Key Points
The c-Mpl activity in downstream signaling and in platelet homeostasis can be functionally separated.
The c-Mpl platelet homeostasis depends on correct processing and surface expression of the receptor, whereas downstream signaling does not.
Abstract
The interaction between thrombopoietin (THPO) and its receptor c-Mpl regulates downstream cytokine signaling and platelet homeostasis. Hereditary mutations of c-Mpl can either result in loss-of-function and thrombocytopenia or in gain-of-function and thrombocythemia (HT), and are important models to analyze the mechanism of c-Mpl activity. We have analyzed the effect of the c-Mpl P106L gain-of-function and the nearby loss-of-function R102P and F104S mutations, which cause HT or thrombocytopenia, respectively, on posttranslational processing, intracellular trafficking, cell surface expression, and cell proliferation. In contrast to R102P and F104S, the P106L mutant confers cytokine-independent growth and stimulates downstream signaling after THPO treatment in Ba/F3 cells. Despite their opposite function, R102P and P106L, both lead to abnormal subcellular receptor distribution, lack of membrane localization, impaired glycosylation, and elevated THPO serum levels in effected patients. These findings indicate that the activation of downstream signaling by c-Mpl P106L does not require correct processing, trafficking, and cell surface expression of c-Mpl, whereas the negative feedback loop controlling THPO serum levels requires cell surface expression of the receptor. Thus, we propose that the P106L mutation functionally separates the activity of c-Mpl in downstream signaling from that in maintaining platelet homeostasis.
Introduction
Platelet production is stimulated by the interaction of the cytokine thrombopoietin (THPO) with its receptor c-Mpl on megakaryocytes and their progenitors with subsequent activation of several downstream pathways, including the Janus kinase/signal transducer and activator of transcription pathway, the phosphatidylinositol 3-kinase pathway, and the mitogen-activated protein kinase pathway. The homeostasis of platelet numbers in the blood is maintained by a negative feedback loop, which requires the clearance of THPO from the plasma by c-Mpl–carrying megakaryocytes and platelets.1 In addition, disruption of the signaling pathway by inactivating THPO or c-Mpl mutations can cause serious thrombocytopenia.2-4 By contrast, activating THPO or c-Mpl mutations can cause hereditary thrombocythemia (HT).5-12 Interestingly, decreased expression of c-Mpl can also result in HT by high THPO levels, although the mechanism for this observation remains unknown.13-15
Genotype analyses in HT have previously identified the transmembrane S505N, the extracellular N35K, and the P106L c-Mpl mutations to occur in Japanese, African American and Arabic populations, respectively.5,9,12,16-18 Furthermore, the c-Mpl S505N mutation in the transmembrane region and the juxtamembrane c-Mpl W515L and W515K gain-of-function mutations have also been identified in patients with acquired diseases, such as essential thrombocytosis, myelofibrosis, and myeloproliferative diseases.19-22 Thus, these mutations identify regions of the receptor that are important for its regulated function, which suggests that overstimulation of the THPO pathway may predispose to clonal hematopoietic disease.22-24
Interestingly, c-Mpl mutations that result in loss of receptor function and have been located in the immediate vicinity of the activating c-Mpl P106L mutation in thrombocytopenia (R102P, F104S), which indicates that amino acids 102 to 106 within the first cytokine receptor homology module are critical for activity.25-28 THPO binding to CRM1 has been shown to initiate signal transduction.29,30 Deletion of this critical domain activates c-Mpl, suggesting an inhibitory function that can be relieved by ligand binding.31
Dimerization of the c-Mpl receptor is known to be essential for its function, although it is unknown if THPO is responsible for dimerization or if THPO binds preformed c-Mpl dimers on the cell surface and induces an activating conformational change of the dimer.30,32-38
Here, we have used rare c-Mpl mutations to gain functional insight into the mechanisms of c-Mpl/THPO activity and regulation. We describe the phenotype of a Qatari family with the Arabic P106L mutation and the functional analysis of this and other mutations in this critical area of the c-Mpl receptor.
Our work provides insights into the glycosylation, the subcellular localization and the homodimerization and heterodimerization of normal and mutated c-Mpl receptors. Specifically, this analysis shows that c-Mpl activation can occur before the receptor is fully glycosylated and transported to the plasma membrane, whereas THPO regulation and degradation requires plasma membrane localization of c-Mpl.
Materials and methods
Genotype analysis
Serum and EDTA blood samples of the patients and their family members were obtained after informed consent had been given. The 5′UTR, the 6 exons of the THPO gene and the coding regions and intron/exon junctions for all 12 exons of the c-Mpl gene were amplified by polymerase chain reaction and were sequenced (GATC Biotech AG, Germany). Primer sequences are available on request.
THPO serum levels
THPO levels from patient serum and Ba/F3 cell culture supernatants, respectively, were determined using a THPO enzyme-linked immunosorbent assay (Human Thrombopoietin Quantikine Kit; R&D Systems, Wiesbaden, Germany).
Plasmid constructs
The c-Mpl–minigene (kind gift of Professor Radek Skoda, Department of Biomedicine, Basel, Switzerland) was inserted between the Xho1 and the Not1 sites of the pCI-neo vector (Promega, Madison, WI). The details of the constructs are described in the supplemental data available on the Blood Web site.
Cell culture and transient transfection
HeLa cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin (P/S) at 37°C and 5% CO2. Cells were transiently transfected by calcium phosphate precipitation in 6-well plates using 3 µg of the test construct DNA.39 For cotransfection experiments, 1.5 µg of each construct was used. When constructs in pCI-neo were transfected, cotransfection of 0.3 µg of pEGFP-N1 DNA (Clontech) was used to control for transfection efficiency. Cells were washed with Tris-buffered saline after 20 hours and harvested 24 hours later.
Stable transfection of Ba/F3 cells
Ba/F3 cells (DSZM, Germany) were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal calf serum, 1% P/S, and 10 ng/mL IL-3 (R&D Systems), if not stated otherwise. The wild-type (WT) and mutant c-Mpl complementary DNA in pCI-neo were transfected into Ba/F3 cells by electroporation according to standard protocols. Stable individual clones were isolated through limiting dilution in 96-well plates and selection with neomycin (Geneticin, Invitrogen). Equal expression of the transfected constructs was confirmed in all clones by immunoblotting (supplemental Figure 4A). Clonal expression of the transfected mutant was validated and splice variants were identified by reverse transcription-polymerase chain reaction analysis spanning the complete coding sequence of the c-Mpl gene (supplemental Figure 4B-C).
Immunoblot analysis
For cytokine depletion, cells were centrifuged at 1000 g for 2 minutes, washed with phosphate-buffered saline and resuspended with Opti-minimum essential medium (MEM) medium (Invitrogen) with 1% P/S, but without addition of serum, IL-3, or neomycin. The details of lysate preparation and antibodies used are shown in the supplemental data.
Cell viability assay
These assays were performed as detailed in the supplemental data.
Immunocytochemistry
These analyses have been performed as detailed in the supplemental data.
Flow cytometry analysis
These analyses have been performed on HeLa cells that were transiently transfected with tagged c-Mpl constructs as detailed in the supplemental data.
Confocal microscopy
This analysis was performed according to the supplemental Methods detailed in the supplemental data.
Statistics
Data are reported as mean ± standard deviation and analyzed by 2-sided Student t test, paired or unpaired, as required.
Results
Clinical phenotype of the patient and mutation analysis
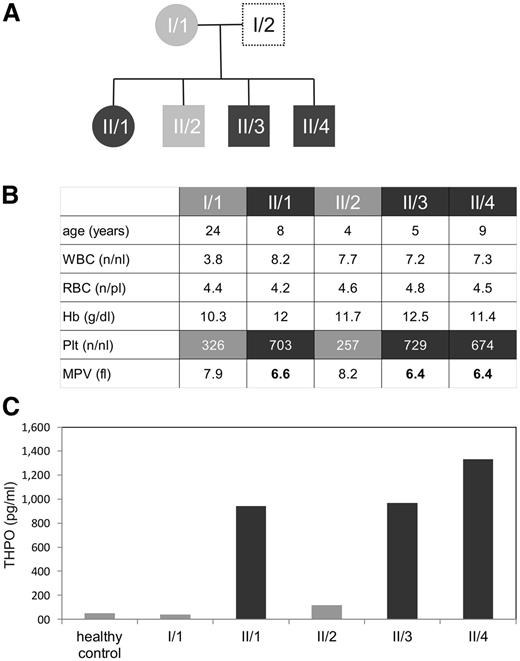
The index patient (II/1, Figure 1A) of Qatari origin was diagnosed with HT and platelet microcytosis (MPV 6.4 fl) in the context of an unrelated intercurrent illness. He was otherwise asymptomatic. The parents of II/1 were first-degree cousins, and family studies showed similar HT and low MPV in 2 brothers (II/3 and II/4), but normal platelet counts in the mother (I/1) and in the third brother (II/2) (Figure 1B). The father was not available for testing. Repetitive full blood counts were normal for all assessed family members (not shown). There was no history of thrombosis in the family.
Affected family members show elevated platelet counts, decreased platelet volume, and highly elevated THPO serum levels. (A) Family tree of the described patients. (B) Table showing age, complete blood counts white blood cells (WBC) (numbers per nanoliter), red blood cells (RBC) (numbers per picoliter), hemoglobin (Hb) (g/dL), platelet count (Plt), and mean platelet volume (MPV) (in fl) of the consanguineous family with HT. The numbers represent a single measurement, but are representative of serial blood test over several months. (C) Serum thrombopoietin levels of a healthy control and members of the described family were measured in pg/mL in serum using a THPO enzyme-linked immunosorbent assay, as described in “Materials and methods.”
Affected family members show elevated platelet counts, decreased platelet volume, and highly elevated THPO serum levels. (A) Family tree of the described patients. (B) Table showing age, complete blood counts white blood cells (WBC) (numbers per nanoliter), red blood cells (RBC) (numbers per picoliter), hemoglobin (Hb) (g/dL), platelet count (Plt), and mean platelet volume (MPV) (in fl) of the consanguineous family with HT. The numbers represent a single measurement, but are representative of serial blood test over several months. (C) Serum thrombopoietin levels of a healthy control and members of the described family were measured in pg/mL in serum using a THPO enzyme-linked immunosorbent assay, as described in “Materials and methods.”
Serum THPO measurements revealed strongly elevated concentrations in the family members with HT and normal (I/1) or marginally elevated (II/2) levels in the 2 family members with normal platelet counts (Figure 1C). Elevated THPO concentrations are known to occur in HT patients with THPO mutations. However, sequencing of the THPO coding region and the 5′UTR revealed no abnormalities. Subsequent sequencing of the c-Mpl gene showed a homozygous mutation at nucleotide 317 of the cDNA (c-Mpl c.317C>T), which leads to a proline to leucine mutation at position 106 in the affected individuals (p.Pro106Leu or P106L). The unaffected family members were heterozygous carriers of this mutation (supplemental Figure 1A). This mutation has been previously reported in Arabic patients with hereditary HT.9
The c-Mpl P106L show constitutive activity that can be further stimulated by THPO
The P106L mutation affects an extracellular domain of the c-Mpl receptor, which is a hotspot for both gain-of-function and loss-of-function mutations.9,25-28
Thus, this domain appears to be of critical physiological importance, which led us to perform detailed functional studies of the P106L gain-of-function and of mutations that have been reported to cause loss of receptor function but are located in close proximity.25-28
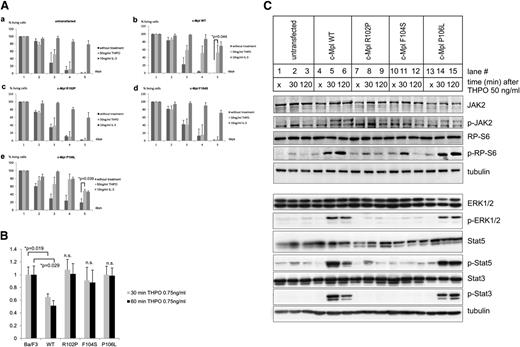
To this end, we designed c-Mpl constructs coding for the WT receptor, the loss-of-function mutations R102P and F104S, and the P106L gain-of-function mutation, and we stably expressed these genes in cytokine-dependent Ba/F3 cells. These cells depend on activated tyrosine kinases for survival and are widely used as a model to study the activation of downstream pathways of cytokine receptors.12,20,30,40 As the tyrosine kinase JAK2 is activated by c-Mpl, cell growth can be measured in a cell viability assay where untransfected Ba/F3 cells serve as controls. Cell growth of c-Mpl WT and P106L transfected Ba/F3 cells was analyzed after stimulation with increasing THPO concentrations from 1 ng to 50 ng/mL. The maximal stimulation was observed at 50 ng/mL THPO (supplemental Figure 3), which was therefore used for the following experiments.
As expected, Ba/F3 cells did not express c-Mpl and untransfected cells depended on IL-3, which could not be substituted by THPO (Figure 2Aa). By contrast, Ba/F3 cells responded to THPO when transfected with the WT c-Mpl gene, which indicated that the transfected construct directed the synthesis of a functional receptor and validated the experimental system (Figure 2Ab). Ba/F3 cells that were transfected with the R102P and F104S mutated c-Mpl constructs did not show any response to THPO (Figure 2Ac-d). This is consistent with the clinically evident loss-of-function phenotype of these mutations.4,25,26
The P106L mutation leads to ligand-independent growth and survival with maintained responsiveness to THPO, but defective THPO clearance. (A) Cell viability assay is shown using 0.4% Trypan blue solution. Depicted in panel A are (a) untransfected Ba/F3 cells and (b) Ba/F3 cells stably transfected with c-Mpl WT, (c) c-Mpl R102P, (d) c-Mpl F104S, (e) c-Mpl P106L, with and without the indicated treatment, respectively. Cells in log growth were plated at a density of 1.5 × 106/well in 6-well plates in serum and IL-3-free Opti-MEM media and assayed for the number of viable cells on 5 subsequent days. Cells left untreated in Opti-MEM served as a negative control and cells stimulated with an additional 20 ng/mL IL-3 to the Opti-MEM media were used as a positive control for maximal growth. Directly after serum and IL-3 depletion, 50 ng/mL THPO was added to the indicated constructs. Results and standard deviations represent the median of 3 independent experiments. (B) THPO uptake analysis is shown from Ba/F3 cell culture supernatants. Cells in the log phase of growth were plated at a density of 2 × 106 in 6-well plates in phenol-red-free RPMI 1640 media with 10% fetal calf serum. Four hours later, the cells were treated with THPO at a final concentration of 0.75 ng/mL. Thirty and 60 minutes after treatment, supernatants were harvested and assayed using a THPO enzyme-linked immunosorbent assay as described in “Materials and methods.” The results show the mean ± standard deviation of 3 independent experiments. (C) Immunoblot analysis is shown of the untransfected and stably transfected Ba/F3-cells 6 hours after IL-3 depletion and addition of serum-free Opti-MEM with 1% P/S. JAK2, RP-S6, ERK1/2, STAT5, STAT3, phospho-JAK2, phospho-RP-S6, phospho-ERK1/2, phospho-STAT5, and phospho-STAT3 were detected using the respective primary antibody as described in “Materials and methods.” Unstimulated cells after 6 hours IL-3 depletion in serum and IL-3-free media (lanes 1, 4, 7, 10, 13, indicated with an “×”) were compared with cells that were first IL-3 depleted for 6 hours and harvested after 30 (lanes 2, 5, 8, 11, and 14) and 120 minutes (lanes 3, 6, 9, 12, and 15) after THPO (50 ng/mL) stimulation, respectively. Equal loading was controlled with an antibody directed against tubulin for each protein preparation. Results are representative of 3 independent experiments. n.s., nonsignificant.
The P106L mutation leads to ligand-independent growth and survival with maintained responsiveness to THPO, but defective THPO clearance. (A) Cell viability assay is shown using 0.4% Trypan blue solution. Depicted in panel A are (a) untransfected Ba/F3 cells and (b) Ba/F3 cells stably transfected with c-Mpl WT, (c) c-Mpl R102P, (d) c-Mpl F104S, (e) c-Mpl P106L, with and without the indicated treatment, respectively. Cells in log growth were plated at a density of 1.5 × 106/well in 6-well plates in serum and IL-3-free Opti-MEM media and assayed for the number of viable cells on 5 subsequent days. Cells left untreated in Opti-MEM served as a negative control and cells stimulated with an additional 20 ng/mL IL-3 to the Opti-MEM media were used as a positive control for maximal growth. Directly after serum and IL-3 depletion, 50 ng/mL THPO was added to the indicated constructs. Results and standard deviations represent the median of 3 independent experiments. (B) THPO uptake analysis is shown from Ba/F3 cell culture supernatants. Cells in the log phase of growth were plated at a density of 2 × 106 in 6-well plates in phenol-red-free RPMI 1640 media with 10% fetal calf serum. Four hours later, the cells were treated with THPO at a final concentration of 0.75 ng/mL. Thirty and 60 minutes after treatment, supernatants were harvested and assayed using a THPO enzyme-linked immunosorbent assay as described in “Materials and methods.” The results show the mean ± standard deviation of 3 independent experiments. (C) Immunoblot analysis is shown of the untransfected and stably transfected Ba/F3-cells 6 hours after IL-3 depletion and addition of serum-free Opti-MEM with 1% P/S. JAK2, RP-S6, ERK1/2, STAT5, STAT3, phospho-JAK2, phospho-RP-S6, phospho-ERK1/2, phospho-STAT5, and phospho-STAT3 were detected using the respective primary antibody as described in “Materials and methods.” Unstimulated cells after 6 hours IL-3 depletion in serum and IL-3-free media (lanes 1, 4, 7, 10, 13, indicated with an “×”) were compared with cells that were first IL-3 depleted for 6 hours and harvested after 30 (lanes 2, 5, 8, 11, and 14) and 120 minutes (lanes 3, 6, 9, 12, and 15) after THPO (50 ng/mL) stimulation, respectively. Equal loading was controlled with an antibody directed against tubulin for each protein preparation. Results are representative of 3 independent experiments. n.s., nonsignificant.
Interestingly, Ba/F3 cells that were expressing the c-Mpl P106L mutant showed prolonged survival in the absence of IL-3 or THPO when compared with untransfected cells or untreated c-Mpl WT cells (Figure 2Ae). However, these cells could be further stimulated by THPO and IL-3. These data indicate that c-Mpl P106L confers constitutive, cytokine-independent activity with remaining response to THPO stimulation.
Because of the raised THPO concentrations in the patients’ serum, we hypothesized that the P106L mutant is incapable of THPO clearance, despite the maintained functional activity of the mutated c-Mpl receptor. Thus, we tested THPO clearance from cell culture supernatants of untransfected and stably transfected Ba/F3 cells, respectively, 30 and 60 minutes after stimulation with THPO. Whereas Ba/F3 cells transfected with c-Mpl WT showed significant clearance of THPO from the supernatant, the cells transfected with the loss-of-function mutants R102P and F104S and the gain-of-function mutant P106L did not show detectable THPO clearance (Figure 2B).
The stimulation of c-Mpl activates the downstream signaling pathway, which can be monitored by determining the phosphorylation status of JAK2, RP-S6, ERK1/2, STAT5, and STAT3 (Figure 2C). Untransfected Ba/F3 cells that were pretreated in serum-free and IL-3-free media showed no detectable phosphorylation of STAT3 and baseline phosphorylation of JAK2, RP-S6, ERK1/2, and STAT5. Ba/F3 cells transfected with the WT construct showed a similar pattern of inactive downstream signaling and the expected activation after THPO treatment. Cells transfected with the loss-of-function c-Mpl mutants R102P and F104S showed the expected lack of response to THPO (Figure 2C). The Ba/F3 cells expressing the P106L mutant showed no significant differences in phosphorylation of JAK2, RP-S6, ERK1/2, and STAT3, and STAT5 compared with the WT construct (Figure 2C, lanes 13-15). These data indicate that the effect on cell growth in P106L transfected cells (Figure 2Ae) cannot be explained by a major constitutive activation of these pathways. Potentially, the constitutive activity of the P106L mutant may be caused by subtle effects on downstream signaling, which cannot be detected by phosphoprotein analysis of signaling molecules. Alternatively, it cannot be excluded that unknown signaling pathways are activated by the P106L mutant.
Full glycosylation is not required for c-Mpl receptor function
It has been reported that the cell surface form of c-Mpl is specifically glycosylated and that the loss of this glycosylation results in the loss of c-Mpl function and platelet production.26 Thus, we hypothesized that the P106L mutant may be more strongly glycosylated than the WT. We tested this hypothesis by determining the glycosylation status of the WT, the R102P, the F104S, and the P106L mutants in transiently transfected HeLa cells with or without treatment with Tunicamycin, which blocks N-linked glycosylation of newly synthesized glycoproteins in the rough endoplasmic reticulum (ER) (Figure 3A). We used HeLa cells for these experiments because they show high protein expression, high levels of glycosylation and are adherent, in contrast to BA/F3 cells, and thus suitable for confocal microscopy. The WT showed the expected glycosylation of the c-Mpl receptor with a mature strongly glycosylated form at 85 kD and the immature weakly glycosylated form at 80 kD.26,41 Both glycosylation forms could be blocked by Tunicamycin (5 or 10 µg/mL, respectively) that led to a nonglycosylated receptor at 70 kD (Figure 3A). A similar glycosylation pattern has also been detected in our stably transfected Ba/F3 cells clones, although the completely glycosylated form of the c-Mpl receptor is less abundant in Ba/F3 than in HeLa cells (supplemental Figure 4A).
Glycosylation of c-Mpl WT and mutants. (A) Immunoblot analysis shown of transiently transfected HeLa cells that have been treated 24 hours before harvest with 5 µg/mL Tunicamycin (lanes 2, 5, 8, 11, and 14), 10 µg/mL Tunicamycin (lanes 3, 6, 9, 12, and 15), or without Tunicamycin (lanes 1, 4, 7, 10, and 13), respectively. Untransfected HeLa cells served as controls (lanes 1-3). The c-Mpl was detected using a primary antibody against c-Mpl. Equal loading was controlled by an antibody directed against actin. Separating lines indicate positions in the blot, whereas the order of lanes was changed using Adobe Photoshop Creative Suite 2 for better understanding (original order was: WT, 106, 102, and 104). (B) The analysis of THPO influence on c-Mpl glycosylation is shown. Immunoblot analysis of transiently transfected HeLa cells. Untransfected HeLa cells served as controls. THPO was added 6 hours before harvest at a concentration of 50 mg/mL (lanes 6-10). The c-Mpl was detected using a primary antibody against c-Mpl. Equal loading was controlled by an antibody directed against tubulin.
Glycosylation of c-Mpl WT and mutants. (A) Immunoblot analysis shown of transiently transfected HeLa cells that have been treated 24 hours before harvest with 5 µg/mL Tunicamycin (lanes 2, 5, 8, 11, and 14), 10 µg/mL Tunicamycin (lanes 3, 6, 9, 12, and 15), or without Tunicamycin (lanes 1, 4, 7, 10, and 13), respectively. Untransfected HeLa cells served as controls (lanes 1-3). The c-Mpl was detected using a primary antibody against c-Mpl. Equal loading was controlled by an antibody directed against actin. Separating lines indicate positions in the blot, whereas the order of lanes was changed using Adobe Photoshop Creative Suite 2 for better understanding (original order was: WT, 106, 102, and 104). (B) The analysis of THPO influence on c-Mpl glycosylation is shown. Immunoblot analysis of transiently transfected HeLa cells. Untransfected HeLa cells served as controls. THPO was added 6 hours before harvest at a concentration of 50 mg/mL (lanes 6-10). The c-Mpl was detected using a primary antibody against c-Mpl. Equal loading was controlled by an antibody directed against tubulin.
The loss-of-function mutation R102P showed incomplete glycosylation of the receptor, which could also be blocked by Tunicamycin.26 This finding is compatible with adequate glycosylation being required for function. The F104S loss-of-function mutation displayed a similar glycosylation pattern as the WT indicating that the mechanism of loss-of-function in this mutant does not work through an alteration of glycosylation and that normal glycosylation is not sufficient for normal function.
Surprisingly, the glycosylation pattern of the receptor with the P106L gain-of-function mutation resembles that of the receptor with the R102P loss-of-function mutation, thus indicating that normal glycosylation is not required for function, although it may be needed for normal regulation.
After stimulation with THPO (Figure 3B), neither c-Mpl WT nor c-Mpl F104S showed a shift between incomplete and full glycosylation, nor did this treatment lead to the occurrence of a fully glycosylated band in the loss-of-function R102P or the gain-of-function P106L mutation. Furthermore, these data show that a reduced glycosylation capacity of the receptor does not necessarily induce loss-of-function, but this can even be associated with gain-of-function.
Subcellular distribution does not correlate with function of R102P, F104S, and P106L mutated c-Mpl receptors
The function of cytokine receptors typically depends on the maturation and transport of receptors through the rough ER and the Golgi apparatus. Therefore, we analyzed the subcellular distribution of the WT and the mutated c-Mpl receptors fused to a C-terminal GFP by confocal microscopy in adherent, transiently transfected HeLa cells.
FLAG-tagged versions of WT and P106L mutated receptors showed equal distribution patterns as the GFP-tagged versions, indicating that the physiological distribution of the receptor is not affected by the fusion with GFP (Figure 4A).
Different subcellular distribution and Golgi localization of c-Mpl WT and mutants. (A) Representative confocal images of transiently transfected HeLa cells. The c-Mpl-GFP minigenes harboring the green fluorescent protein (GFP) expression sequence in frame at the C-terminus were transfected as follows: (Aa-Ac) c-Mpl-WT-GFP, (Ad-Af) c-Mpl-R102P-GFP, (Ag-Ai) c-Mpl-F104S-GFP, and (Aj-Al) c-Mpl-P106L-GFP. The Golgi complex was visualized by a primary mouse antibody against of the Golgi-resident protein GM-130 and a secondary anti-mouse antibody coupled to Alexa 568 (Figure 4A, middle panels Ab, Ae, Ah, and Ak). The panels on the right (Ac, Af, Ai, and Al) represent overlays with the c-Mpl mutants and the Golgi marker. Interfering influence of the GFP-fusion protein on the subcellular distribution was excluded by FLAG-tagged (Am) c-Mpl WT-FLAG and (An) c-Mpl P106L-FLAG constructs harboring the FLAG-Tag at the C-terminus. The FLAG-tag was visualized by a mouse anti-FLAG M2 antibody and a secondary anti-mouse antibody coupled to Alexa 568 as described in “Materials and methods.” The nucleus was visualized by 4,6 diamidino-2-phenylindole staining. Bars in the overlay pictures represent 10 µm. Images were acquired using a Perkin Elmer spinning-disc confocal microscope with a ×100 Nikon oil immersion objective. Cells depicted are representative of the subcellular distribution pattern seen in >90% of the transfected cells. (B). Pearson’s correlation coefficient for the cotransfection experiments described in (Aa-Al) (n = 5). The coefficient was acquired from the Volocity 5.5 software using the automatic threshold function. The complete depicted panel was defined as region of interest (ROI) for the measurements.
Different subcellular distribution and Golgi localization of c-Mpl WT and mutants. (A) Representative confocal images of transiently transfected HeLa cells. The c-Mpl-GFP minigenes harboring the green fluorescent protein (GFP) expression sequence in frame at the C-terminus were transfected as follows: (Aa-Ac) c-Mpl-WT-GFP, (Ad-Af) c-Mpl-R102P-GFP, (Ag-Ai) c-Mpl-F104S-GFP, and (Aj-Al) c-Mpl-P106L-GFP. The Golgi complex was visualized by a primary mouse antibody against of the Golgi-resident protein GM-130 and a secondary anti-mouse antibody coupled to Alexa 568 (Figure 4A, middle panels Ab, Ae, Ah, and Ak). The panels on the right (Ac, Af, Ai, and Al) represent overlays with the c-Mpl mutants and the Golgi marker. Interfering influence of the GFP-fusion protein on the subcellular distribution was excluded by FLAG-tagged (Am) c-Mpl WT-FLAG and (An) c-Mpl P106L-FLAG constructs harboring the FLAG-Tag at the C-terminus. The FLAG-tag was visualized by a mouse anti-FLAG M2 antibody and a secondary anti-mouse antibody coupled to Alexa 568 as described in “Materials and methods.” The nucleus was visualized by 4,6 diamidino-2-phenylindole staining. Bars in the overlay pictures represent 10 µm. Images were acquired using a Perkin Elmer spinning-disc confocal microscope with a ×100 Nikon oil immersion objective. Cells depicted are representative of the subcellular distribution pattern seen in >90% of the transfected cells. (B). Pearson’s correlation coefficient for the cotransfection experiments described in (Aa-Al) (n = 5). The coefficient was acquired from the Volocity 5.5 software using the automatic threshold function. The complete depicted panel was defined as region of interest (ROI) for the measurements.
Colocalization with the Golgi-marker GM-130 was detectable for the WT and the normally glycosylated F104S mutant, which served as positive controls (Figure 4A-B). The R102P loss-of-function and the P106L gain-of-function mutant receptors, which are characterized by their incomplete glycosylation pattern (see above), did not colocalize with the cis-Golgi-marker, suggesting that subcellular transport was arrested at the rough ER. This was validated by colocalization with the ER-marker calnexin (supplemental Figure 2).
These results indicate that incomplete glycosylation correlates with abnormal subcellular distribution. However, neither the glycosylation pattern nor the subcellular distribution predicts the effect of the mutation on receptor activity.
Receptor function can be independent of cell surface localization
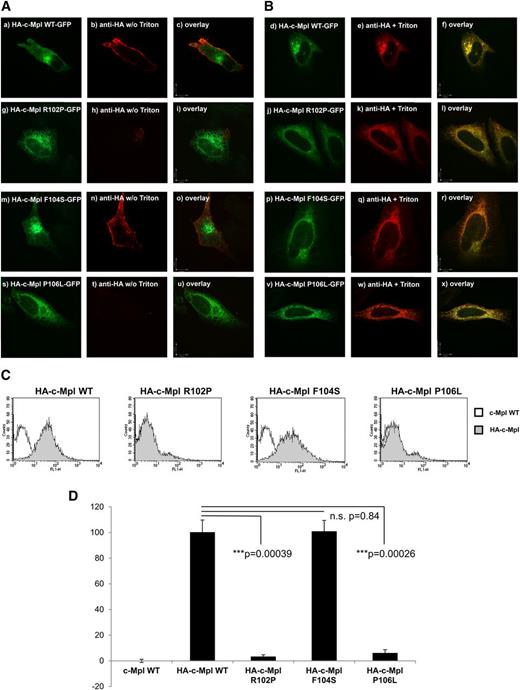
The function of cytokine receptors typically depends on their expression on the cell surface where the interaction with the ligand occurs. Thus, we generated constructs with an extracellular human influenza hemagglutinin (HA)-tag just C-terminally to the secretory leader of the c-Mpl WT, the R102P, the F104S, and the P106L mutant constructs and expressed these constructs in HeLa cells (Figure 5). Nonpermeabilized cells (Figure 5A) were compared with cells that were permeabilized with Triton X-100 (Figure 5B).
Cell surface localization of c-Mpl WT and mutants. (A-B) Representative confocal images of HeLa cells transiently transfected with (Aa-Ac, Bd-Bf) the HA-c-Mpl WT-GFP, (Ag-Ai, Bj-Bl) the HA-c-Mpl R102P-GFP, (Am-Ao, Bp-Br) the HA-c-Mpl F104S-GFP, and (As-Au, Bv-Bx) the HA-c-Mpl P106L-GFP receptor constructs, respectively. (A) An extracellular HA-tag introduced behind the secretory leader of the c-Mpl receptor 3′ of codon 26 was used to detect plasma membrane expression without Triton X-100 permeabilization (Ab, Ah, An, and At). (B) Cells permeabilized with 0.1% Triton X-100 served as a control for the accessibility of the HA-tag and the identical distribution of the constructs harboring the HA-tag (Be, Bk, Bq, and Bw). The HA-tag was visualized with a mouse anti-HA primary antibody and an anti-mouse secondary antibody linked to Alexa 568 as described in “Materials and methods.” Images were acquired using a Perkin Elmer spinning-disc confocal microscope with a ×100 Nikon oil immersion objective. Bars in the overlay pictures represent 10 µm. Cells depicted represent the subcellular distribution pattern seen in >90% of the transfected cells. (C-D) Fluorescent cell sorting of transiently transfected HeLa cells is shown. The c-Mpl WT, R102P, F104S, and P106L constructs harbored the HA-tag at the N-terminus behind the secretory leader. The mCherry plasmid was cotransfected as a control for transfection efficiency. After the harvest, cells were incubated with anti-HA primary antibody followed by secondary anti-mouse IgG conjugated to AlexaFluor 488. Cells transfected with c-Mpl WT served as negative controls. Cells were gated for positive mCherry signal and analyzed as described in “Materials and methods.” (D) Results and standard deviations represent the median of 3 independent experiments. For quantification, HA-c-Mpl WT surface expression was set as 1.0. n.s., nonsignificant.
Cell surface localization of c-Mpl WT and mutants. (A-B) Representative confocal images of HeLa cells transiently transfected with (Aa-Ac, Bd-Bf) the HA-c-Mpl WT-GFP, (Ag-Ai, Bj-Bl) the HA-c-Mpl R102P-GFP, (Am-Ao, Bp-Br) the HA-c-Mpl F104S-GFP, and (As-Au, Bv-Bx) the HA-c-Mpl P106L-GFP receptor constructs, respectively. (A) An extracellular HA-tag introduced behind the secretory leader of the c-Mpl receptor 3′ of codon 26 was used to detect plasma membrane expression without Triton X-100 permeabilization (Ab, Ah, An, and At). (B) Cells permeabilized with 0.1% Triton X-100 served as a control for the accessibility of the HA-tag and the identical distribution of the constructs harboring the HA-tag (Be, Bk, Bq, and Bw). The HA-tag was visualized with a mouse anti-HA primary antibody and an anti-mouse secondary antibody linked to Alexa 568 as described in “Materials and methods.” Images were acquired using a Perkin Elmer spinning-disc confocal microscope with a ×100 Nikon oil immersion objective. Bars in the overlay pictures represent 10 µm. Cells depicted represent the subcellular distribution pattern seen in >90% of the transfected cells. (C-D) Fluorescent cell sorting of transiently transfected HeLa cells is shown. The c-Mpl WT, R102P, F104S, and P106L constructs harbored the HA-tag at the N-terminus behind the secretory leader. The mCherry plasmid was cotransfected as a control for transfection efficiency. After the harvest, cells were incubated with anti-HA primary antibody followed by secondary anti-mouse IgG conjugated to AlexaFluor 488. Cells transfected with c-Mpl WT served as negative controls. Cells were gated for positive mCherry signal and analyzed as described in “Materials and methods.” (D) Results and standard deviations represent the median of 3 independent experiments. For quantification, HA-c-Mpl WT surface expression was set as 1.0. n.s., nonsignificant.
Permeabilized cells served as a positive control for accessibility of the HA-tag of all constructs (Figure 5B). The c-Mpl WT-positive control showed the expected localization on the cell surface in nonpermeabilized cells (Figure 5A). The c-Mpl R102P mutant was used as a negative control because this mutant is known not to be expressed on the cell surface.26 The loss-of-function mutation F104S, which showed a similar glycosylation pattern as the WT, also showed localization on the cell surface (Figure 5An). Surprisingly, however, the P106L gain-of-function mutation did not show any detectable plasma membrane localization in our confocal images (Figure 5At). In order to quantify the surface expression of the c-Mpl mutations analyzed here, we performed flow cytometry analyses (Figure 5C-D). Consistent with the previously published finding of nondetectable c-Mpl P106L mutant receptors on the platelet surface in another family with the c-Mpl P106L mutation and HT, we also observed a lack of detectable c-Mpl P106L surface localization in our cell culture model.9
Taken together, these data indicate that maturation of the receptor in the Golgi apparatus and complete glycosylation correlate with transport to the cell surface. Unexpectedly, however, data obtained with the c-Mpl P106L gain-of-function mutant indicate that the functionality of the receptor, including its response to THPO, does not depend on cell surface localization.
Mutated c-Mpl receptors interact differentially with normal c-Mpl
Considering the recessive mode of inheritance of the c-Mpl mutations, next we tested the hypothesis that the presence of a normal receptor may rescue proper receptor maturation and function. Thus, we coexpressed WT-Mpl and mutant c-Mpl genes carrying C-terminal tags that allowed us to monitor the subcellular distribution of the monomers and the formation of c-Mpl receptor dimers, respectively.
The c-Mpl R102P mutant receptor that was abnormally processed and restricted to the rough ER without Golgi localization (Figure 4Ad-f and supplemental Figure 2) was rescued into the Golgi apparatus by coexpression of the WT construct, and both monomers showed colocalization in the Golgi (Figure 6A-B). Therefore, the R102P mutant may be able to form heterodimers in the rough ER with the WT, which may explain the recessive mode of inheritance of this mutant. Despite the rescue into the Golgi apparatus, WT receptors were unable to rescue R102P mutant receptors into the plasma membrane, as extracellular HA-staining of cells cotransfected with equal amounts of HA-c-Mpl R102P and c-Mpl-WT-GFP constructs did not lead to detection of R102P constructs in the plasma membrane in nonpermeabilized cells (supplemental Figure 1B). Colocalization was observed for the F104S mutant with WT (Figure 4A-B), but not for R102P with F104S mutants (Figure 6A-B).
The c-MPL F104S, but not the P106L-mutated receptors heterodimerize with the WT receptor. (A) Representative confocal images of transiently cotransfected HeLa cells. The (Aa, Ad, and Aj) c-Mpl WT-GFP or (Ag) c-Mpl R102P-GFP, respectively, harboring a GFP expression sequence in frame at the C-terminus and (Ab) c-Mpl R102P-FLAG, (Ae and Ah) F104S-FLAG, or (Ak) P106L-FLAG mutated constructs with a FLAG-tag sequence in frame at the C-terminus were cotransfected with equal amounts of DNA and fixed 48 hours later. The FLAG-tag was visualized with a mouse anti-FLAG M2 primary antibody and an anti-mouse secondary antibody linked to Alexa 568 as described in “Materials and methods.” (Aa, Ab, and Ac) Cotransfection of c-Mpl WT-GFP and c-Mpl R102P-FLAG. (Ad, Ae, and Af) Cotransfection of c-Mpl WT-GFP and c-Mpl F104S-FLAG. (Ag, Ah, and Ai) Cotransfection of c-Mpl R102P-GFP and c-Mpl F104S-FLAG. (Aj, Ak, and Al) Cotransfection of c-Mpl WT-GFP and c-Mpl P106L-FLAG. Panels Ac, Af, Ai, and Al represent the respective overlays of the cotransfection experiments. Images were acquired using a spinning-disc confocal microscope with a ×100 oil immersion objective. Bars represent 10 µm. Cells depicted represent the subcellular distribution pattern seen in >90% of the transfected cells. (B) Pearson’s correlation coefficient of the cotransfection experiments described in (A) (n = 5). The coefficient was acquired with the Volocity 5.5 software using the automatic threshold function. The ROI was defined as a 10 × 10-µm square with the Golgi set as the middle for the ROI. *P = .013; ****P < .0001.
The c-MPL F104S, but not the P106L-mutated receptors heterodimerize with the WT receptor. (A) Representative confocal images of transiently cotransfected HeLa cells. The (Aa, Ad, and Aj) c-Mpl WT-GFP or (Ag) c-Mpl R102P-GFP, respectively, harboring a GFP expression sequence in frame at the C-terminus and (Ab) c-Mpl R102P-FLAG, (Ae and Ah) F104S-FLAG, or (Ak) P106L-FLAG mutated constructs with a FLAG-tag sequence in frame at the C-terminus were cotransfected with equal amounts of DNA and fixed 48 hours later. The FLAG-tag was visualized with a mouse anti-FLAG M2 primary antibody and an anti-mouse secondary antibody linked to Alexa 568 as described in “Materials and methods.” (Aa, Ab, and Ac) Cotransfection of c-Mpl WT-GFP and c-Mpl R102P-FLAG. (Ad, Ae, and Af) Cotransfection of c-Mpl WT-GFP and c-Mpl F104S-FLAG. (Ag, Ah, and Ai) Cotransfection of c-Mpl R102P-GFP and c-Mpl F104S-FLAG. (Aj, Ak, and Al) Cotransfection of c-Mpl WT-GFP and c-Mpl P106L-FLAG. Panels Ac, Af, Ai, and Al represent the respective overlays of the cotransfection experiments. Images were acquired using a spinning-disc confocal microscope with a ×100 oil immersion objective. Bars represent 10 µm. Cells depicted represent the subcellular distribution pattern seen in >90% of the transfected cells. (B) Pearson’s correlation coefficient of the cotransfection experiments described in (A) (n = 5). The coefficient was acquired with the Volocity 5.5 software using the automatic threshold function. The ROI was defined as a 10 × 10-µm square with the Golgi set as the middle for the ROI. *P = .013; ****P < .0001.
Coexpression of the WT-c-Mpl-GFP construct did not alter the subcellular distribution of the P106L-FLAG receptor (Figure 6A-B). These results indicate that coexpression of the P106L mutant and WT-receptors do not interfere with each other’s abnormal and normal processing, respectively, and that the P106L mutant and the WT monomers are not likely to form heterodimers.
Discussion
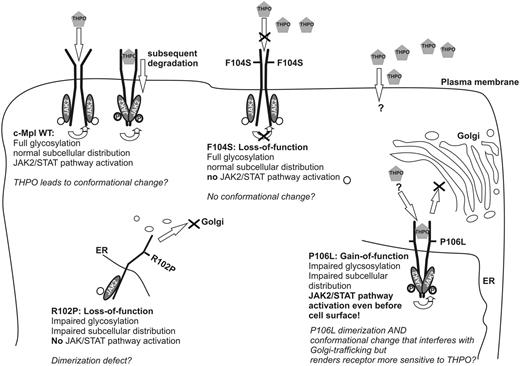
Our detailed functional analysis of rare mutations of the c-Mpl receptor, which either result in loss-of-function and thrombocytopenia or in gain-of-function and HT, allow the surprising conclusion that the functionality of this receptor neither absolutely depends on its normal glycosylation nor on its expression on the cell surface. It remains an open question of how THPO interacts with the abnormally processed receptor that is not detectably expressed on the cell surface of our model system. Notably, the lack of c-Mpl expression and raised THPO levels has also been previously reported on platelets and in serum, respectively, of patients with the P106L mutation.9 The observations in this family thus confirm the validity of our experimental system. In principle, this observation can be explained by 2 alternative models: the P106L receptor may be localized at the cell surface at such a low level that it cannot be detected by fluorescence-activated cell sorter and confocal microscopy. In this scenario, the trace abundance of c-Mpl P106L on the cell surface would not be sufficient for THPO clearance, but these receptor molecules would be highly activated by the markedly elevated THPO levels. Such a model would also be consistent with the previously published data in mouse models showing that low level c-Mpl expression can cause HT.13-15 Alternatively, THPO may be incorporated into the cells by THPO-independent routes, although it is unknown if THPO can bind to other receptors.
Spontaneous or ligand-induced dimerization of c-Mpl is a necessary precondition for c-Mpl receptor activation.30,32-38 The complementation analysis of mutant and WT c-Mpl receptors suggest that dimerization may already occur during the early maturation process of the receptor through the rough ER and Golgi apparatus, which is compatible with similar observations for the EPO and the growth hormone receptors. Such a pathway of receptor maturation would also explain the rescue of the Golgi expression of the nonfunctional R102P mutant into the Golgi apparatus by coexpression of the WT receptor.2,34,42-44 With such a mechanism, the P106L-mutated receptor could form a functionally active homodimer, whose activity would not depend on cell surface expression or on rescue by coexpression of the WT receptor. Indeed, such an intracellular dimeric receptor may be active and particularly sensitive to the low level THPO that may enter the cell through trace levels of the receptor on the cell surface, as discussed above, or through unknown alternative routes (Figure 7). Although the proposed premature dimerization of the c-Mpl P106L mutant receptor hinders ER to Golgi trafficking and proper glycosylation and sorting, the downstream signaling pathway can be activated by THPO in a manner comparable to the properly transported and glycosylated WT receptors. Nevertheless, the ability of binding, internalization and clearance of THPO is affected by the lack of detectable cell surface localization. THPO was neither cleared from cell culture supernatants nor from serum in homozygous patients.9 In heterozygous carriers, the THPO plasma levels are within or just slightly above the normal range. Hence, the functional WT receptors are sufficient to clear THPO from the plasma of these heterozygous individuals, which results in normal platelet counts. By contrast, this physiological negative feedback loop is lost in c-Mpl P106L homozygotes and the raised THPO concentrations stimulate the activity of intracellular P106L homodimers. Therefore, we propose that this mutation separates the function of the mature receptor on downstream signaling from that in maintaining the THPO negative feedback loop that controls platelet numbers in the blood.
Working model. Proposed model of subcellular distribution, THPO-binding, dimerization, conformational change, and JAK2/signal transducer and activator of transcription of c-Mpl WT and R102P, F104S, and P106L mutants.
Working model. Proposed model of subcellular distribution, THPO-binding, dimerization, conformational change, and JAK2/signal transducer and activator of transcription of c-Mpl WT and R102P, F104S, and P106L mutants.
Stable dimerization and ligand-induced or autonomous conformational changes may both be prerequisites for c-Mpl receptor activation.2,35 Several THPO agonists and minibodies that enhance dimerization of the receptor molecules, even before they reach the cell surface, have been recently developed.34,45,46 Distinguishing substances that target the THPO-binding site and exert their effect by facilitating the ligand-induced conformational change from substances that enhance receptor dimerization may be helpful in predicting therapeutic success for different loss-of-function mutations.26,47 For example, R102P could not be stimulated by THPO or THPO-mimicking small molecules that promote signaling of WT c-Mpl, but by a dimerization-inducing minibody.26,48 Consistent with this, c-Mpl R102P receptors did not activate the downstream pathway and mutant receptors were weakly glycosylated and showed an impaired surface localization in our analysis. Because both the loss-of-function mutation R102P and the gain-of-function mutation P106L showed impaired glycosylation and abnormal subcellular distribution, the lack of surface localization does not explain the loss-of-function mutation as R102P. Thus, we hypothesize that R102P has a defect in dimerization with a consecutive altered subcellular distribution and glycosylation.
This study confirms a previous report showing that the F104S homodimeric receptor cannot be activated by THPO.47 This mutation is also likely not to form functional R102P/F104S receptor heterodimers because the F104S mutation does not protect compound heterozygous R102P/F104S patients from developing severe thrombocytopenia.25
Overstimulation of the THPO pathway through either c-Mpl or THPO gain-of-function mutations are thought to contribute to chronic myeloproliferative disorders in patients who are negative for the JAK2 V617F and the CALR mutations.19-21,49-56 The P106L mutation causing familiar HT may have a similar oncogenic potential, although myeloproliferative disorders have not (yet) developed in the families described here or previously.9
In conclusion, our study uncovers that correct glycosylation, subcellular transport, distribution, and cell surface localization are not required for active c-Mpl downstream signaling, but that cell surface localization is required for controlling THPO levels and platelet homeostasis.
We hypothesize that the codons 102 to 106 in the first cytokine receptor homology module play an important role in the dimerization and conformational change of the c-Mpl receptor. Mutations in this area can lead to both gain-of-function and loss-of-function of the c-Mpl receptor. Our findings may represent a therapeutic target for the further development of plasma membrane permeable dimerization-inducing agents that are different from THPO-mimicking agents that require cell surface localization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sven Danckwardt for most helpful discussions, R. Skoda, Basel, Switzerland for the c-Mpl complementary DNA gift, Dr Naima Ali Al Mulla for the patient samples provided by the Department of Pediatrics, Hamad General Hospital, Doha, Qatar, and the Nikon Imaging Center at the University of Heidelberg for providing their excellent facility for imaging.
Authorship
Contribution: C.S., A.-C.K., N.D., I.N.K., C.M.D., C.K., and A.E.K. provided data; C.S., A.-C.K., N.D., I.N.K., C.M.D., and C.K. analyzed the results; C.S., N.H.G., and A.E.K. analyzed data; and C.S. and A.E.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.H.G. is Department of Genetics, University of Cologne, Zuelpicher St 47b, Köln, 50674 Germany.
Correspondence: Andreas E. Kulozik, Im Neuenheimer Feld 430, Heidelberg, D-69120 Germany; e-mail: andreas.kulozik@med.uni-heidelberg.de.