IIIa, P-selectin, and CD41, as previously described.20 Established voltages for forward scatter (FSC) and side scatter (SSC) were used to present platelets in the middle of the dot plot (FSC-SSC). Cells with high FSC-SSC were gated out. A gate was drawn around the platelets and FSC was measured, as previously described.21
Statistical analysis
Results are stated as mean ± standard error of the mean (SEM). Data were analyzed using GraphPad Prism, version 5 (San Diego, CA). Two-way analysis of variance (ANOVA) with Bonferroni posttest was performed to determine differences between the health status of subjects (healthy controls or patients) and agonist treatment, and the possible interactions of each. Platelet counts were analyzed with the Student t test for unpaired observations. Results were considered significant if P was < .05.
Results
Patient demographics and clinical characteristics
No difference was observed between the mean age of the 9 WAS/XLT patients who had their platelet activation measured and healthy controls, 6.9 ± 2.7 years (range, 0.5-26) (Table 1) vs 12.7 ± 4.6 years (range, 1.33-33) (mean ± SEM), respectively (P = .28). There was no correlation between age and any of the responses in platelet activation assays (data not shown). Because WAS/XLT is an X-linked disease, 100% of patients were male; 89% of patients were children. Two patients studied for platelet activation had undergone a splenectomy. In the control group, 63% were children and 38% were male. Three patients received eltrombopag (20 to 75 mg), and had platelet activation markers studied before and after treatment (Table 1). Control subjects did not take any medications at the time of the study. Platelet counts were 373.8 ± 59.9 × 109/L (mean ± SEM) for healthy controls, whereas for the 9 WAS/XLT patients who had their platelet activation measured, platelet counts were 55.9 ± 15.4 × 109/L (mean ± SEM; Table 1). Platelet counts were 18.8 ± 3.8 × 109/L and mean platelet volumes (MPV) were 7.3 ± 0.5 (mean ± SEM) in the 8 WAS/XLT patients prior to initiating eltrombopag.
Platelet activation
Platelet surface markers of activation in WAS/XLT patients compared with controls
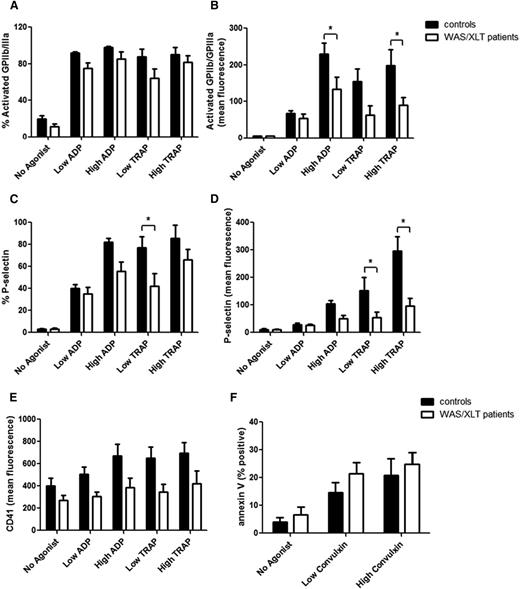
WASP, deficient to a variable degree in WAS/XLT, is important for cytoskeletal movement and could therefore be involved in trafficking of surface proteins and subsequent activation of platelets. Platelet surface expression of activated GPIIb-IIIa (reported by PAC1 binding) and P-selectin were decreased in WAS/XLT patients compared with controls (Figure 1A-D). Significant differences were detected for PAC1 MFI in response to a high concentration of ADP (P < .05) and TRAP (P < .05) (Figure 1B), percentage of cells positive for P-selectin in response to low TRAP (P < .05) (Figure 1C), and P-selectin MFI in response to low (P < .05) and high TRAP (P < .001) (Figure 1D). There was a trend toward lower surface expression levels of CD41 on platelets from WAS/XLT patients compared with controls; however, these differences were not statistically significant (Figure 1E). In contrast, the percentage of annexin V-positive events, as a measure of procoagulant platelets and platelet-derived microparticles, showed no differences between WAS/XLT patients (n = 6) and controls (n = 7) (Figure 1F).
Platelet surface expression of activation markers in controls and WAS/XLT patients. The following markers were measured with and without agonist stimulation: (A) percentage of platelets positive for activated GPIIb-IIIa (measured by PAC1 binding), (B) mean fluorescence for activated GPIIb-IIIa (PAC1), (C) percentage of platelets positive for P-selectin, (D) mean fluorescence for P-selectin, (E), mean fluorescence for CD41, and (F) percentage of cells positive for PS (measured by annexin V binding). Low ADP, 0.5 μM; high ADP, 20 μM; low TRAP, 1.5 μM; high TRAP, 20 μM; low convulxin, 1 ng/mL; and high convulxin, 5 ng/mL. Results are expressed as mean ± SEM with n = 8 (n = 7 for panel F) for controls and n = 9 (n = 6 for panel F) for WAS/XLT patients, and analyzed with 2-way ANOVA with Bonferroni posttest. *Significant difference (P < .05).
Platelet surface expression of activation markers in controls and WAS/XLT patients. The following markers were measured with and without agonist stimulation: (A) percentage of platelets positive for activated GPIIb-IIIa (measured by PAC1 binding), (B) mean fluorescence for activated GPIIb-IIIa (PAC1), (C) percentage of platelets positive for P-selectin, (D) mean fluorescence for P-selectin, (E), mean fluorescence for CD41, and (F) percentage of cells positive for PS (measured by annexin V binding). Low ADP, 0.5 μM; high ADP, 20 μM; low TRAP, 1.5 μM; high TRAP, 20 μM; low convulxin, 1 ng/mL; and high convulxin, 5 ng/mL. Results are expressed as mean ± SEM with n = 8 (n = 7 for panel F) for controls and n = 9 (n = 6 for panel F) for WAS/XLT patients, and analyzed with 2-way ANOVA with Bonferroni posttest. *Significant difference (P < .05).
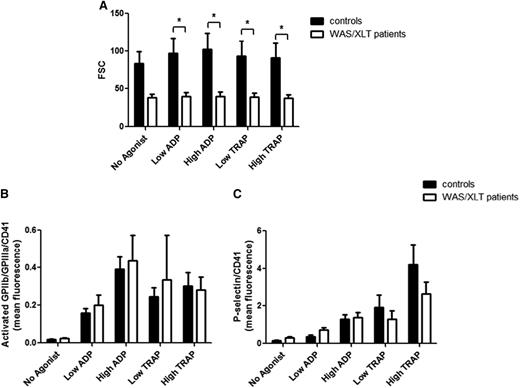
Mean platelet size is well known to be reduced in patients with WAS/XLT,1 as reflected by the reduced FSC of platelets in the present study (Figure 2A). To determine whether differences in platelet surface-activated GPIIb-IIIa and P-selectin between WAS/XLT patients and controls were due to differences in platelet size or were the result of reduced surface expression per unit area, platelet surface PAC1 binding and P-selectin expression were normalized to platelet surface CD41 fluorescence (a surrogate for platelet surface area). When platelet surface-activated GPIIb-IIIa and P-selectin were corrected for platelet size by CD41 MFI, no differences were seen between WAS/XLT patients vs controls (Figure 2B-C). These results indicate that platelet activation measured by PAC1 binding and P-selectin expression is proportional to platelet size in WAS/XLT.
Platelet surface-activated GPIIb-IIIa and P-selectin normalized to platelet surface CD41 fluorescence in controls and WAS/XLT patients. The following markers were measured with and without agonist stimulation: (A) FSC of platelets, (B) ratio of mean fluorescence for activated GPIIb-IIIa (PAC1) and CD41 mean fluorescence, and (C) ratio of mean fluorescence for P-selectin and CD41 mean fluorescence. Low ADP, 0.5 μM; high ADP, 20 μM; low TRAP, 1.5 μM; and high TRAP, 20 μM. Results are expressed as mean ± SEM with n = 8 for controls and n = 9 for WAS/XLT patients, and analyzed with 2-way ANOVA with Bonferroni posttest. *Significant difference (P < .05).
Platelet surface-activated GPIIb-IIIa and P-selectin normalized to platelet surface CD41 fluorescence in controls and WAS/XLT patients. The following markers were measured with and without agonist stimulation: (A) FSC of platelets, (B) ratio of mean fluorescence for activated GPIIb-IIIa (PAC1) and CD41 mean fluorescence, and (C) ratio of mean fluorescence for P-selectin and CD41 mean fluorescence. Low ADP, 0.5 μM; high ADP, 20 μM; low TRAP, 1.5 μM; and high TRAP, 20 μM. Results are expressed as mean ± SEM with n = 8 for controls and n = 9 for WAS/XLT patients, and analyzed with 2-way ANOVA with Bonferroni posttest. *Significant difference (P < .05).
Platelet surface markers of activation in WAS/XLT patients treated with eltrombopag
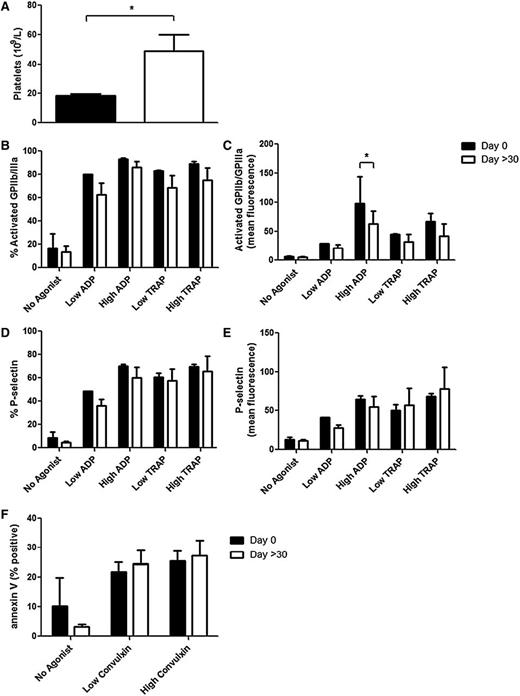
Platelet activation was re-assessed in 3 WAS/XLT patients who were on eltrombopag 20 to 75 mg daily, with time on eltrombopag ranging from 1 to 21.5 months (Table 1). Eltrombopag treatment after these different time points significantly increased platelet counts in these patients from 18.3 ± 1.2 × 109/L to 49.0 ± 11.0 × 109/L (mean ± SEM; Figure 3A). Also, the individual data show an increase in platelet count for each patient (data not shown); thus, eltrombopag produced more platelets in all 3 patients, independent of the time of drug treatment. However, the presence of these new platelets did not result in an improvement in platelet activation, as measured by PAC1 binding, P-selectin expression, and annexin V binding after agonist stimulation (Figure 3B-F), with the exception of high ADP-induced activated GPIIb-IIIa MFI (Figure 3C).
Platelet markers in WAS/XLT patients at day 0 and >30 days of eltrombopag treatment. Platelet count was measured in a Bayer-ADVIA autoanalyzer (A). The following makers were measured with and without agonist stimulation: (B) percentage of platelets positive for activated GPIIb-IIIa (measured by PAC1 binding), (C) mean fluorescence for activated GPIIb-IIIa (PAC1), (D) percentage of platelets positive for P-selectin, (E) mean fluorescence for P-selectin, and (F) percentage of cells positive for PS (measured by annexin V binding). Low ADP, 0.5 μM; high ADP, 20 μM; low TRAP, 1.5 μM; high TRAP, 20 μM; low convulxin, 1 ng/mL; and high convulxin, 5 ng/mL. Results are expressed as mean ± SEM with n = 3 for WAS/XLT patients and analyzed with Student t test (A) or 2-way ANOVA with Bonferroni posttest (B-F). *Significant difference (P < .05).
Platelet markers in WAS/XLT patients at day 0 and >30 days of eltrombopag treatment. Platelet count was measured in a Bayer-ADVIA autoanalyzer (A). The following makers were measured with and without agonist stimulation: (B) percentage of platelets positive for activated GPIIb-IIIa (measured by PAC1 binding), (C) mean fluorescence for activated GPIIb-IIIa (PAC1), (D) percentage of platelets positive for P-selectin, (E) mean fluorescence for P-selectin, and (F) percentage of cells positive for PS (measured by annexin V binding). Low ADP, 0.5 μM; high ADP, 20 μM; low TRAP, 1.5 μM; high TRAP, 20 μM; low convulxin, 1 ng/mL; and high convulxin, 5 ng/mL. Results are expressed as mean ± SEM with n = 3 for WAS/XLT patients and analyzed with Student t test (A) or 2-way ANOVA with Bonferroni posttest (B-F). *Significant difference (P < .05).
Bleeding and platelet counts
Clinical results
Five of 8 WAS/XLT patients (3 XLT and 2 WAS) who were on eltrombopag treatment of at least 1 month (1 adult and 4 children) were clinical responders, as evidenced by achieving at least one platelet count ≥50 × 109/L and double the baseline count, and 6 of 8 had reduced bleeding symptoms (Figure 4; Table 2). Prior to eltrombopag treatment, 2 patients received frequent platelet transfusions, and a third patient reported frequent and severe episodes of epistaxis lasting 30 minutes or more. One responder had experienced an ICH prior to eltrombopag, whereas another had failed hematopoietic stem cell transplantation (HSCT). On eltrombopag, responders and nonresponders who experienced reduced bleeding reported that bruises, petechiae, and epistaxis resolved on their own. None of these patients experienced continued severe bleeding symptoms while on treatment (Table 2). Data on serial liver function tests were available on 4 of the WAS/XLT patients treated with eltrombopag. Serum ALT levels in WAS/XLT patients treated with eltrombopag remained normal with the exception of one transient occurrence of mild transaminitis; this patient continued on therapy. A BM examination was performed in 1 patient, which showed a normocellular marrow with large megakaryocytes and grade 1 (of 4) fibrosis.
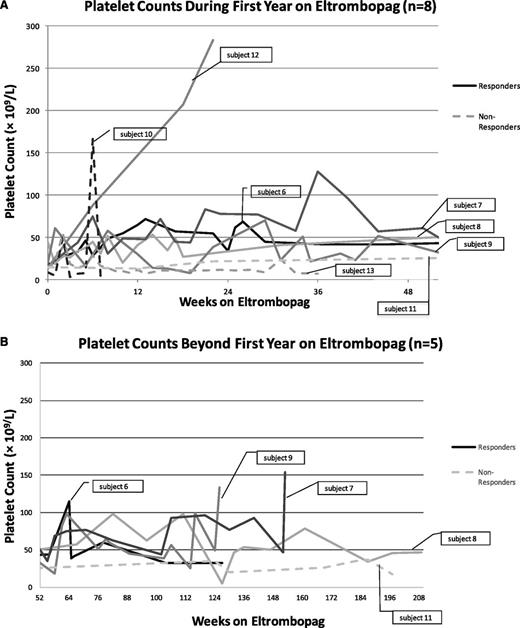
Platelet counts of WAS/XLT patients (n = 8) during treatment with eltrombopag. Eltrombopag doses varied by patient and ranged from 9 mg to 75 mg daily. Patients were considered responders if at least one platelet count increased to ≥50 × 109/L, was double the baseline count, and bleeding was reduced. The temporary rise in platelet count at week 6 to >160 × 109/L in subject 10 (a nonresponder) was the result of a platelet transfusion (A). Five patients continued treatment past 52 weeks (B).
Platelet counts of WAS/XLT patients (n = 8) during treatment with eltrombopag. Eltrombopag doses varied by patient and ranged from 9 mg to 75 mg daily. Patients were considered responders if at least one platelet count increased to ≥50 × 109/L, was double the baseline count, and bleeding was reduced. The temporary rise in platelet count at week 6 to >160 × 109/L in subject 10 (a nonresponder) was the result of a platelet transfusion (A). Five patients continued treatment past 52 weeks (B).
Clinical response to eltrombopag therapy
| Subject number . | Platelet count (109/L) . | Median mean platelet volume . | Dosing (mg/d) . | Bleeding (1 = bleeding before and during tx; 2 = no bleeding before or during tx; 3 = bleeding reduced on tx) . | Change in platelet transfusions while on eltrombopag (0 = no transfusions prior to or during tx) . | Change in WAS/ XLT tx while on eltrombopag (0 = not on other medication prior to or during tx) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline count . | Highest count on epag (wk) . | Still on epag? (Y/N) . | Count at most recent follow- up . | Pre-epag . | On-epag . | Starting dose . | Maximum dose . | ||||
| Responders | |||||||||||
| 6 | 19 | 115 wk 64 | N | 11 20 wks off epag | 7.0 | 6.9 | 50 0.62 mg/kg | 75 0.88 mg/kg | 3 Frequent episodes of epistaxis, at least once per month lasting ≥30 min. On epag, fewer episodes that rarely lasted 10 min | 0 | 0 |
| 7 | 16 | 154 wk 152 | Y | 154 wk 152 | 5.7 | 6.3 | 35 1.9 mg/kg | 75 2.8 mg/kg | 3 Frequent bruising in addition to ICH and very occasional epistaxis that lasted ≥15 min. On epag, no bleeding with increased dose to 75 mg at month 18 | No platelet transfusions required while on epag (had required them prior to therapy for ICH) | Discontinued daily amicar while on epag |
| 8* | 44 | 98 wks 82 and 111 | Y | 47 wk 209 | 5.4 | 6.2 | 9 0.7 mg/kg | 75 3.3 mg/kg | 3 Substantial bruising, oral bleeding, and epistaxis. On epag, fewer episodes of epistaxis and decreased bruising | Less frequent platelet transfusions required (only 1 not related to surgical procedures) | No change in WAS/XLT tx |
| 9 | 20 | 134 wk 126 | Y | 134 wk 126 | 9 | 7.1 | 20 1.6 mg/kg | 60 3.8 mg/kg | 3 Frequent bruising, constant petechiae, and one major bleed when hitting lip. On epag, no bruising or bleeding. Pt is more energetic while on epag | 0 | 0 IVIG ×1 while on epag when pt had a viral infection |
| 12† | 14 | 283 wk 22 | N | 128 24 wks off epag | 6.2 | 5.8 | 25 1.6 mg/kg | 75 5 mg/kg | 2 Before prednisone and epag, bruising and other bleeding. No bleeding on prednisone alone. With addition of epag, no bleeding, including when prednisone was subsequently discontinued | 0 | Discontinued daily prednisone (0.2 mg/kg) while on epag |
| Nonresponders | |||||||||||
| 10 | 9 | 166 wk 6 (after platelet trans-fusion) | N | Lost to follow-up | 7.2 | 8.2 | 50 1.3 mg/kg | 75 2.1 mg/kg | 1 | Frequent platelet transfusions required before and during epag therapy | 0 |
| 11 | 15 | 37 wk 187 | Y | 17 wk 198 | 7.7 | 8.3 | 25 1.1 mg/kg | 75 2.8 mg/kg | 3 Bruising and other minor bleeding. On epag, no bleeding. Pt is more energetic on epag. | Does not require platelet transfusions (had required weekly platelet transfusions prior to epag) | 0 |
| 13 | 13 | 55 wk 2 | N | 30 58 wks off epag, while on romiplostim | 9.5 | 8.2 | 50 weight n/a | 75 weight n/a | 3 On epag, slightly less skin and mucous membrane bleeding | 0 | 0 |
| Subject number . | Platelet count (109/L) . | Median mean platelet volume . | Dosing (mg/d) . | Bleeding (1 = bleeding before and during tx; 2 = no bleeding before or during tx; 3 = bleeding reduced on tx) . | Change in platelet transfusions while on eltrombopag (0 = no transfusions prior to or during tx) . | Change in WAS/ XLT tx while on eltrombopag (0 = not on other medication prior to or during tx) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline count . | Highest count on epag (wk) . | Still on epag? (Y/N) . | Count at most recent follow- up . | Pre-epag . | On-epag . | Starting dose . | Maximum dose . | ||||
| Responders | |||||||||||
| 6 | 19 | 115 wk 64 | N | 11 20 wks off epag | 7.0 | 6.9 | 50 0.62 mg/kg | 75 0.88 mg/kg | 3 Frequent episodes of epistaxis, at least once per month lasting ≥30 min. On epag, fewer episodes that rarely lasted 10 min | 0 | 0 |
| 7 | 16 | 154 wk 152 | Y | 154 wk 152 | 5.7 | 6.3 | 35 1.9 mg/kg | 75 2.8 mg/kg | 3 Frequent bruising in addition to ICH and very occasional epistaxis that lasted ≥15 min. On epag, no bleeding with increased dose to 75 mg at month 18 | No platelet transfusions required while on epag (had required them prior to therapy for ICH) | Discontinued daily amicar while on epag |
| 8* | 44 | 98 wks 82 and 111 | Y | 47 wk 209 | 5.4 | 6.2 | 9 0.7 mg/kg | 75 3.3 mg/kg | 3 Substantial bruising, oral bleeding, and epistaxis. On epag, fewer episodes of epistaxis and decreased bruising | Less frequent platelet transfusions required (only 1 not related to surgical procedures) | No change in WAS/XLT tx |
| 9 | 20 | 134 wk 126 | Y | 134 wk 126 | 9 | 7.1 | 20 1.6 mg/kg | 60 3.8 mg/kg | 3 Frequent bruising, constant petechiae, and one major bleed when hitting lip. On epag, no bruising or bleeding. Pt is more energetic while on epag | 0 | 0 IVIG ×1 while on epag when pt had a viral infection |
| 12† | 14 | 283 wk 22 | N | 128 24 wks off epag | 6.2 | 5.8 | 25 1.6 mg/kg | 75 5 mg/kg | 2 Before prednisone and epag, bruising and other bleeding. No bleeding on prednisone alone. With addition of epag, no bleeding, including when prednisone was subsequently discontinued | 0 | Discontinued daily prednisone (0.2 mg/kg) while on epag |
| Nonresponders | |||||||||||
| 10 | 9 | 166 wk 6 (after platelet trans-fusion) | N | Lost to follow-up | 7.2 | 8.2 | 50 1.3 mg/kg | 75 2.1 mg/kg | 1 | Frequent platelet transfusions required before and during epag therapy | 0 |
| 11 | 15 | 37 wk 187 | Y | 17 wk 198 | 7.7 | 8.3 | 25 1.1 mg/kg | 75 2.8 mg/kg | 3 Bruising and other minor bleeding. On epag, no bleeding. Pt is more energetic on epag. | Does not require platelet transfusions (had required weekly platelet transfusions prior to epag) | 0 |
| 13 | 13 | 55 wk 2 | N | 30 58 wks off epag, while on romiplostim | 9.5 | 8.2 | 50 weight n/a | 75 weight n/a | 3 On epag, slightly less skin and mucous membrane bleeding | 0 | 0 |
Epag, eltrombopag; ICH, intracranial hemorrhage; n/a, not available; Pt, patient; tx, treatment with eltrombopag.
Continuing IVIG every 3 to 4 weeks as prophylaxis for infection.
In view of the uncertainty regarding response to eltrombopag, it was decided to continue the prednisone and add eltrombopag with the plan of discontinuing the prednisone if the eltrombopag elevated the platelet count.
Three eltrombopag-treated patients had platelet counts ≥20 × 109/L above baseline counts and 4 patients reached counts ≥100 × 109/L. Four of 5 clinical responders experienced 50% or more of their on-treatment platelet counts ≥30 × 109/L and 1 patient had >50% of their on-treatment platelet counts ≥50 × 109/L (Figure 4). There was no consistent change in MPV in the 5 eltrombopag-treated responders (Table 2). One clinical responder on eltrombopag received two platelet transfusions subsequent to acute events: one prophylactically for an incidence of head trauma and one for hematemesis during infection. This patient also received prophylactic intravenous immunoglobulin (IVIG) every 3 to 4 weeks; he had been receiving IVIG regularly prior to initiating treatment with eltrombopag, but had also received frequent platelet transfusions despite the IVIG. The duration of eltrombopag use for the 5 responders ranged from 22 to 209 weeks (3 patients are ongoing).
Three WAS patients (1 adult) were nonresponders to eltrombopag. Two had had at least one episode of ICH prior to eltrombopag, 1 was splenectomized, and 1 had failed HSCT. Two patients discontinued eltrombopag due to lack of response, 1 of whom switched to romiplostim therapy with greater effect with the platelet count increasing to >20 × 109/L and having less bleeding. One continued eltrombopag because of a small reduction in bleeding symptoms combined with the lack of available alternative therapy. No patients experienced drug-related serious adverse events on eltrombopag.
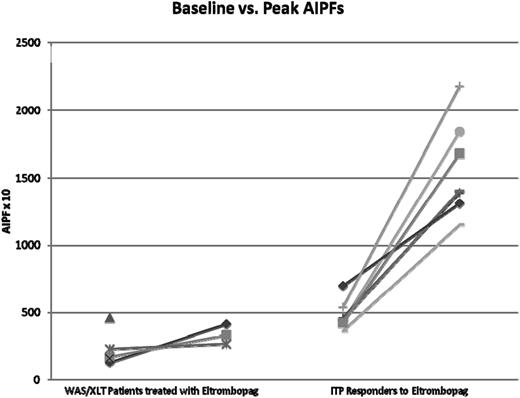
AIPF
AIPF were measured in eltrombopag-treated patients (2 WAS, 3 XLT; post-eltrombopag data were unavailable for 2 patients) (Figure 5). When compared with 7 age-matched chronic ITP patients responsive to eltrombopag, the 3 WAS/XLT patients had substantially and significantly lower baseline AIPFs and peak AIPFs. Increase in AIPF from baseline to peak, peak platelet count, and increase to peak platelet for the WAS/XLT patients were also significantly different from the ITP patients (2-tailed P values < .01 for all measurements; data not shown).
Changes in AIPF in WAS/XLT patients using eltrombopag compared with age-matched chronic ITP patients responding to eltrombopag. Post-eltrombopag data were unavailable for 2 WAS/XLT patients. Two ITP patients had almost superimposable data so that there are 7 patients’ data but only 6 evident lines.
Changes in AIPF in WAS/XLT patients using eltrombopag compared with age-matched chronic ITP patients responding to eltrombopag. Post-eltrombopag data were unavailable for 2 WAS/XLT patients. Two ITP patients had almost superimposable data so that there are 7 patients’ data but only 6 evident lines.
Discussion
Bleeding is a major complication in patients with WAS/XLT, with 30% of patients facing life-threatening bleeding, such as gastrointestinal and ICHs.9 This bleeding tendency is primarily due to the thrombocytopenia observed in WAS/XLT patients; platelet counts are typically <10% of the number of circulating platelets in healthy persons. Given the apparently far higher incidence of serious bleeding in WAS/XLT than observed in comparably thrombocytopenic patients with ITP, two not mutually exclusive hypotheses have been entertained. One is that WAS platelets, absent in WASP, manifest intrinsic platelet function abnormalities. The second is that the microthrombocytopenia is responsible for the bleeding tendency. Thus, it is currently unclear whether, and if so to what degree, platelet dysfunction contributes to the bleeding diathesis in WAS/XLT. We therefore studied the activation state of circulating platelets in WAS/XLT patients with and without agonist stimulation ex vivo and compared these results with those obtained in healthy controls. The platelet surface expression of activated GPIIb-IIIa and P-selectin in response to agonists was lower in WAS/XLT patients compared with healthy controls (Figure 1). However, after correction for surface area using CD41 as a surrogate, the surface expression of activated GPIIb-IIIa and P-selectin in WAS/XLT patients was not different from that of healthy controls (Figure 2). These results indicate that the reduced platelet activation observed in WAS/XLT platelets is primarily due to the small size of the platelets rather than to intrinsic platelet abnormalities.
Several previous studies have examined platelet function in WAS/XLT patients, but the findings have been variable and inconsistent. Some older studies report strongly reduced or absent aggregation in response to multiple agonists in WAS patients.13,22,23 At the time of these studies, there was less understanding of the mechanism of agonist-induced platelet activation and the genetic basis of WAS had not yet been unraveled.3 One study reported that platelet aggregation was normal as were release mechanisms, but a moderate storage pool defect was present in XLT patients.14 Another study with WAS patients showed slightly enhanced platelet aggregation and secretion from dense granules induced by collagen-related peptide and thrombin.15 However, none of these previous platelet function studies took into account the smaller size of WAS/XLT platelets, which could have resulted in false interpretation of the data. Furthermore, the thrombocytopenia that is present in WAS/XLT is well known to interfere with the interpretation of tests of platelet function studies with the exception of flow cytometry.12 The only previous flow cytometric study of platelet function in WAS/XLT which considered the smaller size of platelets in WAS/XLT measured only normal sized platelets by electronic gating in a flow cytometer.16 These normal-sized platelets in WAS showed reduced expression of GPIIb-IIIa and P-selectin after agonist stimulation compared with controls. However, unlike the present study, the mean age of healthy controls in this study16 was 33 years and not age-matched to the WAS patients (mean age, 2.7 years). Furthermore, in this study,16 platelet function was determined in PRP rather than whole blood (as was done in the present study), which may have affected the results by introducing artifactual platelet activation. Variations in study logistics might also explain different findings between studies with WAS/XLT patients. In our study, patient recruitment and platelet activation analysis were performed at two different locations. However, a very short time after blood draw, aliquots were incubated with agonist and antibodies, and samples were fixed before they were sent from the patient recruitment center in New York to the central laboratory in Boston where the analysis was performed. We have previously standardized this approach.20,24-28
Our results show that the reduced platelet activation of WAS/XLT patients compared with age-matched controls can be explained by the smaller size of WAS/XLT platelets, rather than an intrinsic difference in platelet activation (Figure 2). This raises the following question: what is the role of WASP in platelets? A recent study proposed a novel role for WASP downstream of integrin outside-in signaling.18 Agonist-induced GPIIb-IIIa activation measured by PAC1 binding was normal in WAS patients, whereas adherence and spreading on immobilized fibrinogen was decreased, suggesting that WASP does not play a role in the initial platelet response but does play a role with GPIIb-IIIa outside-in responses such as platelet spreading, clot retraction, and platelet plug stability. Also, since WASP might regulate actin filament linkages between the plasma membrane and the cortical cytoskeleton,29 a deficiency of WASP could result in impairments of actin filaments and therefore an increase in PS-positive microparticles.18 This is in agreement with the results observed in our study, although variations between patients were too large to observe statistically significant increases in the percentage of PS-positive microparticles and mean fluorescence (Figure 1F).
The relative contributions to thrombocytopenia of decreased platelet production and increased platelet destruction has not been completely resolved in WAS/XLT patients. The high rate of normalization of the platelet count by splenectomy in these patients implies that accelerated platelet destruction in the spleen plays an important role in the thrombocytopenia of WAS/XLT patients.30 On the other hand, there are several studies demonstrating that platelet production in WAS appears to be impaired.5,31-35 In the present study, compared with thrombocytopenic children with ITP also treated with eltrombopag, platelet production was not only decreased in WAS/XLT patients prior to treatment but the increase in AIPF in response to eltrombopag was also far less (Figure 5). Regardless of the relative roles of accelerated destruction and impaired production, the response to the thrombopoietic agent eltrombopag in 5 out of 8 WAS/XLT patients, and the long-term response in 4 responders (Figure 4B), provides evidence for a novel treatment of WAS/XLT with relatively high efficacy and good tolerability. As is the case in ITP,20 eltrombopag did not improve platelet activation per se in WAS/XLT patients (Figure 3), but increased the platelet count.
Current clinically available therapeutic approaches other than platelet transfusion to increase the platelet count in WAS/XLT include splenectomy and HSCT. However, given the poor response to carbohydrate antigens in WAS/XLT, post-splenectomy pneumococcal sepsis has occurred at a higher rate than in other splenectomized conditions (eg, ITP and hereditary spherocytosis).1 Experimental therapies in WAS are being developed, but in the initial gene therapy trial in Germany by Braun et al and Boztug et al, 7 out of 9 patients who showed correction for WASP expression went on to develop leukemia.36,37 Another recent study using a lentiviral vector for gene transfer in WAS patients showed robust engraftment of gene-corrected cells and no complications after 20 to 32 months.38 Currently, HSCT is considered the optimal therapeutic approach for WAS patients as soon as an appropriate donor is available. In WAS patients, based on our present study, eltrombopag may reduce the risk of hemorrhage by increasing the platelet count and/or platelet turnover until a more definitive therapy is available or while awaiting the effectiveness of other therapy. In XLT, a clinically less severe disorder than WAS, the optimal therapeutic approach is far less clear.39 Our present data suggest that, although additional therapies are needed to address the immunodeficiency, infections, eczema, autoimmunity, and other XLT-related complications, a therapeutic trial of eltrombopag in XLT patients may be beneficial and may avoid a splenectomy (with its increased risk of post-splenectomy sepsis) and also avoid HSCT with its attendant mortality and substantial morbidity (eg, graft-versus-host disease).40 In XLT patients who respond to eltrombopag, it appears that it can be given indefinitely in anticipation of development of a future curative treatment with low morbidity and mortality. Successfully increasing the platelet count and thus reducing bleeding is important, but it is not the only problem facing patients with WAS/XLT. In XLT patients, the risks of autoimmunity and development of malignancy are less than in WAS patients, and thus an effective, safe treatment to reduce bleeding (by increasing the platelet count) has an important role in management of these patients. Nonetheless, use of IVIG and antibiotics as anti-infective treatments are important in not only WAS, but also certain XLT patients and complement the use of eltrombopag.
In addition to monitoring liver function tests, there are two areas of potential concern with eltrombopag: malignancy and marrow fibrosis. Leukemia is less of a risk for WAS/XLT patients than lymphoma.9,41 In myelodysplastic syndrome and aplastic anemia patients, eltrombopag seems to stimulate leukemia albeit at a low rate.42,43 It is unclear whether this is more than a very minor risk in WAS/XLT patients because eltrombopag does not appear to increase the risk of lymphoma.44 BM examinations of pediatric patients on thrombopoietic agents showed a limited degree of fibrosis (1 patient with grade 2), despite some patients having been on treatment for more than 3 years.45 Furthermore, adults with increased grades of fibrosis have demonstrated regression of the fibrosis when eltrombopag was stopped. These results suggest that long-term use of eltrombopag does not substantially increase BM fibrosis and that, if it occurs, it is reversible. However, the effects of long-term use of eltrombopag on the success rate of HSCT, and more specifically the engraftment of stem cells in the BM, have not yet been studied. Two recently completed trials in ITP patients have demonstrated efficacy and safety of eltrombopag in 171 children.46,47 Use of romiplostim or eltrombopag in a retrospective analysis of 33 children with ITP appeared to be safe, effective, and tolerable.45 Eltrombopag has recently been FDA-approved for the treatment of children 6 years and older with chronic ITP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was partly supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medical College (#UL1TR00457); an unrestricted grant from GlaxoSmithKline; Sysmex for providing the XE-2100 instrument; and by the Children’s Cancer and Blood Foundation.
Authorship
Contribution: A.J.G. and E.A.L. analyzed the data and wrote the paper; W.B.M., A.L.F., M.A.B.-L., S.L.C., S.L.B., and M.R.B. participated in study design, sample processing, and data analysis; H.T. and S.R.-V. recruited patients and collected samples; and A.D.M. and J.B.B. designed the study, participated in data analysis, and wrote the paper.
Conflict-of-interest disclosure: A.L.F. and A.D.M. have been principal investigators on research grants to Boston Children’s Hospital from Eisai and Sysmex. J.B.B. receives clinical research support from Amgen, Cangene, GlaxoSmithKline, Genzyme, BiologicTx, Immunomedics, Ligand, Eisai, Shionogi, and Sysmex. J.B.B. has participated in advisory boards for Amgen, GlaxoSmithKline, Ligand, Shionogi, Symphogen, and Eisai. The remaining authors declare no competing financial interests.
Marc R. Barnard died on September 8, 2014.
Correspondence: Alan D. Michelson, Center for Platelet Research Studies, Division of Hematology/Oncology, Boston Children’s Hospital, 300 Longwood Ave, Karp 08213, Boston, MA; e-mail: alan.michelson@childrens.harvard.edu; and James B. Bussel, Division of Hematology/Oncology, Department of Pediatrics and Medicine, New York Presbyterian Hospital, Weill Medical College of Cornell University, 525 East 68th St, Payson 695, New York, NY 10065; e-mail: jbussel@med.cornell.edu.
References
Author notes
A.J.G. and E.A.L. are cofirst authors.
A.D.M. and J.B.B. are cosenior authors.