Key Points
A new subset of human and murine type II NKT-TFH cells against Gaucher lipids that regulate B-cell immunity.
A novel pathway for B-cell help providing a mechanism underlying chronic B-cell activation and gammopathy in metabolic lipid disorders.
Abstract
Chronic inflammation including B-cell activation is commonly observed in both inherited (Gaucher disease [GD]) and acquired disorders of lipid metabolism. However, the cellular mechanisms underlying B-cell activation in these settings remain to be elucidated. Here, we report that β-glucosylceramide 22:0 (βGL1-22) and glucosylsphingosine (LGL1), 2 major sphingolipids accumulated in GD, can be recognized by a distinct subset of CD1d-restricted human and murine type II natural killer T (NKT) cells. Human βGL1-22– and LGL1-reactive CD1d tetramer–positive T cells have a distinct T-cell receptor usage and genomic and cytokine profiles compared with the classical type I NKT cells. In contrast to type I NKT cells, βGL1-22– and LGL1-specific NKT cells constitutively express T-follicular helper (TFH) phenotype. Injection of these lipids leads to an increase in respective lipid-specific type II NKT cells in vivo and downstream induction of germinal center B cells, hypergammaglobulinemia, and production of antilipid antibodies. Human βGL1-22– and LGL1-specific NKT cells can provide efficient cognate help to B cells in vitro. Frequency of LGL1-specific T cells in GD mouse models and patients correlates with disease activity and therapeutic response. Our studies identify a novel type II NKT-mediated pathway for glucosphingolipid-mediated dysregulation of humoral immunity and increased risk of B-cell malignancy observed in metabolic lipid disorders.
Introduction
Natural killer T (NKT) cells are distinct innate lymphocytes that recognize lipid/glycolipid antigens in the context of the major histocompatibility complex (MHC)-like molecule CD1d.1 NKT cells are currently classified into 2 major subsets: type I or invariant NKT (iNKT) cells that express a semi-invariant T-cell receptor (TCR) and recognize the prototypic antigen α-galactosylceramide (α-GalCer), and type II or diverse NKT cells that use diverse TCR α and β chains and do not recognize α-GalCer (reviewed in Godfrey et al2 ). The widely studied type I NKT cells are more prevalent than type II NKT cells in mice as compared with humans, whereas type II NKT cells comprise the dominant subset of human CD1d-restricted T cells.3 Recent studies have begun to implicate a distinct regulatory role for type II NKT cells (or the type I/type II NKT balance) in several settings including autoimmunity, inflammation, obesity, and protection against tumors and pathogens.4-15
Sulfatide was the first antigen recognized as a target for murine type II NKT cells, and sulfatide-reactive T cells are currently the best-studied subset of murine type II NKT cells.4,6 Studies with murine transgenic or sulfatide-reactive NKT cells have suggested that these cells have a diverse but oligoclonal TCR repertoire and distinct genomic profile and mode of TCR binding compared with type I NKT cells.16-19 The spectrum of putative murine type II NKT ligands has now widened, and some of these ligands can be recognized by both type I and type II NKT cells.20-27 Importantly, there are some species-specific differences in ligand recognition between human and murine NKT cells.23,28 Understanding the diversity and functional properties of human type II NKT cells against defined lipids is therefore of great interest in view of their potential immunoregulatory role in several disease states.4,5
Dysregulation of glucosphingolipids (GSLs) has been demonstrated in several metabolic disorders, including Gaucher disease (GD) and obesity.29,30 GD is an inborn error of metabolism due to deficiency of the lysosomal enzyme glucocerebrosidase (acid-β-glucosidase [GBA]).30,31 GBA deficiency leads to progressive lysosomal storage of β-glucosylceramide (β-GlcCer; GL1) and its deacylated product, glucosylsphingosine (Lyso-GL1; LGL1), most conspicuously in the mononuclear phagocytes.32,33 Elevated levels of these lipids can also be detected in circulation, leading to modest elevation in GL1 and a marked increase in LGL1 levels.34 Analysis of fatty acid acyl compositions of spleen from GD patients reveals that β-glucosylceramide 22:0 (βGL1-22) and GL1-24:0 are the most abundant β-GlcCer species.35,36 The accumulation of lipids in GD patients is associated with a chronic progressive inflammatory state with an increase in inflammatory cytokines, activation of macrophages, and high incidence of B-cell activation, manifest as polyclonal and monoclonal gammopathy.32,37-40 Interestingly, chronic inflammation has been observed in glucocerebrosidase-deficient mice with minimal substrate accumulation lacking classically engorged macrophages,37 suggesting involvement of immune cells other than just macrophages in stimulating inflammation and B-cell activation. Here, we have analyzed the host response to GD lipids to gain insights into mechanisms underlying lipid-associated inflammation.
Materials and methods
Mouse and human subjects
Six- to 9-week-old mice on a C57BL/6 background were used. CD1d−/− mice41 and Jα 18−/− on a C57BL/6 background were kindly provided by Dr Peter Cresswell (Yale University, New Haven, CT). The generation of conditional GBA knockout mice has been previously described.42 All mice were bred and maintained in compliance with Yale University’s institutional animal care guidelines. Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated from buffy coats purchased from New York Blood Center or from patients with GD, following informed consents approved by the institutional review board in accordance with the Declaration of Helsinki.
Isolation of human and mice mononuclear cells (MNCs)
CD14+ monocytes were separated from PBMCs with CD14 magnetic beads (Miltenyi Biotec) using the manufacturer’s protocol. MNCs from thymus, spleen, and liver were isolated following a protocol described earlier.43
Antibodies and flow cytometry
Data were acquired with the LSRII system (BD Biosciences) and FACSCalibur (BD Biosciences) and analyzed with FlowJo (Tree Star). Doublets were excluded with FSC1-FSC-H linearity. Mouse antibodies (clones) were as follows: anti-CD3e(145-2C11), anti-CD4(RM4-5), anti-CD8(53-6.7), anti-CD19(1D3), anti-B220(RA3-6B2), anti-F4/80(45-4801), anti-IgM(eB121-15F9), anti-IgD(11-26c), anti-CD279/PD1(J43), anti-CD185/CXCR5(SPRCL5), anti-MHC II(M5/114.15.2) (from eBioscience), anti-BCl-6(K112-91), anti-foxp3(MF23), anti-ICOS(15F9), and anti-IL-21(FFA21). The human antibodies anti-CD3e (SK7), anti-CD4(RPA-T4), anti-CD8(SK1), anti-CD45 RA(H100), anti-CD45RO(UCHL1), anti-CD56(MY31), anti-IFN-γ(B27), isotype control (MOPC-21), anti-CD19(SJ25C1), anti-CD27 (M-T271), and anti-IL-21(3A3-N2.1) were from BD Pharmingen; anti-TCR Vα24(C15) and anti-TCR Vβ11 (C21) were from Beckman Coulter; and anti-CD62L(FN50), anti-PLZF(Mags.21F7), anti- CD279/PD1(J105), anti-CD185/CXCR5(MU5UBEE), anti-CD38(HB7), anti-FAS(15A7), and anti-GL7(GL7) were from eBioscience.
Lipids and CD1d tetramer loading
NKT ligand α-galactosyl-ceramide (α-GalCer) was kindly provided by Kirin Breweries (Tokyo, Japan). β-D-GlcCer d18:1-C24:1(15Z),C12:0 was from Avanti Polar Lipids. β-D-GlcCer d18:1-C22:0 (thin-layer chromatography purity >98%) and lysoglucocerebroside (thin-layer chromatography purity >98%) were from Matreya. Mouse and human empty CD1d tetramer were obtained from the National Institutes of Health (NIH) tetramer facility (Atlanta, GA). A molar loading ratio (lipid to CD1d) of 200:1 was used for tetramerization studies for both mouse and human tetramers as used in other studies.21,25 The unloaded multimer control was prepared by mixing CD1d tetramer with the carrier only.
Stimulation of NKT cells in culture
For stimulation with anti-CD3/CD28 beads (Miltenyi Biotec), sorted tetramer-positive cells were stimulated with anti-CD3/CD28 beads in a 96-well U-bottom plate. For some experiments, sorted βGL1-22/LGL1/α-GalCer tetramer-positive cells were cultured with CD1d-expressing cell line (C1rd cells; kindly provided by Dr Mark Exley, Boston) or mock-transfected control cells at a T-cell: antigen-presenting cell (APC) ratio of 1: 0, as described previously,22,44 with or without lipid pulsing.
TCR sequencing
Injection of lipids
C57BL/6, CD1d−/−, or Jα18−/− mice were injected intraperitoneally with phosphate-buffered saline, α-GalCer (4 μg/mouse), or βGL1-22 or LGL1 (200 μg/mouse) each week for 6 weeks in a volume of 100 μL. The mice were sacrificed 7 days after the last injection.
Microarray analysis
RNA from CD1d tetramer-sorted β-GL122/LGL1–specific T cells or iNKT cells was amplified, labeled, and hybridized on the Affymetrix Human Genome U133 Plus 2.0 microarray chips. The data were analyzed with GeneSpring GX 12.5 (Agilent Technologies) and Partek Genomice Suite (6.6) software, as described previously.47,48
Statistics
The Student t test was used to compare data between 2 groups. Significance was set at P < .05. Correlation coefficient between 2 variables was calculated with the Pearson correlation test.
Results
Detection of human CD1d-restricted T cells specific for βGL1-22 and LGL-1
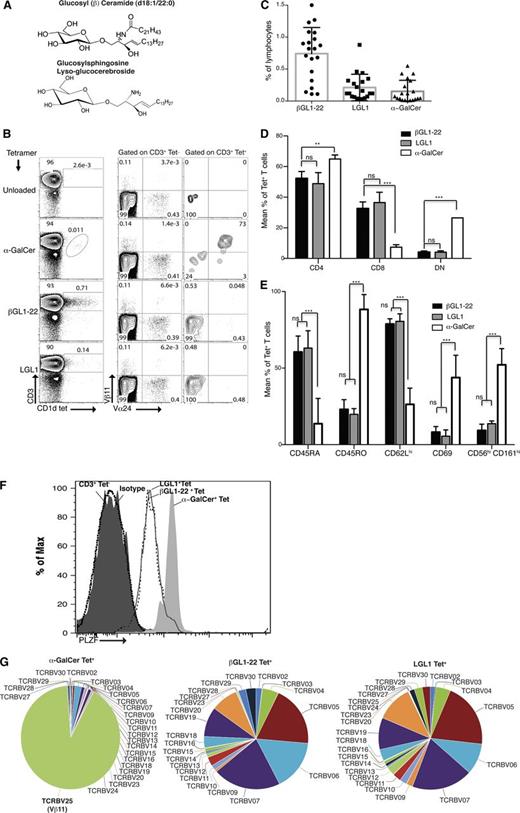
In order to detect human CD1d-restricted T cells specific for the GSLs βGL1-22 and its deacylated product, LGL1 (Figure 1A), βGL1-22– and LGL1-loaded human CD1d tetramers were used to stain freshly isolated human PBMCs from healthy blood donors. βGL1-22/LGL1–loaded CD1d tetramer-positive cells consisted predominantly of Vα24−Vβ11−CD3ε+ T cells (Figure 1B), whereas α-GalCer–loaded tetramer-positive cells were primarily Vα24+Vβ11+ T cells. These reagents specifically stained only CD3ε+ T cells, and there was no staining of CD3ε− cells (data not shown). Frequency of βGL1-22–specific T cells was higher than LGL1-specific T cells in PBMCs from healthy donors (Figure 1C). βGL1-22/LGL1 tetramer-positive T cells were predominantly CD4+ or CD8+ T cells, as opposed to α-GalCer tetramer-positive T cells, which consisted mainly of CD4+ and double-negative (DN) cells (Figure 1D). βGL1-22/LGL1 tetramer-positive cells displayed naive phenotype (CD45RAhi, CD62Lhi, CD69neg/lo) and had low expression of natural killer (NK)-cell markers like CD56 and CD161. In contrast, α-GalCer tetramer-positive T cells revealed an effector/memory phenotype (CD45ROhi, CD62Llo, CD69hi) and displayed classical NK-cell markers like CD56 and CD161 (Figure 1E). Both βGL1-22 and LGL1 tetramer-positive T cells were found to express NKT-associated transcription factor PLZF49 similar to type I NKT cells, but at lower levels (Figure 1F). To gain additional insight into the overall spectrum of TCR use by βGL1-22/LGL1 tetramer-positive T cells, we analyzed Vβ chain usage of sorted βGL1-22/LGL1–specific T cells by TCR sequencing, which revealed diverse Vβ receptor usage among βGL1-22/LGL1–reactive T cells. Together, these data demonstrate that βGL1-22– and LGL1-specific CD1d-restricted human T cells can be detected in human PBMCs, use diverse TCRs, express PLZF, and are phenotypically distinct from α-GalCer–reactive type I NKT cells.
Detection and characterization of surface phenotype and TCR repertoire of βGL1-22– and LGL1-reactive T cells. (A) The chemical structure of glucosylceramide (top panel) and glucosylsphingosine (bottom panel) used in this study. (B) Panel showing staining of human PBMCs with CD1d tetramers loaded with α-GalCer, βGL1-22, or LGL1. Invariant TCR usage was monitored using anti-Vα24 and anti-Vβ11 antibodies. Numbers in the contour plots show percentages of cells in CD3+ tetrameter-negative (Tet−) and CD3+ tetrameter-positive (Tet+) gates, respectively. (C) Plot summarizing percentages of lymphocytes staining in freshly isolated PBMCs from healthy donors for CD1d tetramer loaded with βGL1-22, LGL1, or α-GalCer. Data are presented as mean ± standard error of the mean (SEM), with each dot indicating 1 donor (n = 20). (D) Compiled results from analyses of 5 different human PBMCs showing the mean percentages of CD4+, CD8+, and CD4−CD8− (DN) expressing βGL1-22, LGL1, or α-GalCer tetramer-positive T cells. Error is presented as SEM among donors (***P < .0001; **P < .001). (E) Compiled results from analyses of 5 different human PBMCs showing mean percentages of CD45RA, CD45RO, CD62L, CD69, CD56, and CD161 expression by human βGL1-22, LGL1, or α-GalCer CD1d tetramer-positive T cells. Error is presented as SEM among donors (***P < .0001). (F) Intracellular flow cytometry with anti-PLZF monoclonal antibody (mAb) of α-GalCer tetramer-positive (gray shaded), βGL1-22 tetramer-positive (dashed line), or LGL1 tetramer-positive (solid line) and conventional T cells CD3+Tet− (black shaded) from freshly isolated human PBMCs as indicated. The isotype control is the dotted line histogram. Data are representative of 4 experiments. (G) TCR sequencing analysis of in vitro expanded and sorted CD1d-α-GalCer, βGL1-22, or LGL1 tetramer-positive T cells. Pie charts representing the TCRVβ repertoire usage by α-GalCer–, βGL122–, and LGL1-specific T cells. Data are representative of 3 separate experiments.
Detection and characterization of surface phenotype and TCR repertoire of βGL1-22– and LGL1-reactive T cells. (A) The chemical structure of glucosylceramide (top panel) and glucosylsphingosine (bottom panel) used in this study. (B) Panel showing staining of human PBMCs with CD1d tetramers loaded with α-GalCer, βGL1-22, or LGL1. Invariant TCR usage was monitored using anti-Vα24 and anti-Vβ11 antibodies. Numbers in the contour plots show percentages of cells in CD3+ tetrameter-negative (Tet−) and CD3+ tetrameter-positive (Tet+) gates, respectively. (C) Plot summarizing percentages of lymphocytes staining in freshly isolated PBMCs from healthy donors for CD1d tetramer loaded with βGL1-22, LGL1, or α-GalCer. Data are presented as mean ± standard error of the mean (SEM), with each dot indicating 1 donor (n = 20). (D) Compiled results from analyses of 5 different human PBMCs showing the mean percentages of CD4+, CD8+, and CD4−CD8− (DN) expressing βGL1-22, LGL1, or α-GalCer tetramer-positive T cells. Error is presented as SEM among donors (***P < .0001; **P < .001). (E) Compiled results from analyses of 5 different human PBMCs showing mean percentages of CD45RA, CD45RO, CD62L, CD69, CD56, and CD161 expression by human βGL1-22, LGL1, or α-GalCer CD1d tetramer-positive T cells. Error is presented as SEM among donors (***P < .0001). (F) Intracellular flow cytometry with anti-PLZF monoclonal antibody (mAb) of α-GalCer tetramer-positive (gray shaded), βGL1-22 tetramer-positive (dashed line), or LGL1 tetramer-positive (solid line) and conventional T cells CD3+Tet− (black shaded) from freshly isolated human PBMCs as indicated. The isotype control is the dotted line histogram. Data are representative of 4 experiments. (G) TCR sequencing analysis of in vitro expanded and sorted CD1d-α-GalCer, βGL1-22, or LGL1 tetramer-positive T cells. Pie charts representing the TCRVβ repertoire usage by α-GalCer–, βGL122–, and LGL1-specific T cells. Data are representative of 3 separate experiments.
Antigen specificity of βGL1-22– and LGL1-specific type II NKT cells
In order to further validate the ligand-specificity of tetramer-positive cells, we analyzed the capacity of tetramer-positive cells to respond to specific lipids in culture. Freshly isolated human PBMCs were cultured with α-GalCer, βGL1-22, or LGL1, and interferon-γ (IFN-γ) production in response to the glycolipids was measured by intracellular staining. We found that βGL1-22/LGL1 was able to stimulate IFN-γ production, and this effect was completely blocked by mAb specific to CD1d (Figure 2A-B). Compared with type I NKT cells, βGL1-22/LGL1 type II NKT cells secreted lower levels of IFN-γ (see MFI in Figure 2B), suggesting a lower stimulatory capacity of these ligands. Coculture of purified tetramer-positive cells with CD1d-expressing C1R cells confirmed that βGL1-22/LGL1 tetramer-positive T-cell responses to βGL1-22/LGL1 pulsed APCs were CD1d dependent, as the CD1d-negative parental cell line pulsed with either βGL1-22 or LGL1 did not stimulate cytokine secretion (Figure 2C). In order to further confirm antigen specificity, βGL1-22/LGL1–specific CD1d-restricted T cells were expanded in culture using respective lipid-loaded human monocyte–derived dendritic cells (DCs). βGL1-22/LGL1–loaded DCs led to expansion of lipid-specific T cells without concurrent expansion of invariant TCR (iTCR)-positive (Vα24+Vβ11+) cells (including in tetramer-negative fraction), unlike α-GalCer–loaded DCs, which led to clear expansion of Vα24+Vβ11+ T cells (Figure 2D). The diverse TCR-V-β repertoire of expanded T cells was also confirmed with the use of a flow cytometry–based V-β repertoire assay (supplemental Figure 1, available on the Blood Web site). Together, these data demonstrate that βGL1-22/LGL1–loaded CD1d tetramers detect T cells that respond functionally to the specific lipid in a CD1d-dependent manner.
Antigen specificity, gene expression, and cytokine profile of βGL1-22– and LGL1-specific type II NKT cells. (A) IFN-γ production by intracellular staining of freshly isolated PBMCs cultured with α-GalCer (1 μg/mL) or βGL1-22 and LGL1 (20 µg/mL) for 12 hours is shown. Live and dead staining was performed using the live/dead fixable dead cell stain kit. IFN-γ expression is represented in terms of mean fluorescence intensity (MFI) following stimulation in tetramer-positive cells vs tetramer-negative cells as a control. (B) Bar graphs depict the IFN-γ production by CD3+ α-GalCer, βGL1-22, or LGL1 tetramer-positive T cells in human PBMCs stimulated with α-GalCer (1 μg/mL) or βGL1-22 and LGL1 (20 µg/mL) in the presence of either CD1d blocking (CD1d42) or isotype control mAb. Data are representative of 3 experiments. (C) Bar graphs showing IFN-γ production by sorted βGL1-22, LGL1, or α-GalCer tetramer-positive T-cell cultures in response to CD1d-transfected (C1rD-CD1d) or untransfected (C1rD-Mock) APCs with indicated antigens. Vehicle-pulsed CD1d-transfected APCs served as control. IFN-γ secretion from the culture supernatants collected after 48 hours was monitored by Luminex. Data are representative of 3 experiments (* indicates saturating IFN-γ levels). (D) Representative fluorescence-activated cell sorter (FACS) analysis showing expansion of βGL1-22, LGL1, or α-GalCer tetramer-positive T-cell cultures in response to monocyte-derived DCs pulsed with either vehicle or βGL1-22, LGL1, and α-GalCer. Expansion of iTCR was monitored using anti-Vα24 and anti-Vβ11 antibodies. Numbers in the contour plots show percentages of cells in CD3+ tetramer-positive and CD3+ tetramer-negative gates, respectively. (E) Principal component (PC) analysis of global gene expression profiles of sorted βGL1-22/LGL1–specific type II NKT and α-GalCer–specific type I NKT cells, either before or after anti-CD3/28 stimulation. Each data point/shape represents a microarray, with all 6 arrays presented on PC analysis plot either before or after stimulation. The α-GalCer array shows some separation from βGL1-22/LGL1 arrays in both stimulated and unstimulated conditions. Given PC1 describes the largest amount of data variance (40.0%), the aggregation of the arrays is mostly accounted for by the sample location. The percentage values in parentheses indicate the proportion of the total variance described in each PC: PC1 (x-axis), PC2 (y-axis), and PC3 (z-axis). (F) Differentially expressed genes in sorted βGL1-22/LGL1–specific type II NKT compared with α-GalCer–specific type I NKT. Bar graphs showing fold change in expression levels of selected genes related to transcription, differentiation, and cytokine and chemokine signaling compared with type I NKT cells. (G-H) Cytokine expression by flow-sorted CD1d-βGL1-22+, CD1d-LGL1+, or α-GalCer+ T-cell populations in response to stimulation (G) stimulation with anti-CD3/CD28 beads and (H) stimulation with CD1d or mock-transfected lipid-loaded APCs. Culture supernatants collected after 48 hours were analyzed for 39 different analytes by Luminex. Analytes that showed significant levels when compared with unstimulated control are shown. Data are shown as mean (±SEM) of 3 similar experiments. DMSO, dimethylsulfoxide; MDC; macrophage-derived chemokine; MIP-1β, macrophage inflammatory protein 1β.
Antigen specificity, gene expression, and cytokine profile of βGL1-22– and LGL1-specific type II NKT cells. (A) IFN-γ production by intracellular staining of freshly isolated PBMCs cultured with α-GalCer (1 μg/mL) or βGL1-22 and LGL1 (20 µg/mL) for 12 hours is shown. Live and dead staining was performed using the live/dead fixable dead cell stain kit. IFN-γ expression is represented in terms of mean fluorescence intensity (MFI) following stimulation in tetramer-positive cells vs tetramer-negative cells as a control. (B) Bar graphs depict the IFN-γ production by CD3+ α-GalCer, βGL1-22, or LGL1 tetramer-positive T cells in human PBMCs stimulated with α-GalCer (1 μg/mL) or βGL1-22 and LGL1 (20 µg/mL) in the presence of either CD1d blocking (CD1d42) or isotype control mAb. Data are representative of 3 experiments. (C) Bar graphs showing IFN-γ production by sorted βGL1-22, LGL1, or α-GalCer tetramer-positive T-cell cultures in response to CD1d-transfected (C1rD-CD1d) or untransfected (C1rD-Mock) APCs with indicated antigens. Vehicle-pulsed CD1d-transfected APCs served as control. IFN-γ secretion from the culture supernatants collected after 48 hours was monitored by Luminex. Data are representative of 3 experiments (* indicates saturating IFN-γ levels). (D) Representative fluorescence-activated cell sorter (FACS) analysis showing expansion of βGL1-22, LGL1, or α-GalCer tetramer-positive T-cell cultures in response to monocyte-derived DCs pulsed with either vehicle or βGL1-22, LGL1, and α-GalCer. Expansion of iTCR was monitored using anti-Vα24 and anti-Vβ11 antibodies. Numbers in the contour plots show percentages of cells in CD3+ tetramer-positive and CD3+ tetramer-negative gates, respectively. (E) Principal component (PC) analysis of global gene expression profiles of sorted βGL1-22/LGL1–specific type II NKT and α-GalCer–specific type I NKT cells, either before or after anti-CD3/28 stimulation. Each data point/shape represents a microarray, with all 6 arrays presented on PC analysis plot either before or after stimulation. The α-GalCer array shows some separation from βGL1-22/LGL1 arrays in both stimulated and unstimulated conditions. Given PC1 describes the largest amount of data variance (40.0%), the aggregation of the arrays is mostly accounted for by the sample location. The percentage values in parentheses indicate the proportion of the total variance described in each PC: PC1 (x-axis), PC2 (y-axis), and PC3 (z-axis). (F) Differentially expressed genes in sorted βGL1-22/LGL1–specific type II NKT compared with α-GalCer–specific type I NKT. Bar graphs showing fold change in expression levels of selected genes related to transcription, differentiation, and cytokine and chemokine signaling compared with type I NKT cells. (G-H) Cytokine expression by flow-sorted CD1d-βGL1-22+, CD1d-LGL1+, or α-GalCer+ T-cell populations in response to stimulation (G) stimulation with anti-CD3/CD28 beads and (H) stimulation with CD1d or mock-transfected lipid-loaded APCs. Culture supernatants collected after 48 hours were analyzed for 39 different analytes by Luminex. Analytes that showed significant levels when compared with unstimulated control are shown. Data are shown as mean (±SEM) of 3 similar experiments. DMSO, dimethylsulfoxide; MDC; macrophage-derived chemokine; MIP-1β, macrophage inflammatory protein 1β.
βGL1-22/LGL1–specific type II human NKT cells have different genomic and cytokine profile compared with type I NKT cells
The ability to detect and isolate βGL1-22/LGL1–specific human type II NKT cells allowed us to compare the global gene expression profiles of these cells with type I NKT cells using microarray analysis. Principal component analysis revealed that the gene expression profile signature for βGL1-22 and LGL1-specific T cells both before and after activation with anti-CD3/CD28 beads is distinct from that of type I NKT cells (Figure 2E and supplemental Table 1). Selected genes that are differentially expressed by βGL1-22/LGL1–specific type II NKT (relative to type I NKT) are shown in Figure 2F. Metacore analysis revealed that the top differentially expressed pathways involved DAP12 and Th17 pathways (supplemental Table 2). To further confirm the differences observed with respect to cytokines, we compared the cytokine secretion of sorted βGL1-22/LGL1 tetramer-positive cells with type I NKT cells in response to anti-CD3 stimulation. Compared with type I NKT cells, βGL1-22– and LGL1-specific T cells secreted higher levels of interleukin-5 (IL-5), IL-6, IL-10, IL-13, IL-17, granulocyte macrophage colony-stimulating factor, macrophage-derived chemokine, and macrophage inflammatory protein 1β (Figure 2G). This response was CD1d dependent, as it was not observed in cocultures with CD1d-deficient APCs (Figure 2H), corroborating the antigen specificity of βGL1-22/LGL1–specific type II NKT cells. Together, these data demonstrate that the human type II NKT cells identified here have a distinct genomic and cytokine profile compared with type I NKT cells, alluding to their potential for distinct roles in regulating human immune response.
Detection and phenotypic characterization of murine βGL1-22– and LGL1-specific CD1d-restricted T cells
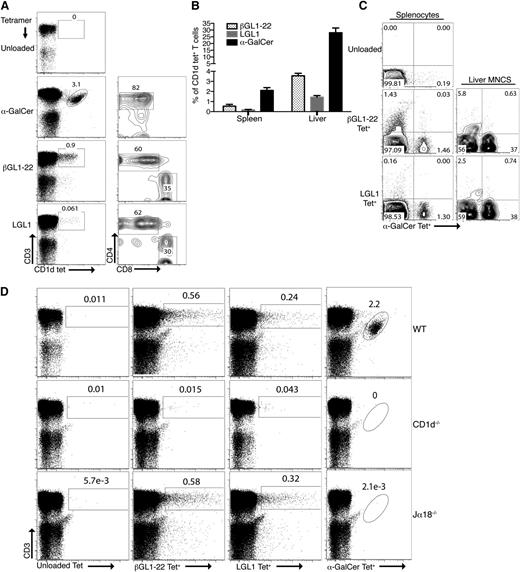
Murine βGL1-22 and LGL1 tetramer-positive T cells are composed of both CD4+ (∼60% and 62%) and CD8+ T cells (∼35.1% and 29.6%), respectively, in contrast to α-GalCer tetramer-positive type I NKT cells, which are predominantly composed of CD4+ T cells (∼82%) (Figure 3A). βGL1-22 tetramer-positive and LGL1 tetramer-positive cells constitute ∼0.9% and 0.06% of lymphocytes in spleen and ∼4% and 1.5% of lymphocytes in liver, respectively, whereas α-GalCer tetramer-positive type I NKTs represent ∼2% of lymphocytes in spleen and ∼30% of lymphocytes in liver (Figure 3B). Frequency of βGL1-22–specific T cells was higher than LGL1-specific T cells in both splenocytes and liver MNCs (Figure 3B). To determine whether murine βGL1-22/LGL1–reactive T cells are a type II NKT population similar to human PBMCs, splenic as well as liver MNCs were simultaneously stained with α-GalCer and βGL1-22/LGL1–loaded CD1d tetramers conjugated to different fluorochromes. Both βGL1-22– and LGL1-specific T cells had a nonoverlapping profile with type I NKT cells (Figure 3C). This observation is further validated by the detection of βGL1-22/LGL1 tetramer-positive, but not α-GalCer tetramer-positive, cells in Jα18−/− mice, which completely lack type I NKT cells. Both βGL1-22– and LGL1-specific T cells were deficient in splenocytes from CD1d−/− mice, consistent with their CD1d-dependent nature (Figure 3D).
Detection and phenotypical characterization of βGL1-22– and LGL1-specific type II NKT cells in mice. (A) α-GalCer, βGL1-22, or LGL1 tetrameter-positive cells in the spleen of C57BL/6 mice were analyzed for the expression CD4 and CD8 expression. (B) Frequency of CD1d-βGL1-22, LGL1, or α-GalCer tetrameter-positive T cells in spleen and liver of C57BL/6 mice (n = 5). (C) Representative FACS analysis of CD3ε+ T cells costained with α-GalCer-CD1d tetramer and unloaded tetramer or βGL1-22/LGL1 tetramer from C57BL/6 mice splenocytes and liver MNCs. Results are representative of 3 experiments. (D) Flow cytometric analysis representing staining of splenocytes from WT (C57BL/6), CD1d−/−, or Jα18−/− mice with CD1d tetramers loaded with βGL1-22 or LGL1 or α-GalCer. Representative data from at least 3 experiments are shown.
Detection and phenotypical characterization of βGL1-22– and LGL1-specific type II NKT cells in mice. (A) α-GalCer, βGL1-22, or LGL1 tetrameter-positive cells in the spleen of C57BL/6 mice were analyzed for the expression CD4 and CD8 expression. (B) Frequency of CD1d-βGL1-22, LGL1, or α-GalCer tetrameter-positive T cells in spleen and liver of C57BL/6 mice (n = 5). (C) Representative FACS analysis of CD3ε+ T cells costained with α-GalCer-CD1d tetramer and unloaded tetramer or βGL1-22/LGL1 tetramer from C57BL/6 mice splenocytes and liver MNCs. Results are representative of 3 experiments. (D) Flow cytometric analysis representing staining of splenocytes from WT (C57BL/6), CD1d−/−, or Jα18−/− mice with CD1d tetramers loaded with βGL1-22 or LGL1 or α-GalCer. Representative data from at least 3 experiments are shown.
βGL1-22/LGL1 type II NKT cells show constitutive expression of TFH phenotype
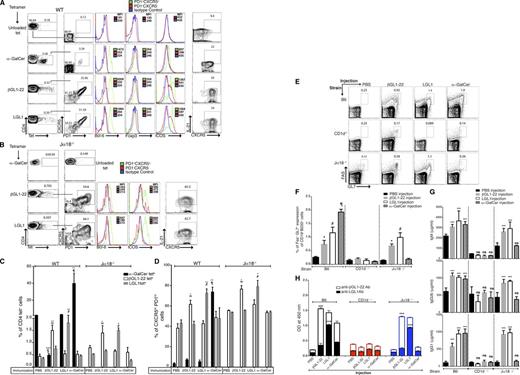
Transcriptional profiling data of βGL1-22– and LGL1-specific T cells revealed expression of genes associated with TFH phenotype. Therefore, we examined if βGL1-22– or LGL1-specific type II NKT cells acquire the typical CXCR5hi PD-1hi ICOShi Bcl-6hi TFH phenotype upon immunization with βGL1-22 or LGL1. Phenotypic analysis revealed that βGL1-22– and LGL1-specific T cells showed constitutive expression of TFH phenotype (defined as CXCR5hi PD-1hi ICOS hi Bcl-6hi foxp3neg IL-21+) in wild-type (WT) (Figure 4A) as well as Jα18−/− mice (Figure 4B). Interestingly, α-GalCer CD1d tetramer-positive T cells showed TFH phenotype in only 3% of iNKT cells at steady-state level (Figure 4A). Injection of βGL1-22 or LGL1 increased the frequency of antigen-specific T cells in vivo in the spleen of WT as well as Jα18−/− mice (Figure 4C and supplemental Figures 2 and 3) exhibiting a sustained upregulation of TFH markers CXCR5hi PD-1hi (Figure 4D), ICOS (supplemental Figure 4), and IL-21 (supplemental Figure 5). Contrary to the findings at steady-state level, TFH phenotype was also induced in type I NKT cells following activation by α-GalCer in a Jα18-dependent manner, as expected (Figure 4D). Injection of the lipids in CD1d−/− mice did not result in the induction of either lipid-specific T cells or TFH markers (data not shown). Injection of lipids did not lead to changes in CD8+ T cells (data not shown). Notably, injection of βGL1-22 in WT mice led to reduction in α-GalCer CD1d tetramer-positive cells (Figure 4C) concurrent with activation of ICOS and IL-21 expression (supplemental Figures 4 and 5), underscoring a possible crosstalk between βGL1-22–specific type II NKT cells and type I NKT cells.5,7,50 Together, these data show that murine βGL1-22– and LGL1-specific type II murine NKT cells respond to ligand-specific stimulation in vivo and constitutively express TFH phenotype, in contrast to type I NKT cells, wherein TFH phenotype requires stimulation by α-GalCer.
βGL1-22/LGL1–specific type II murine NKT cells display NKTFH phenotype and provide B-cell help with the induction of GC B cells and IgG and lipid-reactive antibodies in C57BL/6 and Jα18−/− mice. (A-B) Spleen cells from WT (A, C57BL/6) mice or (B, Jα18−/− mice) obtained 7 days after immunization with α-GalCer, βGL1-22, LGL1, or vehicle alone were stained to assess CXCR5, PD-1, ICOS, Bcl-6, and IL-21 expression among CD4+ tetrameter-positive NKT cells. Numbers in plots indicate percentage of the cells included in the gates. The histogram on the right shows differences in Bcl-6, Foxp3, and ICOS expression as reflected by MFI between follicular helper (PD-1+CXCR5+) T cells and nonfollicular helper (PD-1−CXCR5−) cells (top) as control. Panel on extreme right shows staining for IL-21. Data for MFI on isotype controls are on pooled cells. (C-D) Percentages of CD4+ CD1d tetrameter-positive cells (C) and CXCR5hi PD-1hi NKT-TFH cells (D) in spleens of WT and Jα18−/− mice after immunizations with vehicle or α-GalCer, βGL1-22, or LGL1. The results represent 1 of 3 comparable experiments, each performed with at least 3 mice per group is shown. § represents comparison of α-GalCer tetrameter-positive cells between WT mice injected with vehicle and βGL1-22 (P < .0001); ∞ represents comparison of βGL1-22 tetrameter-positive cells between vehicle and βGL1-22 immunized mice (WT and Jα18−/−); # represents comparison of LGL1 tetrameter-positive cells between vehicle and LGL1-immunized mice (WT and Jα18−/−); ¶ represents comparison of α-GalCer tetrameter-positive cells between WT mice injected with vehicle and α-GalCer. ***P < .0001; **P < .001; *P < .01. (E) Representative contour plots showing the percentage of FAS+GL-7+ GC B cells among total B cells (CD19+B220+) in WT, CD1d−/−, and Jα18−/− mice splenocytes 7 days after α-GalCer, βGL1-22, or LGL1 immunization. (F) Complied results showing the frequency of GC B cells in WT, CD1d−/−, and Jα18−/− mice splenocytes 7 days after α-GalCer, βGL1-22, or LGL1 immunization. Data are presented as mean ≥ SEM (n = 6). (G) Serum from WT, CD1d−/−, and Jα18−/− mice immunized with α-GalCer, βGL1-22, or LGL1 was collected, and total immunoglobulin of the indicated isotypes and IgM were measured by ELISA. Data are presented as mean ± SEM (n = 4). (H) Stacked bar graph showing the presence of anti- βGL1-22 or LGL1 antibodies in the sera of WT, CD1d−/−, and Jα18−/− mice immunized with α-GalCer, βGL1-22, or LGL1. ***P < .0001; **P < .001; *P < .01. ns, nonsignificant; PBS, phosphate-buffered saline.
βGL1-22/LGL1–specific type II murine NKT cells display NKTFH phenotype and provide B-cell help with the induction of GC B cells and IgG and lipid-reactive antibodies in C57BL/6 and Jα18−/− mice. (A-B) Spleen cells from WT (A, C57BL/6) mice or (B, Jα18−/− mice) obtained 7 days after immunization with α-GalCer, βGL1-22, LGL1, or vehicle alone were stained to assess CXCR5, PD-1, ICOS, Bcl-6, and IL-21 expression among CD4+ tetrameter-positive NKT cells. Numbers in plots indicate percentage of the cells included in the gates. The histogram on the right shows differences in Bcl-6, Foxp3, and ICOS expression as reflected by MFI between follicular helper (PD-1+CXCR5+) T cells and nonfollicular helper (PD-1−CXCR5−) cells (top) as control. Panel on extreme right shows staining for IL-21. Data for MFI on isotype controls are on pooled cells. (C-D) Percentages of CD4+ CD1d tetrameter-positive cells (C) and CXCR5hi PD-1hi NKT-TFH cells (D) in spleens of WT and Jα18−/− mice after immunizations with vehicle or α-GalCer, βGL1-22, or LGL1. The results represent 1 of 3 comparable experiments, each performed with at least 3 mice per group is shown. § represents comparison of α-GalCer tetrameter-positive cells between WT mice injected with vehicle and βGL1-22 (P < .0001); ∞ represents comparison of βGL1-22 tetrameter-positive cells between vehicle and βGL1-22 immunized mice (WT and Jα18−/−); # represents comparison of LGL1 tetrameter-positive cells between vehicle and LGL1-immunized mice (WT and Jα18−/−); ¶ represents comparison of α-GalCer tetrameter-positive cells between WT mice injected with vehicle and α-GalCer. ***P < .0001; **P < .001; *P < .01. (E) Representative contour plots showing the percentage of FAS+GL-7+ GC B cells among total B cells (CD19+B220+) in WT, CD1d−/−, and Jα18−/− mice splenocytes 7 days after α-GalCer, βGL1-22, or LGL1 immunization. (F) Complied results showing the frequency of GC B cells in WT, CD1d−/−, and Jα18−/− mice splenocytes 7 days after α-GalCer, βGL1-22, or LGL1 immunization. Data are presented as mean ≥ SEM (n = 6). (G) Serum from WT, CD1d−/−, and Jα18−/− mice immunized with α-GalCer, βGL1-22, or LGL1 was collected, and total immunoglobulin of the indicated isotypes and IgM were measured by ELISA. Data are presented as mean ± SEM (n = 4). (H) Stacked bar graph showing the presence of anti- βGL1-22 or LGL1 antibodies in the sera of WT, CD1d−/−, and Jα18−/− mice immunized with α-GalCer, βGL1-22, or LGL1. ***P < .0001; **P < .001; *P < .01. ns, nonsignificant; PBS, phosphate-buffered saline.
Murine βGL1-22/LGL1 type II NKT cells can provide help to B cells in vivo
Injection with βGL1-22 or LGL1 in mice led to the induction of FAS+GL-7+ splenic germinal center (GC) B cells in WT and Jα18−/− mice, but not in CD1d−/− mice (Figure 4E and 4F). Increase in TFH and GC B cells was also associated with an increase in total immunoglobulin G1 (IgG1), IgG2b, and IgM in WT and Jα18−/− mice injected with respective lipids, but not in CD1d−/− mice, confirming CD1d-restricted help provided by βGL1-22– and LGL1-specific type II NKT cells to B cells (Figure 4G). In order to evaluate the effect of these lipids on the induction of lipid-specific antibodies, we analyzed the sera from these mice for lipid-specific antibodies by enzyme-linked immunosorbent assay (ELISA). Injection of βGL1-22 and LGL1 led to the induction of corresponding lipid-specific antibodies in WT and Jα18−/− mice, but not in CD1d-deficient mice (Figure 4H). Injection of α-GalCer also led to some increase in βGL1-22-specific antibodies, albeit less than following the injection of specific ligands. Together, these data indicate that lipid-mediated activation of βGL1-22/LGL1–specific type II NKT cells is associated with the induction of GC B cells and lipid-specific antibodies in vivo in a CD1d-dependent manner.
Human type II NKT-mediated help to B cells
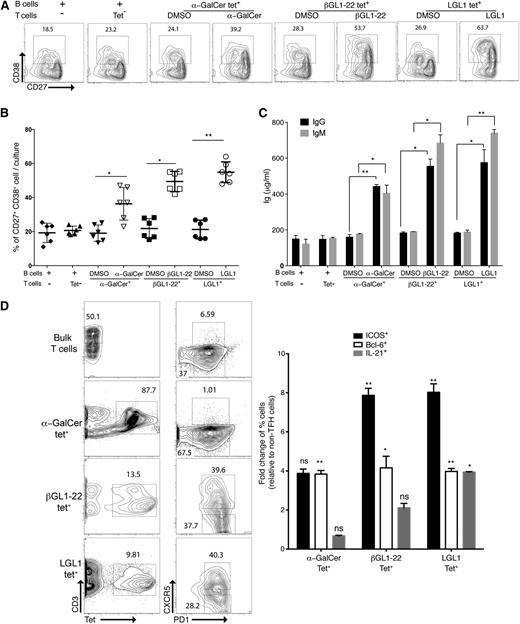
Coculture of human βGL1-22 and LGL1-specific type II NKT cells and autologous B cells induced expansion of CD27hiCD38hi plasmablasts in the presence of respective lipids from cocultured B cells (Figure 5A-B). B cells pulsed with vehicle or cultured with tetrameter-negative cells did not show any significant effect on the frequency of CD27hiCD38hi cells (Figure 5B). Coculture of βGL1-22 and LGL1-specific type II NKT cells with autologous B cells induced greater immunoglobulin secretion relative to B cells cultured alone or with non-NKT cells (Figure 5C). Parallel to B-cell differentiation into plasmablasts in the coculture, βGL1-22– and LGL1-specific type II NKT cells were found to express TFH markers (CXCR5hiPD1hi ICOShi Bcl-6hi, IL-21+) (Figure 5D). Interestingly α-GalCer tetramer-positive T cells did lead to some induction of plasmablasts and immunoglobulin, albeit less than with type II NKT cells (Figure 5A,C), despite not having TFH phenotype (Figure 5D) in these cultures. Together, these results demonstrate that human βGL1-22– and LGL1-specific type II NKT cells can provide efficient help to B cells.
βGL1-22/LGL1–specific type II human NKT cells provides more efficient B-cell help than type I NKT cells. Sorted human α-GalCer, βGL1-22, or LGL1 tetrameter-positive cells were incubated with purified autologous CD19+ B cells in the presence of vehicle or respective lipids. After 5 to 6 days of culture, cells and supernatants were harvested. The frequency of B cells with CD27hi CD38hi phenotype was determined by flow cytometry (A-B) and the levels of secreted IgM and IgG were measured by ELISA (C). (A) Representative contour plot showing the frequency of CD27hi CD38hi cells. (B) Compiled results from analyses of 3 different experiments showing the percentages of CD27hi CD38hi cells. (C) Levels of secreted immunoglobulin measured by ELISA. (D) FACS plot showing the presence of TFH (PD1+CXCR5+) and non-TFH (PD1−CXCR5−) phenotype in α-GalCer, βGL1-22, or LGL1 tetrameter-positive T cells or bulk T cells. Panel on the right shows fold change (relative to non-TFH cells) of percent positive cells for ICOS, Bcl6, and IL-21. **P < .001; *P < .01. DMSO, dimethylsulfoxide.
βGL1-22/LGL1–specific type II human NKT cells provides more efficient B-cell help than type I NKT cells. Sorted human α-GalCer, βGL1-22, or LGL1 tetrameter-positive cells were incubated with purified autologous CD19+ B cells in the presence of vehicle or respective lipids. After 5 to 6 days of culture, cells and supernatants were harvested. The frequency of B cells with CD27hi CD38hi phenotype was determined by flow cytometry (A-B) and the levels of secreted IgM and IgG were measured by ELISA (C). (A) Representative contour plot showing the frequency of CD27hi CD38hi cells. (B) Compiled results from analyses of 3 different experiments showing the percentages of CD27hi CD38hi cells. (C) Levels of secreted immunoglobulin measured by ELISA. (D) FACS plot showing the presence of TFH (PD1+CXCR5+) and non-TFH (PD1−CXCR5−) phenotype in α-GalCer, βGL1-22, or LGL1 tetrameter-positive T cells or bulk T cells. Panel on the right shows fold change (relative to non-TFH cells) of percent positive cells for ICOS, Bcl6, and IL-21. **P < .001; *P < .01. DMSO, dimethylsulfoxide.
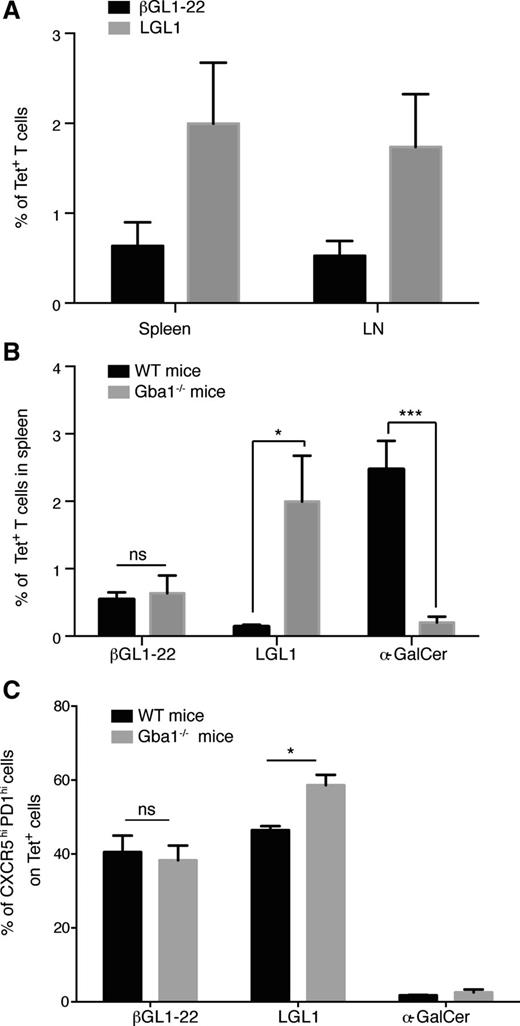
Alteration in GL1/LGL1-specific T cells in a murine model of GD
In order to test if these NKT cells are altered in vivo in the context of a disease state associated with alteration of corresponding lipids, we analyzed mouse models and patients with GD. Mistry et al recently developed a murine model that faithfully recapitulates the clinical phenotype of type I human GD.42 Therefore, we analyzed T cells from the spleen and lymph nodes of these mice for the presence of βGL1-22– and LGL1-reactive T cells using CD1d tetramers (Figure 6A). Compared with WT control mice, GBA−/− mice exhibited a >20-fold increase of LGL1-specific T cells (Figure 6B). In contrast, compared with the WT control mice, the proportion of type I NKT cells was strikingly reduced in GBA−/− mice (Figure 6B). Therefore, lysosomal GSL accumulation in GBA−/− mice was associated with an altered balance of type I vs type II NKT cells. Concomitant to increase in LGL1-specific T cells in GBA−/− mice, upregulation of TFH markers in these cells was also observed (Figure 6C).
Changes in βGL1-22– and LGL1-reactive T cells in a murine model of GD. (A) Bar graphs summarizing the percentage of CD1d-βGL1-22/LGL1 tetrameter-positive T cells in spleen and lymph node (LN) of GBA−/− mice. (B) Comparison of frequency of CD1d-βGL1-22, LGL1, and α-GalCer tetrameter-positive T cells between WT C57BL/6 (WT) and GBA1-deficient (GBA−/−) mice splenocytes. Data are presented as mean ± SEM from 3 different mice. (C) Compiled results comparing the percentage of CXCR5hi PD-1hi tetrameter-positive T cells between WT and GBA−/− mice. Data are presented as mean ± SEM from 3 different mice. *P < .01.
Changes in βGL1-22– and LGL1-reactive T cells in a murine model of GD. (A) Bar graphs summarizing the percentage of CD1d-βGL1-22/LGL1 tetrameter-positive T cells in spleen and lymph node (LN) of GBA−/− mice. (B) Comparison of frequency of CD1d-βGL1-22, LGL1, and α-GalCer tetrameter-positive T cells between WT C57BL/6 (WT) and GBA1-deficient (GBA−/−) mice splenocytes. Data are presented as mean ± SEM from 3 different mice. (C) Compiled results comparing the percentage of CXCR5hi PD-1hi tetrameter-positive T cells between WT and GBA−/− mice. Data are presented as mean ± SEM from 3 different mice. *P < .01.
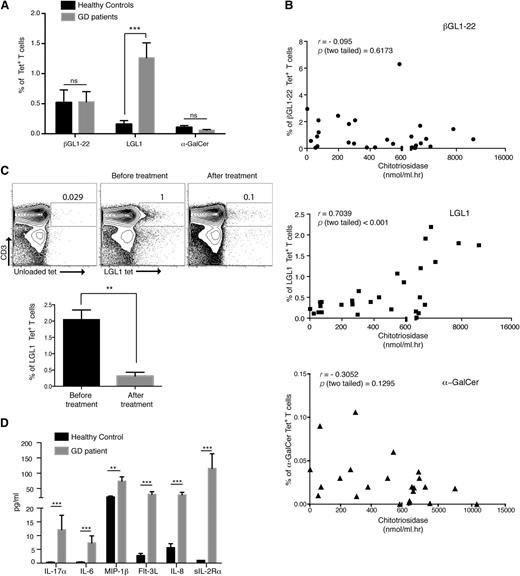
Alteration of LGL1-specific T cells in type 1 GD patients and its correlation with clinical disease
To extend the findings from GD mice to patients, we examined the presence of βGL1-22– and LGL1-specific T cells as well as type I NKT cells in PBMCs from GD patients (n = 20) and compared the findings to healthy donors. Clinical features of the type 1 GD patient cohort are shown in supplemental Table 3. In contrast to healthy donors, the majority of βGL1-22– and LGL1-specific T cells had a CD45RO+ memory and TFH phenotype, consistent with ligand-mediated activation in vivo (supplemental Figures 6 and 7). The proportion of circulating LGL1-specific T cells was significantly increased in GD patients (percent mean ± SEM: 0.16 ± 0.06 vs 1.263 ± 0.25, P < .001), whereas the proportion of type I NKT cells was not significantly different (Figure 7A). The percentage of LGL1-specific, but not βGL1-22–specific, T cells or type 1 NKT cells correlated with the serum level of chitotriosidase, an established biomarker of clinical disease activity in GD (R = 0.70; P < .001) (Figure 7B). The increase in LGL1-specific T cells was most noticeable in untreated patients (n = 5), and the levels of these T cells declined after enzyme-replacement therapy (Figure 7C). Analysis of cytokine profile in the serum of GD patients revealed an increase in several cytokines (IL-17, IL-6, macrophage inflammatory protein 1β, fms-related tyrosine kinase 3 ligand, IL-8, and soluble IL-2 receptor) (Figure 7D), which correlated with the cytokine profile of LGL1-reactive T cells. Together, these data show that as with GD mice, GSL accumulation in GD patients is associated with an altered balance of type I and II NKT cells and, importantly, that disease severity in the GD patients correlates with circulating LGL1-specific NKT cells.
Changes in βGL1-22/LGL1–specific T cells in GD patients correlates with disease severity. (A) Percentages of CD1d− βGL1-22–, LGL1-, or α-GalCer–specific T cells were compared between healthy controls and GD patients. (B) Graph shows the correlation between percentage of CD1d-βGL1-22 tetrameter-positive cells, LGL1 tetrameter-positive cells, or type I NKT cells and chitotriosidase activity in type 1 GD patients. (C) Representative dot plot representing the changes in the percentage of LGL1-specific T cells in GD patients before and after treatment (top). Lower panel shows graph summarizing changes in the percentage of LGL1-specific T cells in GD patients before and after treatment (n = 4) (bottom). (D) Multiplex cytokine analysis of sera from GD patients (n = 29) and healthy subjects (n = 15). Analytes with significant differences compared with healthy control sera are shown. Data are shown as mean + SEM. ***P < .0001; **P < .001; *P < .01. MIP-1β, macrophage inflammatory protein 1β; sIL-2Rα, soluble IL-2 receptor α.
Changes in βGL1-22/LGL1–specific T cells in GD patients correlates with disease severity. (A) Percentages of CD1d− βGL1-22–, LGL1-, or α-GalCer–specific T cells were compared between healthy controls and GD patients. (B) Graph shows the correlation between percentage of CD1d-βGL1-22 tetrameter-positive cells, LGL1 tetrameter-positive cells, or type I NKT cells and chitotriosidase activity in type 1 GD patients. (C) Representative dot plot representing the changes in the percentage of LGL1-specific T cells in GD patients before and after treatment (top). Lower panel shows graph summarizing changes in the percentage of LGL1-specific T cells in GD patients before and after treatment (n = 4) (bottom). (D) Multiplex cytokine analysis of sera from GD patients (n = 29) and healthy subjects (n = 15). Analytes with significant differences compared with healthy control sera are shown. Data are shown as mean + SEM. ***P < .0001; **P < .001; *P < .01. MIP-1β, macrophage inflammatory protein 1β; sIL-2Rα, soluble IL-2 receptor α.
Discussion
Polyclonal type II NKT cells were initially discovered in the context of MHCII−/− mice and are currently identified as CD1d-restricted T cells lacking iTCR.51,52 In contrast to type I NKT cells, information on functional properties of human type II NKT cells and their dysregulation in the context of human disease is extremely limited. We focused our studies on 2 distinct GSLs, βGL1-22 and LGL1, which are known to be dysregulated in several human metabolic disorders. We show that in contrast to type I NKT cells,53 βGL1-22– and LGL1-specific type II NKT cells constitutively express TFH phenotype. We also show that these cells have a distinct cytokine profile and provide robust help to B cells. This study therefore identifies a novel pathway mediated by type II NKT-TFH cells for B-cell immunity and inflammation.
β-Glucosylceramide with acyl chains (12:0 and 24:1) was initially identified as a ligand for type I NKT cells.21 However, recent studies using sulfatide-reactive type II NKT cell hybridoma XV19 and Jα18-deficient IL-4 reporter mice report recognition of β-GlcCer (12:0 and 24:1) by murine type II NKT cells.24,26 In contrast to initial findings from Brennan et al, our data pointed to β-glucosylceramide as a ligand for type II as opposed to type I NKT cells. Recent studies have attributed Brennan’s initial findings to a contaminating minor lipid species, consistent with endogenous α-linked glucosylceramide.54-56 These new studies therefore provide further support to our conclusion that β-GlcCer is primarily a type II ligand. Interestingly, in a small minority of donors/experiments (supplemental Figure 8), we did observe an increase in human iNKT cells following stimulation with β-GlcCer. This was not specific for individual lipid preparations but rather individual donors and tracked with higher baseline iNKT cells. In light of new studies, underlying reasons may include contaminating lipids or variations in endogenous α-anomeric lipid antigens in humans, which need further study.
In contrast to βGL1-22, LGL1-specific T cells were found to belong exclusively to the polyclonal human type II NKT subset. Phenotypic characterization, TCR sequencing, and transcriptional and cytokine profiling provided additional confirmation that human βGL1-22/LGL1–specific T cells are distinct from type I NKT cells. Notably, these cells express the NKT-lineage factor PLZF, albeit at lower level than in type I NKT cells. In addition to its well-documented role in type I NKT cells, PLZF was recently implicated in the differentiation of type II NKT cells as well.26,57 Crosstalk between type I and II NKT cells is increasingly implicated in the regulation of immunity to pathogens, tumors, and autoimmunity.5,7,50,58 The mechanism underlying the crosstalk between NKT subsets requires further study and may be more prominent with specific ligands. For example, in our studies, injection of βGL1-22 (but not LGL1) led to reduction in type I NKT cells in vivo. Similar findings have been made in other studies wherein activation of type II NKT cells led to decline in type I NKT cells in vivo.50 Although human T cells specific for both βGL1-22 and LGL1 can be detected using lipid-loaded CD1d tetramers, the broad pattern of staining that we observed suggests a range of binding affinities. Further studies are needed to better understand the biological significance of this variation. This may be particularly important in the context of TCR usage, as it is possible that there may be some oligoclonality, particularly with higher-affinity type II NKT cells. Oligoclonality has also been observed with sulfatide-reactive type II NKT cells.16
An important and novel aspect of these studies is the potential role of type II NKT-TFH cells in providing help to B cells and regulating antibody responses. Several studies have demonstrated that type I NKT cells can express BCL6 upon stimulation and co-opt the TFH pathway to provide direct or indirect help to B-cell responses against model antigens and pathogens.53,59-65 Initial studies suggested that type I NKT-induced antibody responses were rapid but transient, although a recent study challenged this view by showing that type I NKT cells can promote prolonged antibody responses to pneumococcal capsular polysaccharides by extrafollicular B-cell responses.53,59-61,66 Intriguingly, βGL1-22/LGL1–specific T cells were found to constitutively express TFH phenotype (CXCR5hi PD1hi ICOShi BCL6+IL-21+) in nearly 40% to 50% of lipid-reactive CD1d tetramer-positive T cells at steady state as opposed to type I NKT cells, which differentiated into TFH cells only after activation by α-GalCer. A specific expansion of CD1d-restricted lipid-reactive tetramer-positive cell numbers was observed following injection of either βGL1-22 or LGL1 in WT/Jα18−/− mice, but not in CD1d−/− mice. Differences in TFH phenotype and B-cell help between type I and II NKT cells are particularly prominent with human NKT cells, wherein type I NKT have only a weak TFH phenotype.67 Therefore, type II (as opposed to type I) NKT cells may be the major contributors to NKT-mediated B-cell help in humans, particularly as the relative proportions of type I vs II NKT cells are reversed between mice and humans.3
In order to study changes in βGL1-22/LGL1–specific T cells in a disease setting,34 we analyzed patients and mouse models with GD. It is of interest that βGL1-22/LGL1–specific T cells in GD patients have a memory phenotype consistent with their in vivo activation due to dysregulation of these lipids. Analysis of the phenotype of these cells in cord blood would also be of interest. Although a causative link between the increase in LGL1-specific T cells and clinical phenotype of GD remains to be established, the observed correlation between levels of LGL1-specific T cells and disease activity and the reduction in the level of these T cells in vivo upon clinical improvement following therapy suggest that host immune response to lipids may contribute to the pathogenesis of chronic inflammation in GD. The finding that LGL1-specific T cells (including in GD patients) are enriched for a TFH phenotype and lead to GC B-cell activation with production of antibodies suggests potential role of these cells in providing help for B-cell activation and increased risk of B-cell malignancy, such as myeloma observed in GD.68-70 Further studies are needed to evaluate this possibility.
As the GSL ligands studied here, particularly LGL1, are commonly altered in several inflammatory states, our findings should prompt an evaluation of human LGL1-specific type II NKT cells in several clinical settings.29 One clinical setting of particular interest is obesity, wherein an increase in these GSL ligands and changes in type II NKT cells have been previously implicated, although antigenic specificity of these T cells was not examined.14,15,29,71 Further studies of subsets of human type II NKT cells described herein may have broad implications in metabolic disorders associated with inflammation.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anumeha Shah for technical help with Luminex assays, Cindy Desmarais at Adaptive Biotechnologies for help with TCR sequencing, and Kavita M. Dhodapkar for discussions. They acknowledge the NIH Tetramer Core Facility (contract HHSN27201300006C) for provision of unloaded human and murine CD1d tetramers.
M.V.D. is supported in part by funds from the NIH National Cancer Institute (CA135110, CA106802, CA156689) and the Leukemia and Lymphoma Society. P.K.M. is supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (65932) and Center of Excellence Grant in Clinical Translational Research from Genzyme, a Sanofi company.
Authorship
Contribution: S.N. designed and performed the majority of experiments, analyzed data, and wrote the manuscript; C.S.B. performed experiments and analyzed data; R.V. analyzed data; J.L. and R.Y. performed some experiments; G.M.P. provided patient materials and analyzed data; P.K.M. provided mouse models of GD and patient materials, and helped design some of the experiments, interpret data, and write the paper; and M.V.D. developed and supervised the entire project, designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for G.M.P. is Mater Misericordiae University Hospital, Dublin, Ireland.
Correspondence: Madhav V. Dhodapkar, 333 Cedar St, WWW 211b, Yale University School of Medicine, New Haven, CT 06515; e-mail: madhav.dhodapkar@yale.edu.