Key Points
A new technology is presented to assess apparent affinities of FVIII-specific antibodies, differentiated for isotypes and IgG subclasses.
Affinities of FVIII-specific antibodies in patients with FVIII inhibitors are up to 100-fold higher than in patients without inhibitors.
Abstract
Recently, we reported that distinct immunoglobulin (Ig) isotypes and IgG subclasses of factor VIII (FVIII)-specific antibodies are found in different cohorts of patients with hemophilia A and in healthy individuals. Prompted by these findings, we further investigated the distinguishing properties among the different populations of FVIII-specific antibodies. We hypothesized that the affinity of antibodies would discriminate between the neutralizing and nonneutralizing antibodies found in different study cohorts. To test this idea, we established a competition-based enzyme-linked immunosorbent assay technology to assess the apparent affinities for each isotype and IgG subclass of FVIII-specific antibodies without the need for antibody purification. We present a unique data set of apparent affinities of FVIII-specific antibodies found in healthy individuals, patients with congenital hemophilia A with and without FVIII inhibitors, and patients with acquired hemophilia A. Our data indicate that FVIII-specific antibodies found in patients with FVIII inhibitors have an up to 100-fold higher apparent affinity than that of antibodies found in patients without inhibitors and in healthy individuals. High-affinity FVIII-specific antibodies could be retrospectively detected in longitudinal samples of an individual patient with FVIII inhibitors 543 days before the first positive Bethesda assay. This finding suggests that these antibodies might serve as potential biomarkers for evolving FVIII inhibitor responses.
Introduction
Neutralizing antibodies against factor VIII (FVIII), also known as FVIII inhibitors, remain the major challenge in treating congenital hemophilia A with FVIII products.1 They may also arise as autoantibodies, causing the rare bleeding disorder acquired hemophilia A.2 The underlying immune mechanisms leading to the development of these antibodies are poorly understood. Recently, we reported the prevalence of isotypes and immunoglobulin (Ig)G subclasses of FVIII-specific antibodies in cohorts of patients with congenital hemophilia A with and without FVIII inhibitors, in healthy individuals, and in patients with acquired hemophilia A.3 Our data showed the distinct spectra of isotypes and IgG subclasses of antibodies present in the different study cohorts. FVIII-specific IgG4 emerged as a distinguishing subclass because it was found exclusively in patients with FVIII inhibitors. At the same time, the identity of the immune regulatory pathways that give rise to the generation of neutralizing and nonneutralizing antibody populations remains elusive.
Antibody responses are initiated through the recognition of antigens by specific B-cell receptors expressed on naïve B cells and the subsequent induction of clonal expansion and differentiation of these B cells into antibody-producing plasma cells. Antibody-producing plasma cells can arise from different pathways: follicular pathways in specialized germinal centers within B-cell follicles of secondary lymphoid organs; and extrafollicular pathways outside B-cell follicles. The nature of the differentiation pathway has substantial consequences for the survival and location of the resulting plasma cells and for the quality and quantity of the antibodies they secrete. Plasma cells that develop in follicular differentiation pathways in germinal centers are predominantly long-lived. They migrate to the bone marrow where they can survive in specific plasma cell niches. The antibodies they produce are switched from IgM to IgG or IgA and have high affinity.4,5 Plasma cells that arise from extrafollicular pathways are predominantly short-lived and nonmigratory. The antibodies they secrete are generally of low or moderate affinity.5 Depending on the type of antigen and the surrounding microenvironment, extrafollicular pathways of B-cell differentiation can occur in either a CD4+ T-cell dependent or -independent manner.5 The spleen encloses an additional extrafollicular compartment—the marginal zone—that houses nonrecirculating B cells.6 Given sufficient stimulatory signals, activated marginal-zone B cells can rapidly differentiate into short-lived plasma cells independently of T-cell help.7 The antibodies produced under such conditions are considered mostly of low affinity.5
Clearly, the quality of circulating antibodies (eg, their isotypes and affinities) can provide information reflecting the underlying immune mechanisms involved in their generation. On their own, the signals produced by monovalent protein antigens such as FVIII are considered to be insufficient to induce clonal expansion of B cells and their differentiation into antibody-producing plasma cells. Additional activation signals provided by interactions with other immune cells or by the triggering of innate immune receptors are thought to be required.4
Aschenbach et al8 and Mayr et al9 previously reported that high-affinity autoantibodies against insulin are found in healthy children who are at high risk for developing type 1 diabetes mellitus.8,9 Alternatively, children who have low-affinity autoantibodies against insulin are at low risk for eventually developing the disease. These data indicate that the affinity of antibodies not only might be indicative of the underlying immune mechanism involved in their generation but also might serve as a potential biomarker for evolving immunopathology. With these findings in mind, we decided to investigate whether the affinity of circulating FVIII-specific antibodies can differentiate neutralizing antibodies (as found in patients with FVIII inhibitors) from nonneutralizing antibodies (as found in some patients without FVIII inhibitors and in some healthy individuals).
Antibody affinities have been studied using a variety of approaches, including label-free biosensor technologies such as surface plasmon resonance (SPR) and biolayer interferometry (BLI), as well as indirect and competitive immunoassays based on chemiluminescence or fluorescence labeling. Label-free biosensor technologies allow the analysis of association and dissociation kinetics of antibodies binding to their respective antigens.10 However, purified antibodies are required for this purpose. Competitive immunoassays provide apparent affinities in equilibrium and analyze free antibodies present in a solution after preincubation of the antibody solution with preselected molar concentrations of the respective antigen.11 Assuming equimolar interaction between antibodies and antigen, this approach enables apparent affinity determination without antibody purification. Traditionally, neither label-free biosensor technologies nor competitive immunoassay technologies have allowed the assessment of low-affinity antibodies in a mixture with high-affinity antibodies when both antibody populations recognize the same antigen. Bobrovnik et al12 and Stevens et al13 introduced a novel approach that combines competition-based enzyme-linked immunosorbent assay (ELISA) technology with nonlinear regression modeling of the ELISA graphs to facilitate the analysis of 2 antibody clusters with low and high apparent affinity in the same sample. We applied this approach to the analysis of FVIII-specific antibodies present in human plasma samples and established a competition-based ELISA platform that combines the advantages of low sample consumption, no need for antibody purification, the potential to assess apparent affinities of low-affinity and high-affinity antibody clusters in the same sample, and the ability to specify apparent affinities of individual antibody isotypes and IgG subclasses. Moreover, this technology enables the cost-effective analysis of large cohorts of plasma samples without the need for sophisticated equipment.
For the first time, we present a unique data set reflecting the apparent affinities of FVIII-specific antibodies, differentiated for immunoglobulin isotypes and IgG subclasses, as found in patients with and without FVIII inhibitors and in healthy individuals.
Materials and methods
Human plasma samples
All patient samples were received after the patients or their guardians gave written informed consent and with approval from the local ethics committees.
Healthy individuals.
Plasma samples from 634 healthy individuals were screened for the presence of FVIII-specific antibodies. Seventeen plasma samples (from 13 women and 4 men; median age 38 years, interquartile range [IQR]: 24-48 years) contained FVIII-specific antibodies with titers ≥1:80. Information on ethnicity of plasma donors was not available. One 22-year-old healthy woman, positive for FVIII-specific IgG1, was monitored for almost 3 years.
Patients with severe congenital hemophilia A.
Plasma samples from white patients with severe congenital hemophilia A (FVIII <1%) were obtained from the Medical University Vienna, Vienna, Austria; the Medical University Bonn, Bonn, Germany; the Medical School Hannover, Hannover, Germany; and the Institute of Hematology and Transfusion Medicine, Warsaw, Poland.
Twenty-four samples were obtained from patients with persistent FVIII inhibitors (median titer 10 BU/mL, IQR: 5-29 BU/mL), with a median time from inhibitor diagnosis of 58 months (IQR: 11-175 months) and a median historic peak inhibitor titer of 136 BU/mL (IQR: 87-213 BU/mL). All samples contained FVIII-specific antibodies with titers ≥1:80.
Seventy-seven samples were obtained from patients with no history of FVIII inhibitors after at least 100 exposure days to FVIII products. Four samples (median age 36 years, IQR: 26-43 years) contained FVIII-specific antibodies with titers ≥1:80.
In addition, longitudinal plasma samples collected over 4 years from a patient with severe congenital hemophilia A with FVIII inhibitors were analyzed retrospectively. Plasma samples were collected both before and after inhibitor diagnosis. A brief description of the medical history of this patient is given in the supplemental Data, Online Supplement 1, available on the Blood Web site.
Patients with acquired hemophilia A.
Plasma samples from 19 white patients with acquired hemophilia A (14 women and 5 men; median age 76 years, IQR: 67-81 years) were obtained from the Medical University Vienna; the Medical School Hannover; the Medical School Greifswald, Greifswald, Germany; and the Institute of Hematology and Transfusion Medicine. Samples with median inhibitor titers of 10 BU/mL (IQR: 6-37 BU/mL) were collected at first diagnosis, before the start of interventional therapy. All samples contained FVIII-specific antibodies with titers ≥1:80. In addition, longitudinal plasma samples obtained from a 79-year-old male patient were analyzed retrospectively. A description of the medical history of this patient is given in the supplemental Data, Online Supplement 2.
Human recombinant FVIII
Full-length recombinant human FVIII (FVIII; Baxter BioScience) was used for all analytical assays.
Detection of FVIII-specific antibodies
FVIII-specific antibodies of immunoglobulin isotypes IgA and IgM and of IgG subclasses 1 to 4 were analyzed using a highly sensitive and fully validated ELISA platform as described previously.3 In addition, we used a fully validated ELISA for the detection of FVIII-specific total IgG antibodies. Based on the validation of the ELISA platform, the confirmation of antibody specificity for FVIII requires antibody titers ≥1:80.3 Samples with confirmed specificity for FVIII qualified for the assessment of apparent affinity. Antibody specificity for FVIII had to be confirmed in longitudinal sample analyses at least once to qualify for affinity assessment.
Detection of neutralizing antibodies
The Nijmegen modification of the Bethesda assay14 and a commercially available kit (Technoclone) with a cutoff of 1 BU/mL were used to detect neutralizing antibodies.
Affinity ELISA platform for the assessment of the apparent affinity of FVIII-specific antibodies
The apparent affinities of FVIII-specific antibodies for all immunoglobulin isotypes and IgG subclasses were assessed using a competition-based ELISA approach. The test principle, technical details, and validation of the affinity ELISA platform are described in the supplemental Data, Online Supplement 3. In brief, the affinity assessment is based on the availability of antibody for binding to FVIII-coated ELISA plates after competition with FVIII in solution. Data for apparent affinities (KA [M−1]) were derived from nonlinear regression modeling of competition ELISA Δ optical densities as described previously.13
SPR, BLI, and SET
The experimental details for affinity measurements of an FVIII-specific monoclonal IgG1 antibody (AbD Serotec) using SPR, BLI, and solution equilibrium titration (SET) are given in the supplemental Data, Online Supplement 4.
Statistical analysis
Differences in apparent affinities of FVIII-specific IgG subclasses between different cohorts were tested using the GENMOD Procedure of the SAS software (v.9.3) without including covariates. Differences for FVIII-specific IgA and IgM were not tested because of the low numbers of nonzero values in all study cohorts. The negative binomial, Poisson, zero-inflated negative binomial, and zero-inflated Poisson models were fitted to the data set according to IgG subclass. Because the models use different error structures, they are considered nonnested.15 Among those models achieving convergence, model fit was compared using the Clarke test.16 The zero-inflated negative binomial model 17 was found to provide the best fit for all antibody classes. The overall significance level for comparisons of IgG subclass data was set to 5%. Multiplicity was adjusted using the Bonferroni-Holm procedure.18
To differentiate between the affinities of potentially pathogenic and nonpathogenic IgG1 immune responses, cut points were calculated between corresponding cohorts by means of the receiver operating characteristic curves. Specificity and sensitivity were calculated for each IgG1 measurement (m) used for the receiver operating characteristic curve. From these data, the Youden index was calculated as sensitivity m + specificity m − 1. The measurement with the highest Youden index was selected as the cut point to discriminate between the groups.19 Cutoff calculation for other IgG subclasses of FVIII-specific antibodies could not be done because of the low number of nonzero values in the noninhibitor cohorts.
Results
Assessment of apparent affinities for FVIII-specific antibodies
We established a competition-based affinity ELISA platform to assess apparent affinities of FVIII-specific antibodies in human plasma samples. Initially, we used 2 representative samples to define the appropriate range of FVIII concentrations to be used in antibody competition for the detection of both high-affinity and low-affinity antibodies: (1) a high-affinity monoclonal human FVIII-specific IgG1 antibody spiked into human plasma, free from FVIII-specific antibodies; and (2) plasma from a healthy individual with FVIII-specific IgG1 antibodies with a titer of 1:640. FVIII concentrations of 0, 0.9, 1.4, 2.1, 3.1, 4.7, 14.0, and 42.1 nM were found to be optimal for the affinity assessment of total IgG, IgG1, IgG2, IgG3, and IgA antibodies. FVIII concentrations of 0, 0.016, 0.031, 0.063, 0.125, 0.25, 0.5, and 1 nM were found to be optimal for the affinity assessment of IgG4 antibodies. This differentiation was necessary because of the higher affinities observed for FVIII-specific IgG4. We determined whether the affinity ELISA could identify low-affinity antibodies in the presence of high-affinity antibodies by spiking the high-affinity monoclonal IgG1 antibody into the plasma sample with low-affinity IgG1 and comparing this mixture with the individual antibody preparations. We used a nonlinear regression model as described by Stevens et al13 and predefined statistical acceptance criteria for model selection to analyze the binding curves (Figure 1) and to calculate apparent affinities (Table 1). Model 1 was best fitted to describe the competition curves of the high-affinity monoclonal antibody (KA = 4.9 × 109 M−1) and the low-affinity antibody-containing plasma sample (KA = 9.1 × 107 M−1). Model 2, which identified 2 antibody clusters with high apparent affinity (KA = 5.4 × 109 M−1) and low apparent affinity (KA = 9.9 × 107 M−1), provided the best fit to describe the binding curve of the mixed antibody preparation (Table 1). The 2 apparent affinities identified were almost identical to the results calculated for the individual antibody preparations. We conclude that the affinity ELISA platform enables the detection of 2 major antibody clusters with low and high affinities in a single plasma sample.
Representative FVIII competition curves of FVIII-specific antibody samples used for affinity ELISA validation. Samples containing FVIII-specific antibodies were preincubated with different concentrations of FVIII in solution (FVIII [nM]) and subsequently tested for the presence of remaining free antibodies (ΔOD-blank). A nonlinear distribution of FVIII concentrations was used for optimal coverage of different competition behavior in individual samples. A monoclonal human FVIII-specific IgG1 antibody spiked into human plasma not containing any FVIII-binding antibodies (sample A; circles) requires lower FVIII concentrations for competition than a human plasma sample containing low-affinity FVIII-specific IgG1 antibodies (sample B; triangles). A mixture of samples A and B was used to mimic bimodal affinity distribution (sample C; diamonds).
Representative FVIII competition curves of FVIII-specific antibody samples used for affinity ELISA validation. Samples containing FVIII-specific antibodies were preincubated with different concentrations of FVIII in solution (FVIII [nM]) and subsequently tested for the presence of remaining free antibodies (ΔOD-blank). A nonlinear distribution of FVIII concentrations was used for optimal coverage of different competition behavior in individual samples. A monoclonal human FVIII-specific IgG1 antibody spiked into human plasma not containing any FVIII-binding antibodies (sample A; circles) requires lower FVIII concentrations for competition than a human plasma sample containing low-affinity FVIII-specific IgG1 antibodies (sample B; triangles). A mixture of samples A and B was used to mimic bimodal affinity distribution (sample C; diamonds).
Model selection for the determination of apparent affinities
| Sample* . | R2 > 0.7 . | Lower limit of 95% CI > 0 . | F test . | Selected model . | Population 1 KA [M−1] . | Population 2 KA [M−1] . | ||
|---|---|---|---|---|---|---|---|---|
| M1 . | M2 . | M1 . | M2 . | |||||
| A | Yes | Yes | Yes | No | NA | M1 | 4.9 × 109 | NA |
| B | Yes | Yes | Yes | No | NA | M1 | 9.1 × 107 | NA |
| C | Yes | Yes | Yes | Yes | M2 | M2 | 5.4 × 109 | 9.9 × 107 |
| Sample* . | R2 > 0.7 . | Lower limit of 95% CI > 0 . | F test . | Selected model . | Population 1 KA [M−1] . | Population 2 KA [M−1] . | ||
|---|---|---|---|---|---|---|---|---|
| M1 . | M2 . | M1 . | M2 . | |||||
| A | Yes | Yes | Yes | No | NA | M1 | 4.9 × 109 | NA |
| B | Yes | Yes | Yes | No | NA | M1 | 9.1 × 107 | NA |
| C | Yes | Yes | Yes | Yes | M2 | M2 | 5.4 × 109 | 9.9 × 107 |
An explanation of the model-selection strategy can be found in “Materials and methods.” Model 1 (homogenous apparent affinity distribution), assuming 1 major antibody cluster, resulted in the best description of the FVIII competition curves for samples A and B. In contrast, model 2 (bimodal apparent affinity distribution), assuming 2 major antibody clusters with distinct affinities, provided the best fit to describe the competition curve of sample C.
Sample A: monoclonal human FVIII-specific IgG1 antibody spiked into human plasma not containing any FVIII-binding antibodies; sample B: human plasma from a healthy individual with FVIII-specific IgG1, diluted with human plasma not containing FVIII-binding antibodies; and sample C: a mixture of samples A and B.
CI, confidence interval; M1, model 1; M2, model 2; NA, not applicable; R2, coefficient of determination; KA, apparent affinity.
To validate the stability of the affinity ELISA for total IgG, we tested the method for intrarun and interrun reproducibility and for the freeze/thaw-cycle robustness. In addition, we tested the corresponding affinity ELISA for assessment of FVIII-specific IgG1 for intrarun reproducibility. The coefficient of variation (CV) for all tested conditions was <25% (Table 2), confirming the assay’s reproducibility and robustness. We also considered the polyclonal nature of antibody-containing clinical samples obtained from patients with FVIII inhibitors. We tested the reproducibility and robustness of the affinity ELISA to assess the apparent affinity of 1 or 2 major antibody clusters in 2 representative clinical samples (samples D and E in Table 3). The affinity ELISA reproducibly detected 1 major antibody cluster in sample D and 2 major antibody clusters with different apparent affinities in sample E (Table 3). The specific values for the apparent affinity had a CV ≤25% when only 1 antibody cluster was present in the sample (sample D) and had CVs between 30% and 51% when 2 antibody clusters with different affinities were present in the sample (sample E).
Summary of results obtained during affinity ELISA validation
| Assay validation criteria . | Sample A* . | Sample B† . | Sample C‡ . | |
|---|---|---|---|---|
| Population 1 (high affinity) . | Population 2 (low affinity) . | |||
| Total IgG | ||||
| Intraassay stability | 10 | 5 | 15 | 9 |
| Interassay stability | 18 | 11 | 17 | 5 |
| Robustness (freeze/thaw) | 20 | 4 | 8 | 8 |
| IgG1 | ||||
| Intraassay stability | 5 | 2 | 5 | 11 |
| Assay validation criteria . | Sample A* . | Sample B† . | Sample C‡ . | |
|---|---|---|---|---|
| Population 1 (high affinity) . | Population 2 (low affinity) . | |||
| Total IgG | ||||
| Intraassay stability | 10 | 5 | 15 | 9 |
| Interassay stability | 18 | 11 | 17 | 5 |
| Robustness (freeze/thaw) | 20 | 4 | 8 | 8 |
| IgG1 | ||||
| Intraassay stability | 5 | 2 | 5 | 11 |
Data are CVs in percent.
Sample A: monoclonal human FVIII-specific IgG1 antibody spiked into human plasma not containing any FVIII-binding antibodies.
Sample B: human plasma from a healthy individual with FVIII-specific IgG1 diluted with human plasma not containing FVIII-binding antibodies.
Sample C: a mixture of samples A and B.
Reproducibility of affinity ELISA results for representative samples of FVIII inhibitor patients
| Total IgG assay validation criteria . | Sample D* . | Sample E* . | |
|---|---|---|---|
| Population 1 (high affinity) . | Population 2 (low affinity) . | ||
| Intraassay stability | 11 | 30 | 43 |
| Interassay stability | 25 | 41 | 41 |
| Robustness (freeze/thaw) | 8 | 51 | 37 |
| Total IgG assay validation criteria . | Sample D* . | Sample E* . | |
|---|---|---|---|
| Population 1 (high affinity) . | Population 2 (low affinity) . | ||
| Intraassay stability | 11 | 30 | 43 |
| Interassay stability | 25 | 41 | 41 |
| Robustness (freeze/thaw) | 8 | 51 | 37 |
Data are CVs in percent.
Samples D and E: human plasma from 2 subjects with severe congenital
hemophilia A and FVIII inhibitor, diluted with human plasma not containing
FVIII-binding antibodies.
Next, we asked how the results obtained with the competition-based affinity ELISA compare with the results obtained with alternative technologies. We used the purified FVIII-specific human monoclonal IgG1 antibody to compare the affinity ELISA with the competition-based SET technology, the direct binding SPR technology using a carboxymethylated sensor chip (C1) or a carboxymethylated dextran-coated sensor chip (CM5), and the direct binding BLI technology. The results of the affinity ELISA (KA = 5.3 × 109 M−1) are in excellent agreement with the results obtained using the SET platform (KA = 4.2 × 109 M−1) and are in good agreement with the results generated using the label-free platforms BLI (KA = 2.3 × 108 M−1) and SPR when using the C1 sensor chip (KA = 1.5 × 109 M−1) but not the CM5 sensor chip (KA = 5.1 × 107 M−1). Interestingly, the results obtained with the SPR technology were dependent on the type of sensor chip used, which confirms a previous report.20
Purified antibodies are required when quantifying affinity using direct-binding approaches. Nevertheless, we used the direct-binding technology BLI to run a qualitative comparison of the binding characteristics of all samples used for the validation of the affinity ELISA (see the supplemental Data, Online Supplement 5). This comparison indicates that differences in apparent affinity as quantified by the affinity ELISA are also detectable in the association and dissociation analysis using BLI.
In summary, we established a competition-based affinity ELISA platform to assess apparent affinities of FVIII-specific antibodies in human plasma samples.
Apparent affinity of FVIII-specific antibodies in different study cohorts
We screened plasma samples containing FVIII-specific antibodies with titers ≥1:80 from cohorts of patients with hemophilia and healthy individuals as presented in Figure 2.
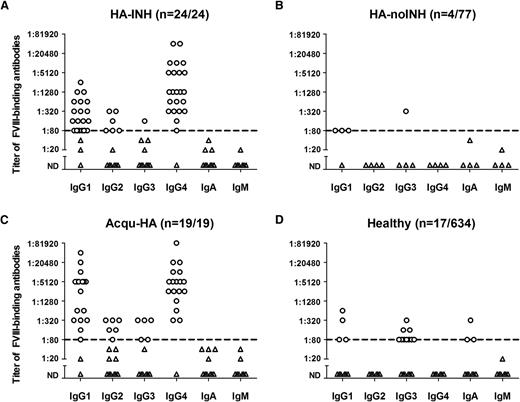
Titers of FVIII-specific antibodies assessed for individual immunoglobulin isotypes and IgG subclasses. Presented are the results of all plasma samples that contained FVIII-specific antibodies with a titer ≥1:80 of at least 1 immunoglobulin isotype or IgG subclass. (A) Twenty-four of 24 patients with severe hemophilia A and FVIII inhibitors (HA-INH). (B) Four of 77 patients with severe hemophilia A without FVIII inhibitors (HA-noINH). (C) Nineteen of 19 patients with acquired hemophilia A (Acqu-HA). (D) Seventeen of 634 healthy individuals (Healthy). A titer of 1:20 represents the screening cutoff of the method to differentiate positive and negative samples. The dotted line at a titer of 1:80 represents the lower limit of detection for FVIII-specific antibodies.3 Circles depict positive results for FVIII-specific antibodies; triangles represent results that were below the lower limit of detection for FVIII-specific antibodies. ND, not detectable.
Titers of FVIII-specific antibodies assessed for individual immunoglobulin isotypes and IgG subclasses. Presented are the results of all plasma samples that contained FVIII-specific antibodies with a titer ≥1:80 of at least 1 immunoglobulin isotype or IgG subclass. (A) Twenty-four of 24 patients with severe hemophilia A and FVIII inhibitors (HA-INH). (B) Four of 77 patients with severe hemophilia A without FVIII inhibitors (HA-noINH). (C) Nineteen of 19 patients with acquired hemophilia A (Acqu-HA). (D) Seventeen of 634 healthy individuals (Healthy). A titer of 1:20 represents the screening cutoff of the method to differentiate positive and negative samples. The dotted line at a titer of 1:80 represents the lower limit of detection for FVIII-specific antibodies.3 Circles depict positive results for FVIII-specific antibodies; triangles represent results that were below the lower limit of detection for FVIII-specific antibodies. ND, not detectable.
The pattern of apparent affinities differentiated for individual antibody isotypes and IgG subclasses showed clear differences between patients with hemophilia with and without FVIII inhibitors (Figure 3A-B) and between patients with acquired hemophilia A and healthy individuals (Figure 3C-D). The highest apparent affinities were seen for FVIII-specific IgG4 present in samples from patients with FVIII inhibitors. A proportion of samples in each study group contained 2 major antibody clusters with different apparent affinities (Figure 3).
Apparent affinities of FVIII-specific antibodies assessed for individual immunoglobulin isotypes and IgG subclasses. Presented are apparent affinities KA [M−1] of FVIII-specific antibodies found in the different study cohorts presented in Figure 2. All samples containing FVIII-specific antibodies with a titer ≥1:80 as presented in Figure 2 were included in the analysis. (A) Patients with severe hemophilia A and FVIII inhibitors (HA-INH). (B) Patients with severe hemophilia A without FVIII inhibitors (HA-noINH). (C) Patients with acquired hemophilia A (Acqu-HA). (D) Healthy individuals (Healthy). Some samples in each study cohort contained 2 populations of FVIII-specific antibodies with distinct apparent affinities. These 2 populations present in the same sample are indicated by an open circle and a closed circle connected by a straight line.
Apparent affinities of FVIII-specific antibodies assessed for individual immunoglobulin isotypes and IgG subclasses. Presented are apparent affinities KA [M−1] of FVIII-specific antibodies found in the different study cohorts presented in Figure 2. All samples containing FVIII-specific antibodies with a titer ≥1:80 as presented in Figure 2 were included in the analysis. (A) Patients with severe hemophilia A and FVIII inhibitors (HA-INH). (B) Patients with severe hemophilia A without FVIII inhibitors (HA-noINH). (C) Patients with acquired hemophilia A (Acqu-HA). (D) Healthy individuals (Healthy). Some samples in each study cohort contained 2 populations of FVIII-specific antibodies with distinct apparent affinities. These 2 populations present in the same sample are indicated by an open circle and a closed circle connected by a straight line.
FVIII-specific IgG1 was unique because it was found in all study groups. The apparent affinity pattern of FVIII-specific IgG1 found in healthy individuals was similar to the affinity pattern of the lower-affinity IgG1 antibody cluster found in patients who expressed 2 FVIII-specific IgG1 antibody clusters with different affinities (Figure 3).
When we compared the apparent affinities of FVIII-specific IgG1 from patients with and without FVIII inhibitors, we found that patients with inhibitors expressed significantly higher affinities (Table 4). On the basis of this finding, we calculated an affinity cut point KA to differentiate between neutralizing and nonneutralizing FVIII-specific IgG1. The cut point KA was 1.3 × 109 M−1 (Table 4).
Statistical comparison and cut point calculation to differentiate between pathogenic and nonpathogenic FVIII-specific IgG1
| Comparison . | Adjusted P . | Sensitivity . | Specificity . | Cut point KA [M−1] . |
|---|---|---|---|---|
| KA HA-INH IgG1 > KA HA-noINH IgG1 | <.0001 | 0.875 | 0.987 | 1.3 × 109 |
| KA Acqu-HA IgG1 > KA Healthy IgG1 | <.0001 | 0.895 | 1.000 | 2.5 × 109 |
| Comparison . | Adjusted P . | Sensitivity . | Specificity . | Cut point KA [M−1] . |
|---|---|---|---|---|
| KA HA-INH IgG1 > KA HA-noINH IgG1 | <.0001 | 0.875 | 0.987 | 1.3 × 109 |
| KA Acqu-HA IgG1 > KA Healthy IgG1 | <.0001 | 0.895 | 1.000 | 2.5 × 109 |
Statistical cut point calculation for apparent affinities of FVIII-specific IgG1, comparing congenital hemophilia A patients with (HA-INH) and without (HA-noINH) FVIII inhibitors, and comparing patients with acquired hemophilia A (Acqu-HA) with healthy individuals (Healthy), using the Youden index approach as described in “Materials and methods.”
When we compared the apparent affinities of FVIII-specific IgG1 from patients with acquired hemophilia A to those from healthy individuals, we found that the patients expressed significantly higher affinities (Table 4). We also calculated an affinity cut point KA to differentiate between neutralizing IgG1 (as found in patients) and nonneutralizing IgG1 (as found in healthy individuals). The cut point KA was 2.5 × 109 M−1 (Table 4), which is similar to the cut point KA of 1.3 × 109 M−1 found for FVIII-specific IgG1 in patients with congenital hemophilia A with and without inhibitors.
Additionally, we compared the apparent affinities of FVIII-specific antibodies found in patients with congenital hemophilia A and inhibitors to those in patients with acquired hemophilia A. Patients with congenital hemophilia A and inhibitors expressed significantly higher affinities for FVIII-specific IgG2 and IgG4, but not for FVIII-specific IgG1 and IgG3 (see the supplemental Data, Online Supplement 6).
We conclude that the apparent affinities of FVIII-specific antibodies are significantly higher in patients with FVIII inhibitors compared to those in patients without FVIII inhibitors and in healthy individuals. FVIII-specific IgG4 found in patients with congenital hemophilia A and FVIII inhibitors expresses the highest affinity of all IgG subclasses.
Longitudinal monitoring of FVIII-specific antibodies
After we had established the patterns of apparent affinities of FVIII-specific antibodies in the different study cohorts, we were interested to know whether the various species of FVIII-specific antibodies persist during long-term follow-up. Moreover, we wanted to determine whether high-affinity antibodies could be detected before the diagnosis of FVIII inhibitors. We retrospectively studied longitudinal samples from a patient with congenital hemophilia A and FVIII inhibitors, a patient with acquired hemophilia A, and a healthy individual.
The samples from the patient with congenital hemophilia A were taken at different time points before and after FVIII inhibitor diagnosis as defined by positive Bethesda assay. Data presented in Figure 4 demonstrate that high-affinity FVIII-specific antibodies were already detectable 543 days before the first FVIII inhibitor diagnosis. Importantly, these antibodies contained not only high-affinity IgG1 but also high-affinity IgG4, which differentiates neutralizing FVIII inhibitors from nonneutralizing FVIII-specific antibodies. These high-affinity antibodies persisted for the whole observation period but increased in titer over time. Together with the high-affinity antibodies, low-affinity FVIII-specific antibodies were detectable at 543 and 444 days before the diagnosis of FVIII inhibitors. These low-affinity antibodies were not detectable at later time points.
Longitudinal monitoring of antibodies against FVIII in a patient with congenital hemophilia A. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Antibody data for IgM, IgA, IgG2, and IgG3 were below the cutoff of the antibody assays and are, therefore, not shown. Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinity include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles] and IgG4 [open green diamonds]); and apparent affinities of FVIII-specific antibodies for low-affinity IgG1 clusters (open blue bars) and IgG4 clusters (open green bars) and for high-affinity IgG1 clusters (filled blue bars) and IgG4 clusters (filled green bars). The details of the medical history for this patient are included in the supplemental Data, Online Supplement 1.
Longitudinal monitoring of antibodies against FVIII in a patient with congenital hemophilia A. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Antibody data for IgM, IgA, IgG2, and IgG3 were below the cutoff of the antibody assays and are, therefore, not shown. Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinity include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles] and IgG4 [open green diamonds]); and apparent affinities of FVIII-specific antibodies for low-affinity IgG1 clusters (open blue bars) and IgG4 clusters (open green bars) and for high-affinity IgG1 clusters (filled blue bars) and IgG4 clusters (filled green bars). The details of the medical history for this patient are included in the supplemental Data, Online Supplement 1.
The samples from the patient with acquired hemophilia A were taken at day 10, 31, and 57 after initial FVIII inhibitor diagnosis. Data presented in Figure 5 demonstrate that high-affinity FVIII-specific IgG1 and IgG4 antibodies persisted during the whole observation period, even at day 31 when the FVIII inhibitor had disappeared.
Longitudinal monitoring of antibodies against FVIII in a patient with acquired hemophilia A. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Antibody data for IgM, IgA, IgG2, and IgG3 were below the cutoff of the antibody assays and are, therefore, not shown. Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinity include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles] and IgG4 [open green diamonds]); and apparent affinities of FVIII-specific antibody clusters for IgG1 (filled blue bars) and IgG4 (filled green bars). The details of the medical history for this patient are included in the supplemental Data, Online Supplement 2.
Longitudinal monitoring of antibodies against FVIII in a patient with acquired hemophilia A. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Antibody data for IgM, IgA, IgG2, and IgG3 were below the cutoff of the antibody assays and are, therefore, not shown. Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinity include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles] and IgG4 [open green diamonds]); and apparent affinities of FVIII-specific antibody clusters for IgG1 (filled blue bars) and IgG4 (filled green bars). The details of the medical history for this patient are included in the supplemental Data, Online Supplement 2.
The samples from the healthy individual covered a period of more than 3 years. The initial sample contained FVIII-specific IgG1 antibodies with a titer of 1:640 and a KA of 1 × 108 M−1. During subsequent follow-up at day 63, 366, 798, 987, and 1001, we did not see any significant change in antibody titer or apparent affinity (Figure 6). None of the samples was positive for FVIII inhibitors, which indicates that low-affinity FVIII-specific antibodies can persist long-term.
Longitudinal monitoring of antibodies against FVIII present in a healthy individual. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinities include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles]); and apparent affinities of FVIII-specific antibody cluster (IgG1 [filled blue bars]).
Longitudinal monitoring of antibodies against FVIII present in a healthy individual. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinities include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles]); and apparent affinities of FVIII-specific antibody cluster (IgG1 [filled blue bars]).
In summary, high-affinity FVIII-specific IgG1 and IgG4 might indicate evolving or persisting FVIII inhibitor responses, even when FVIII inhibitors are not detectable.
Discussion
The regulation of antibody responses against FVIII in patients with hemophilia A and in healthy individuals is not well understood, which is why a better characterization of FVIII-specific immune responses is required. The affinity of antibodies to their respective antigen is an important feature, reflecting the maturation stage of antibody responses and potentially implicating immune regulatory pathways involved in their generation. We present the first study that provides a comprehensive data set that assesses the apparent affinities of FVIII-specific antibodies, separated for individual isotypes and IgG subclasses, in different cohorts of patients with congenital or acquired hemophilia A and in healthy individuals.
The strength of the affinity ELISA platform that we established in this study is illustrated by low sample consumption, no need for antibody purification, the potential to assess low-affinity antibody clusters in mixtures with high-affinity antibody clusters, and the ability to specify apparent affinities of individual antibody isotypes and IgG subclasses. The ELISA-based technology reproducibly detects 1 or 2 antibody clusters of different affinities in the same plasma sample. The absolute values for the apparent affinities represent estimates that are reflected by CVs up to 51% when 2 antibody clusters with different apparent affinities are present in the same plasma sample. Due to the polyclonal nature of the antibody response, the values for apparent affinities determined by nonlinear regression models can be only estimates of average affinities of a particular antibody cluster. Although separate analysis of association and dissociation kinetic parameters is not feasible with this technology, we believe that the advantages outweigh this limitation. Importantly, this platform has the potential of distinguishing between the apparent affinities of different antibody isotypes and IgG subclasses, which will improve our understanding of the regulation of neutralizing and nonneutralizing FVIII-specific antibodies. Data generated with the affinity ELISA platform are in good agreement with alternative technologies such as SPR and BLI.
Our results reveal an up to 100-fold difference between apparent affinities of FVIII-specific antibodies found in patients with FVIII inhibitors and those found in patients without inhibitors and in healthy individuals. On the basis of recent findings,4,5,7 we postulate that the higher-affinity antibodies found in patients with FVIII inhibitors are generated by plasma cells that arise from follicular differentiation pathways in germinal centers and subsequently migrate to specific plasma cell niches in the bone marrow where they can survive long-term. The lower-affinity antibodies found in patients without FVIII inhibitors and in healthy individuals, however, are more likely to be produced by plasma cells arising from extrafollicular differentiation pathways or from nonrecirculating marginal-zone B cells.
FVIII-specific IgG4, which was exclusively present in samples from patients with FVIII inhibitors, expressed the highest apparent affinity, confirming the importance of IgG4 in neutralizing antibody responses against FVIII. The class switch to IgG4 has been described to depend on a type 2 helper CD4+ T-cell response.21 Growing evidence links IgG4 to interleukin-10-producing regulatory CD4+ T cells.22-24 The question arises as to which mechanisms cause the high affinity of FVIII-specific IgG4. Recently, Collins et al25 proposed a model of sequential IgG class switching in secondary germinal center responses, where primary memory B cells reenter a germinal center in a secondary response, undergo additional somatic hypermutations, and switch to a more downstream IgG subclass. Their model is based on data indicating that the number of somatic hypermutations in V(D)J gene sequences of the different IgG subclasses corresponds to the position of each constant region gene within the immunoglobulin heavy chain locus, that is IgG3 < IgG1 < IgG2 < IgG4.25 The number of somatic hypermutations is directly related to the number of cell divisions in germinal centers and correlates with affinity maturation of the B-cell receptor.26 This model could explain the higher affinity of FVIII-specific IgG4 compared with FVIII-specific IgG1 in patients with congenital hemophilia A and FVIII inhibitors. It would also predict that high-affinity FVIII-specific IgG1 is likely to occur prior to high-affinity FVIII-specific IgG4 in the course of the development of FVIII inhibitors.
Another question is whether the appearance of high-affinity antibodies against FVIII could provide a suitable biomarker for the early detection of evolving FVIII inhibitor responses. The retrospective analysis of longitudinal samples from a patient with severe hemophilia A indicated that high-affinity IgG1 and IgG4 were detectable as early as 543 days before the first detection of FVIII inhibitors. Analyzing cohorts of different patient populations, we previously demonstrated that FVIII-specific IgG4 was found in patients with FVIII inhibitors but not in patients without inhibitors or in healthy individuals.3 Therefore, we believe that even low titers of high-affinity FVIII-specific IgG4 might indicate an evolving FVIII inhibitor. If this hypothesis could be confirmed in a prospective clinical study, it might offer an opportunity for early immune intervention to prevent the clinical manifestation of FVIII inhibitors.
The presence of high-affinity FVIII-specific antibodies might also provide helpful information for monitoring patients with acquired hemophilia A, which is only diagnosed after the FVIII inhibitor has already been established. The presence or absence of high-affinity antibodies might be a suitable biomarker for assessing treatment efficacy. Our retrospective analysis of longitudinal samples illustrated that the presence of high-affinity antibodies in the absence of detectable FVIII inhibitors might be indicative of an early relapse associated with the reappearance of FVIII inhibitors.
The origin and biological significance of low-affinity antibodies against self-proteins such as FVIII as seen in some healthy individuals is still a matter of debate. Previously, Cohen27 suggested that autoantibodies present in healthy individuals are biomarkers of the immunologic homunculus, the body’s system to sense the current immunogenic states and ensure immune homeostasis. Considering the low affinity, we would expect that FVIII-specific antibodies in healthy individuals arise from T cell–independent immune pathways. Interestingly, we observed persistent low-affinity antibodies without any change in titer or affinity in a healthy individual whom we monitored for almost 3 years. Until recently, T cell–independent antibody responses were believed to result in only short-lived plasma cells lacking the generation of immunologic memory.5 However, it is becoming increasingly clear that T cell–independent antibody responses can generate both memory B cells and long-lived plasma cells.28-30 There is evidence that a specific subpopulation of B cells, termed B1b cells, can differentiate into memory B cells and long-lived plasma cells in a T cell–independent fashion. Additional signals, such as triggering of innate immune receptors, are assumed necessary to complement signals coming through B-cell receptors in order to provide sufficient stimulus for B1b cells to differentiate into memory B cells and long-lived plasma cells.29 Future studies will show whether B1b cells are responsible for producing persistent low-affinity antibodies against FVIII in healthy individuals.
In summary, we present the first comprehensive assessment of apparent affinities of FVIII-specific antibodies found in different cohorts of patients with congenital or acquired hemophilia A and in healthy individuals. We believe that the appearance or disappearance of high-affinity FVIII-specific antibodies might provide useful biomarkers for evolving or disappearing FVIII inhibitor responses. Prospective, longitudinal clinical studies are needed to provide further support for this hypothesis.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fatima Al-Awadi, Neriman Duman, Damir Fetahagic, Margit Pichler, and Michaela Schädler for excellent technical assistance, and Elise Langdon-Neuner for editing the manuscript.
This work was supported by Baxter BioScience.
Authorship
Contribution: C.J.H. and B.M.R. designed the research; C.J.H., S.F.J.W., M.H., P.A., F.M.H., G.S., and J.K. established validated assay formats; C.J.H., S.F.J.W., and M.H. performed experiments; C.J.H., S.F.J.W., M.H., P.A., F.M.H., G.S., J.K., and B.M.R. analyzed and interpreted data; J.-P.L. performed statistical modeling of apparent affinities; J.O., A.T., C.M., J.W., A.G., and P.N.K. provided patient samples and interpreted data; F.S. interpreted data; and C.J.H. and B.M.R. wrote the paper.
Conflict-of-interest disclosure: C.J.H., S.F.J.W., M.H., P.A., F.M.H., J.-P.L., G.S., J.K., F.S., and B.M.R. are employees of Baxter BioScience, which provided support for this study. The remaining authors declare no competing financial interests.
Correspondence: Birgit M. Reipert, Baxter BioScience, Industriestrasse 72, A-1220 Vienna, Austria; e-mail: birgit_reipert@baxter.com.

![Figure 1. Representative FVIII competition curves of FVIII-specific antibody samples used for affinity ELISA validation. Samples containing FVIII-specific antibodies were preincubated with different concentrations of FVIII in solution (FVIII [nM]) and subsequently tested for the presence of remaining free antibodies (ΔOD-blank). A nonlinear distribution of FVIII concentrations was used for optimal coverage of different competition behavior in individual samples. A monoclonal human FVIII-specific IgG1 antibody spiked into human plasma not containing any FVIII-binding antibodies (sample A; circles) requires lower FVIII concentrations for competition than a human plasma sample containing low-affinity FVIII-specific IgG1 antibodies (sample B; triangles). A mixture of samples A and B was used to mimic bimodal affinity distribution (sample C; diamonds).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/7/10.1182_blood-2014-09-598268/4/m_1180f1.jpeg?Expires=1769887727&Signature=iQfbIEb4K-RA7M6IOZAgwjsmjbk42O3RtS1hZhpEdDtwxfedkRbPP34ooMlwIJCA309wHYRCG0Ba8EmFWeYv4A~dbgpvxq-FWBvQl3m7akfDYpo4BQLP5fwrdKV~q96mhy~YnoUwB3lXV9AxndxHyBBcF2qGVOlOL4CF8CIBPLonafWzoaJnMpXWvhuBXqGOyj3URDrBPU7HK7mt3GVLhgPArpPF~QTCoxbRQqG0Y3VVBedcqtSnAxzxlD770EBnNmQOVESXs7AlaVyko~RKf7BADhSAJ0VE2TAZeKcUBs02xMlMndowTJsrHflhSPD72UQFqTsqIUkzu5IqcKyO7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Apparent affinities of FVIII-specific antibodies assessed for individual immunoglobulin isotypes and IgG subclasses. Presented are apparent affinities KA [M−1] of FVIII-specific antibodies found in the different study cohorts presented in Figure 2. All samples containing FVIII-specific antibodies with a titer ≥1:80 as presented in Figure 2 were included in the analysis. (A) Patients with severe hemophilia A and FVIII inhibitors (HA-INH). (B) Patients with severe hemophilia A without FVIII inhibitors (HA-noINH). (C) Patients with acquired hemophilia A (Acqu-HA). (D) Healthy individuals (Healthy). Some samples in each study cohort contained 2 populations of FVIII-specific antibodies with distinct apparent affinities. These 2 populations present in the same sample are indicated by an open circle and a closed circle connected by a straight line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/7/10.1182_blood-2014-09-598268/4/m_1180f3.jpeg?Expires=1769887727&Signature=4iXqux0BwBiQ1aRr0egsHQKyihkSmXp-C6t9ikcUt8anAQHecSSRR0w6MHjsSg6fhBq-qPLZulr7i9ZrPiuOuBdMYD~ffbK5GIn4PzedgH3Il6NZuayIsnzQrvokFGaJZeiJ8NPHQCNjjB9E~d8ppJ0MFy6OKoCpjgQa-G1XQPTSb6SLi8YLnK9KIhAvFg062a7brelKpyop4IEAt0KyDgB52qU5Hi2tHyu8JoelYFUqlUUV2vXem795tTATdJ1GznD4d0JnqmvJuUOCSJiX-syWi6qYahrResRvZww8JX6tDcc3HKw3VtMobXVxT739RxdyMcOBo0kHcgRWMT4vrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Longitudinal monitoring of antibodies against FVIII in a patient with congenital hemophilia A. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Antibody data for IgM, IgA, IgG2, and IgG3 were below the cutoff of the antibody assays and are, therefore, not shown. Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinity include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles] and IgG4 [open green diamonds]); and apparent affinities of FVIII-specific antibodies for low-affinity IgG1 clusters (open blue bars) and IgG4 clusters (open green bars) and for high-affinity IgG1 clusters (filled blue bars) and IgG4 clusters (filled green bars). The details of the medical history for this patient are included in the supplemental Data, Online Supplement 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/7/10.1182_blood-2014-09-598268/4/m_1180f4.jpeg?Expires=1769887727&Signature=q4fY7aKFNocT5gbdvFRz-8CRJF~TdMA8RnC176dEap6QLfq0V5LBCA4FCksMyT8vMjUyEDfhOkDgpgt266X3B3kntbxqMGKP-1go~hofU2Rzx20Cj2hqyUOfLOItdq64LWt3mclQKDmOXd5uIgGJz~YfIgwZ4AXaVuTtjOME5X3t4l2ingQ5tilkSHg1EmEGTHfvZuBwXRE4IJVl5Z4j93EiQm~yk6-Kf6nKbhqlapG9TUbj6f1gU6TbDHBOEsbdKASxfUOVlguKgYy4K1iir1vGbMEQw1o-hRp3L5E3YRTUk5hScK0ociCrAPbGZW39qWC59MFOZC-Y4UB3LVHj~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Longitudinal monitoring of antibodies against FVIII in a patient with acquired hemophilia A. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Antibody data for IgM, IgA, IgG2, and IgG3 were below the cutoff of the antibody assays and are, therefore, not shown. Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinity include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles] and IgG4 [open green diamonds]); and apparent affinities of FVIII-specific antibody clusters for IgG1 (filled blue bars) and IgG4 (filled green bars). The details of the medical history for this patient are included in the supplemental Data, Online Supplement 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/7/10.1182_blood-2014-09-598268/4/m_1180f5.jpeg?Expires=1769887727&Signature=NqTBrddmv61lb~DXydfGkzBF33yOraOGF-L-zVBlDSXTjc~mg-bdQsw7JkF7W9SlvZ1X03h6XyGeoSW~j5JT8yZ0TvDN1r0qr3--CW8wh~1MVwW8mZzHMrsWViuixIkACqz0rCH6ZPTPgVOOdaXjwn3xxlQ3ycXVQjCqbrVpZPOoXI6kd1G9QOPehG36XXXsGlVi7uRaMw7ZwDBMQpgrDPECmKaVXEycxqcrzVmPK5kHq96DdZGtoRuBlgIXwVq2l1vtbVERtfBlTj4fkHPwE2ijI-fXHANeTrDSdSVAkoP-rEcFxUiaSD5vKoFMKr5A~ArPYiBXrqXj1~s1OEKkSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Longitudinal monitoring of antibodies against FVIII present in a healthy individual. Presented are the results of a longitudinal monitoring of antibodies against FVIII, including titers of binding antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (A); Bethesda titers of FVIII inhibitors (A-B); and apparent affinities of antibodies, differentiated for individual immunoglobulin isotypes and IgG subclasses (B). Data for titers of antibodies against FVIII include the variation of the method (±1 titer step). Data for apparent affinities include the 95% CI. Presented are Bethesda titers of FVIII inhibitors (BU/mL; filled red diamonds); titers of antibodies against FVIII (IgG1 [open blue circles]); and apparent affinities of FVIII-specific antibody cluster (IgG1 [filled blue bars]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/7/10.1182_blood-2014-09-598268/4/m_1180f6.jpeg?Expires=1769887727&Signature=iD7Ls43oNPpSP6H~sZZAT~wNcUPlx2WK1WiCm6XINeN6f-~EvmGyvDP8X9r8t3nnWflsYfXAKQ2rMe2wpUt3VAXKoTIAAP0kBkTbijkThKyjuVw1culZz7lomqExAEQ4LX15hXboV1G3dLTZ7q27ibSyni5eQ1nzkBXe0pmLKmU2P5uO2NjCFUvJx63Ivc~6kqD-mF-1rjW98lAeo4b-oF7Rbn0le9knDf9WKMTOykH0tg~YS18aS9Jm8Tf1OIygziDA98-jmyGs368MNMBDr88AbC5FhPVt~QNwhJpMybU-JADRoPFw3zBhzLe5XkltnrrfGCTho4e71LvjYvXe0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal