Key Points
P-Rex and Vav Rac-GEFs cooperate in leukocyte recruitment during inflammation by facilitating leukocyte adhesion to the vascular endothelium.
P-Rex/Vav expression in platelets is required for vascular adhesion and recruitment of neutrophils and eosinophils into lung tissue.
Abstract
The small GTPase Rac is required for neutrophil recruitment during inflammation, but its guanine-nucleotide exchange factor (GEF) activators seem dispensable for this process, which led us to investigate the possibility of cooperation between Rac-GEF families. Thioglycollate-induced neutrophil recruitment into the peritoneum was more severely impaired in P-Rex1−/− Vav1−/− (P1V1) or P-Rex1−/− Vav3−/− (P1V3) mice than in P-Rex null or Vav null mice, suggesting cooperation between P-Rex and Vav Rac-GEFs in this process. Neutrophil transmigration and airway infiltration were all but lost in P1V1 and P1V3 mice during lipopolysaccharide (LPS)-induced pulmonary inflammation, with altered intercellular adhesion molecule 1-dependent slow neutrophil rolling and strongly reduced L- and E-selectin–dependent adhesion in airway postcapillary venules. Analysis of adhesion molecule expression, neutrophil adhesion, spreading, and migration suggested that these defects were only partially neutrophil-intrinsic and were not obviously involving vascular endothelial cells. Instead, P1V1 and P1V3 platelets recapitulated the impairment of LPS-induced intravascular neutrophil adhesion and recruitment, showing P-Rex and Vav expression in platelets to be crucial. Similarly, during ovalbumin-induced allergic inflammation, pulmonary recruitment of P1V1 and P1V3 eosinophils, monocytes, and lymphocytes was compromised in a platelet-dependent manner, and airway inflammation was essentially abolished, resulting in improved airway responsiveness. Therefore, platelet P-Rex and Vav family Rac-GEFs play important proinflammatory roles in leukocyte recruitment.
Introduction
During inflammation, neutrophils are rapidly recruited from the bloodstream into inflamed tissues where they mount proinflammatory and antimicrobial responses.1 Recruitment occurs in a cascade of steps, beginning with the upregulation of P-selectin on the surface of endothelial cells that line postcapillary venules. P-selectin captures neutrophils from the bloodstream by engaging P-selectin glycoprotein ligand 1 (PSGL1) on their surface, enabling them to roll along the intraluminal wall. When captured, L-selectin on the neutrophil surface engages endothelial PSGL1 to support rolling, and endothelial E-selectin engages neutrophil PSGL1, among other counterligands, to slow rolling down. Binding of the neutrophil integrins LFA1 and Mac1 to their endothelial ligand intercellular adhesion molecule 1 (ICAM1) confers firm adhesion, and Mac1 enables the cells to crawl along the vessel wall before they actively transmigrate into the inflamed tissue by para- or transcellular routes.2 This recruitment cascade has largely been elucidated in the inflamed cremaster muscle and mesenteric circulation, but the importance of individual selectins and integrins varies between organs.2 For example, murine β2-integrin deficiency impairs neutrophil recruitment to the skin, but not the peritoneum or lung,3 and P-selectin/ICAM1 deficiency abolishes peritoneal, but not pulmonary recruitment.4 The mechanisms governing pulmonary leukocyte recruitment are particularly ill understood,2 but new intravital techniques to monitor airways and alveoli have emerged5-7 that enable such studies.
The small G protein Rac is essential for actomyosin cytoskeletal dynamics, and thus controls cell adhesion and migration.8 Rac-dependent human neutrophil immunodeficiency syndrome, characterized by severe recurring bacterial infections and poor wound healing, is caused by an inactivating Rac2 mutation leading to impaired integrin-dependent neutrophil adhesion, L-selectin–dependent rolling, and chemotaxis.9,10 In mouse neutrophils, Rac1 confers directional migration,11 Rac2 F-actin formation, integrin-dependent spreading, L-selectin–dependent rolling, and migration,12,13 and the related isoform RhoG confers full polarization.14 Conditional Rac1-deficiency impairs recruitment during sterile peritonitis15 or acute fMLP-induced lung inflammation,16 Rac2−/− mice show reduced recruitment during sterile peritonitis13 or immune-complex–induced acute lung injury,17 and combined Rac1/Rac2-deficiency abolishes recruitment during acute lung inflammation18 and delays accumulation in synovial fluid during infection-triggered arthritis and disease development.19
Rac can be activated by at least 20 different guanine-nucleotide exchange factors (GEFs). Differential tissue distribution and coupling to upstream pathways largely determines the GEFs that activate Rac within a given situation.20 Two Dbl-type Rac-GEF families, P-Rex and Vav, activate Rac in mouse neutrophils. Among the P-Rex family, P-Rex1, but not P-Rex2, is expressed,21,22 and among the Vav family, Vav3 is fourfold more abundant than Vav1 and 120-fold more than Vav2.23 P-Rex1−/− or P-Rex null (P-Rex1−/−P-Rex2−/−) neutrophils show mild defects in GPCR-dependent Rac2 and RhoG activity, actin polymerization, and migration,14,21,24,25 and additional defects in E-selectin–dependent slow rolling and Mac1-dependent crawling under flow.26 Similarly, Vav1−/− neutrophils have mildly impaired GPCR-dependent F-actin polymerization and chemotaxis27 and reduced Mac1-dependent crawling under flow,28 but Vav1−/− Vav3−/− or Vav null (Vav1−/− Vav2−/− Vav3−/−) cells adhere and chemotax normally, despite reduced spreading, with attenuated adhesion under flow.21,23 Consequently, neutrophil recruitment is somewhat reduced in P-Rex1−/− mice, but largely normal in Vav1−/− mice during sterile peritonitis,24,25,27,28 and normal in Vav1−/− Vav3−/− mice during sterile peritonitis23 or on immune complex deposition in the skin or lung29 or in Vav null mice during bacterial lung inflammation.30
Hence neither P-Rex family nor Vav family Rac-GEFs alone are required for neutrophil recruitment. Given that Rac is essential, this suggests either that another Rac-GEF family activates Rac during this process or that the P-Rex and Vav families cooperate. We have shown that P-Rex1 and Vav1 cooperate in regulating GPCR-dependent neutrophil responses.21 Therefore we hypothesized that individual Rac-GEFs from the P-Rex and Vav families might also cooperate in neutrophil recruitment.
Methods
Mice
P-Rex1−/− Vav1−/− (P1V1), P-Rex1−/− Vav3−/− (P1V3),21 P-Rex null (P-Rex1−/− P-Rex2−/−),31 and Vav null (Vav1−/− Vav2−/− Vav3−/−)32 mouse strains were described previously and were tested against controls of the appropriate genetic background and upon previous bone marrow transplantation or platelet reconstitution where indicated.
Peritonitis
Sterile peritonitis was induced intraperitoneally with 0.25 mL 3% thioglycollate (TGC) and peritoneal lavages performed after 1.5 hours, unless otherwise indicated. To assess vascular permeability, 150 μL of 9.4 mg/mL Evans blue was injected IV before TGC challenge.
LPS-induced acute lung inflammation
Mice were stimulated intranasally with 200 μg/kg lipopolysaccharide (LPS) or were sham treated, and bronchoalveolar lavages, intravital microscopy, or histological analysis was performed 4 hours later.
Ovalbumin-induced allergic lung inflammation and lung function
Mice were immunized intraperitoneally with 10 μg/400 μL ovalbumin (Ova) or were sham immunized, were exposed to aerosolized Ova on days 15 to 17 to induce allergic inflammation, and lavages, intravital microscopy, histology, or lung function assays were performed 24 hours after the final allergen challenge. Lung function was measured as described previously.33
Detailed protocols are provided in the supplemental Methods (see the Blood Web site).
Results
P-Rex and Vav family Rac-GEFs cooperate in neutrophil recruitment during sterile peritonitis
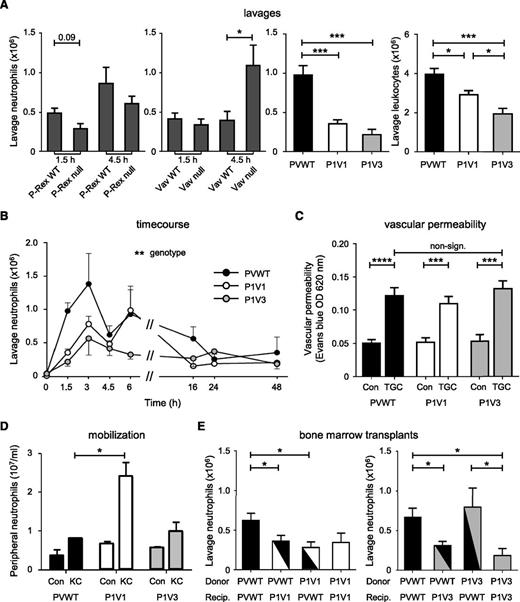
To test whether cooperation between Rac-GEF families occurs, we assessed neutrophil recruitment during TGC-dependent sterile peritonitis. As expected, recruitment was somewhat impaired, although nonsignificantly, in P-Rex null mice, similarly to Rac2−/− mice (both Rac1 and Rac2 are required18,19 ), and recruitment was normal or increased in Vav null mice, depending on time, compared with wild-type mice of the appropriate genetic background (Figure 1A and supplemental Figure 1A). In contrast, 64% and 78% fewer neutrophils were recruited in P-Rex1−/− Vav1−/− (P1V1) and P-Rex1−/− Vav3−/− (P1V3) than in P-Rex/Vav wild-type (PVWT) mice after 1.5 hours. Neutrophils accounted for reduced total peritoneal leukocytes in P1V1 and P1V3 mice, whereas resident peritoneal macrophages and lymphocytes were largely unaffected (Figure 1A). As the impairment is stronger in P1V1 and P1V3 mice than P-Rex null or Vav null mice, we concluded that individual Rac-GEFs from the P-Rex and Vav families cooperate in neutrophil recruitment.
P-Rex and Vav family Rac-GEFs cooperate in peritoneal neutrophil recruitment. (A) Stronger impairment of neutrophil recruitment in P1V1 or P1V3 than P-Rex null or Vav null mice. Sterile peritonitis was induced with TGC, comparing GEF-deficient strains to wild-type mice of appropriate genetic background, and peritoneal lavages were performed after 1.5 hours, or 4.5 hours where indicated, and were analyzed for neutrophils or total leukocytes (right). Data are mean ± standard error of the mean (SEM) of 3 experiments with 5 to 16 mice/group; statistics Kruskal-Wallis test with Dunn’s multiple comparisons or Mann-Whitney U test between genotypes. (B) Time course. Sterile peritonitis was induced as mentioned earlier, except for different times. Data are mean ± SEM of n = 3 to 20 mice/time point and genotype; statistics 2-way analysis of variance (ANOVA) with Bonferroni multiple comparisons. (C) Normal vascular permeability. Evans Blue dye was IV injected 30 minutes before TGC or mock challenge, and peritoneal vascular permeability was assessed 1.5 hours after challenge. Data are mean ± SEM of 3 experiments with 5 to 23 mice/group; statistics unpaired Student t test between sham- and TGC-treated mice and 1-way ANOVA for genotypes. (D) Peripheral neutrophils and neutrophil mobilization. Mice were IV injected with 50 nM KC or were sham treated and neutrophil numbers in the circulation assessed after 1 hour. Data are mean ± SEM of n = 4 to 11 mice/genotype; statistics as in (B). (E) Bone marrow transplants. Mice were irradiated and their hematopoietic systems were reconstituted with donor bone marrow, as indicated, before TGC peritonitis was induced and neutrophil recruitment was determined, as in (A). Data are mean ± SEM of 3 experiments with 5 to 12 mice/group for P1V1 and with 4 to 11 mice/group for P1V3; statistics 1-way ANOVA.
P-Rex and Vav family Rac-GEFs cooperate in peritoneal neutrophil recruitment. (A) Stronger impairment of neutrophil recruitment in P1V1 or P1V3 than P-Rex null or Vav null mice. Sterile peritonitis was induced with TGC, comparing GEF-deficient strains to wild-type mice of appropriate genetic background, and peritoneal lavages were performed after 1.5 hours, or 4.5 hours where indicated, and were analyzed for neutrophils or total leukocytes (right). Data are mean ± standard error of the mean (SEM) of 3 experiments with 5 to 16 mice/group; statistics Kruskal-Wallis test with Dunn’s multiple comparisons or Mann-Whitney U test between genotypes. (B) Time course. Sterile peritonitis was induced as mentioned earlier, except for different times. Data are mean ± SEM of n = 3 to 20 mice/time point and genotype; statistics 2-way analysis of variance (ANOVA) with Bonferroni multiple comparisons. (C) Normal vascular permeability. Evans Blue dye was IV injected 30 minutes before TGC or mock challenge, and peritoneal vascular permeability was assessed 1.5 hours after challenge. Data are mean ± SEM of 3 experiments with 5 to 23 mice/group; statistics unpaired Student t test between sham- and TGC-treated mice and 1-way ANOVA for genotypes. (D) Peripheral neutrophils and neutrophil mobilization. Mice were IV injected with 50 nM KC or were sham treated and neutrophil numbers in the circulation assessed after 1 hour. Data are mean ± SEM of n = 4 to 11 mice/genotype; statistics as in (B). (E) Bone marrow transplants. Mice were irradiated and their hematopoietic systems were reconstituted with donor bone marrow, as indicated, before TGC peritonitis was induced and neutrophil recruitment was determined, as in (A). Data are mean ± SEM of 3 experiments with 5 to 12 mice/group for P1V1 and with 4 to 11 mice/group for P1V3; statistics 1-way ANOVA.
A time course in PVWT mice showed that TGC-induced peritoneal neutrophil recruitment occurs in 2 phases and is largely resolved by 24 hours. Recruitment was impaired during the early phase in P1V1 mice, but throughout in P1V3 mice (Figure 1B), suggesting different mechanisms. TGC-induced Rac activity was lower in P1V1 and P1V3 than in PVWT peritoneal leukocytes isolated 1.5 hours after challenge, roughly correlating with neutrophil recruitment. However, P1V1 cells showed a stronger reduction in Rac2 activity and P1V3 cells in Rac1 activity, demonstrating further recruitment-related differences (supplemental Figure 1B). Vascular permeability was normal under conditions in which both P1V1 and P1V3 mice showed strongly impaired neutrophil recruitment (Figure 1C), both strains showed slight neutrophilia, and keratinocyte chemoattractant (mouse IL-8, KC)-induced neutrophil mobilization from bone marrow into the bloodstream was increased in P1V1 and was normal in P1V3 mice (Figure 1D). Therefore, vascular permeability, peripheral neutrophil numbers, or neutrophil mobilization do not underlie the impaired neutrophil recruitment.
For further characterization, we transplanted P1V1 or P1V3 bone marrow cells into irradiated PVWT mice and vice versa. Both hematopoietic P1V1 cells in PVWT mice and the inverse created impairment for TGC-induced peritoneal neutrophil recruitment, suggesting that both hematopoietic-intrinsic and hematopoietic-extrinsic factors contribute. In contrast, recruitment was impaired in P1V3 mice with PVWT hematopoietic cells but not the inverse, suggesting that the P1V3 defect was largely from the tissue environment (Figure 1E).
To identify neutrophil-intrinsic contributing factors, we assessed adhesion and chemotaxis. Adhesion to monolayers of bEND5 endothelial cells was reduced in basal or fMLP-stimulated P1V1 neutrophils and fMLP-stimulated P1V3 cells, and both P1V1 and P1V3 cells spread significantly less than PVWT cells (supplemental Figure 2A-B). Using fMLP gradients in an EZ-Taxiscan chamber, we observed that chemotaxis of P1V1 neutrophils was impaired, whereas P1V3 neutrophils migrated better than PVWT cells (supplemental Figure 2C and supplemental Video 1). P1V1 cells migrated less per se, with reduced directionality and speed resulting in shorter distance, both accumulated (meandering path) and Euclidean (as the crow flies), whereas P1V3 cells migrated with increased speed and normal directionality, resulting in farther distance (supplemental Figure 2D-F). Therefore, in agreement with previous work,21 P1V1 neutrophils have stronger intrinsic defects than P1V3 neutrophils, despite both mouse strains showing similar impairments in neutrophil recruitment in vivo.
P-Rex and Vav mediate neutrophil recruitment during LPS-induced acute lung inflammation
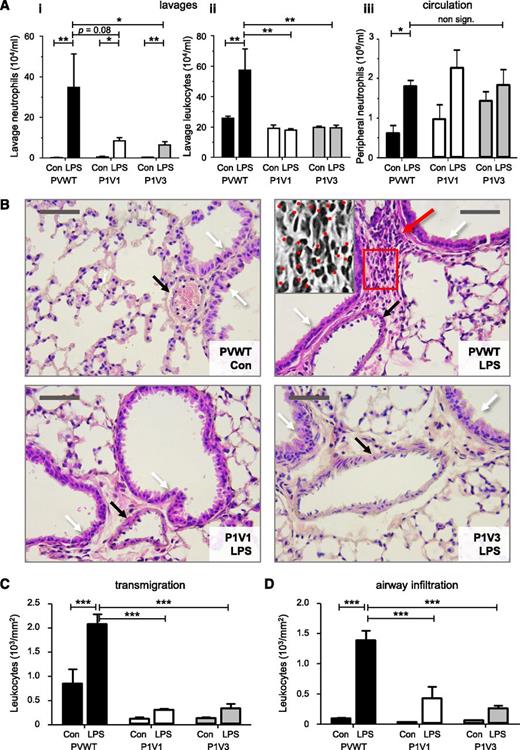
To assess neutrophil recruitment in another pathophysiological condition, we investigated LPS-dependent acute lung inflammation. An intranasal LPS challenge induced robust pulmonary neutrophil recruitment in PVWT mice within 4 hours, but recruitment was reduced fivefold in P1V1 and P1V3 mice (Figure 2A) to a level similar to that observed in Rac2−/− mice (supplemental Figure 3), although peripheral neutrophil, pulmonary TNFα and IL-6, and peripheral KC levels were normal (Figure 2A and supplemental Figure 4A-C). Histological analysis similarly showed abundant neutrophils in LPS-inflamed lungs of PVWT mice and few in P1V1 and P1V3 mice (Figure 2B). Neutrophil transmigration was reduced by 85% in both P1V1 and P1V3 mice, and infiltration of the airway walls by 82% and 85%, respectively (Figure 2C-D). Therefore, Rac-GEF deficiency protects mice from acute lung inflammation.
P-Rex and Vav are required for neutrophil recruitment to the acutely LPS-inflamed lung. (A) Reduced pulmonary neutrophil recruitment in P1V1 and P1V3 mice during LPS-induced acute lung inflammation. Mice were challenged intranasally with 200 μg/kg LPS or carrier (Con), and pulmonary recruitment of neutrophils (i) or total leukocytes (ii) were assessed after 4 hours by lung lavage, as were neutrophil numbers in peripheral blood (iii). Data are mean ± SEM of 3 experiments with 5 to 8 mice/group; statistics unpaired Student t test between sham and LPS treatment and Kruskal-Wallis test with Dunn’s multiple comparisons (i,ii) or 2-way ANOVA with Bonferroni multiple comparisons (iii) for genotypes. (B) Reduced LPS-induced neutrophil infiltration into lung tissue of P1V1 and P1V3 mice. H&E-stained mouse lung histology slides prepared after LPS challenge as in (A). Black arrows indicate blood vessels, white arrows airway walls, and the red arrow infiltrated neutrophils. Photographs represent 4 to 9 mice per group. The size bar represents 50 μm. The grey-scale insert in the PVWT/LPS panel is a ×2.5 magnification of the red frame. Neutrophils are identified by their characteristic donut- or horseshoe-shaped nuclei and are marked with dots. (C,D) Reduced leukocyte transmigration and airway infiltration in lung tissue of P1V1 and P1V3 mice. Leukocyte transmigration into lung tissue (C) and airway infiltration (D) in response to LPS challenge quantitated from histology slides, as in (B), as leukocytes within a radius of 50 μm of blood vessels or 50 μm of the airway wall, respectively, with multiple measurements per slide. Data are mean ± SEM of n = 4 to 9 mice per genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons.
P-Rex and Vav are required for neutrophil recruitment to the acutely LPS-inflamed lung. (A) Reduced pulmonary neutrophil recruitment in P1V1 and P1V3 mice during LPS-induced acute lung inflammation. Mice were challenged intranasally with 200 μg/kg LPS or carrier (Con), and pulmonary recruitment of neutrophils (i) or total leukocytes (ii) were assessed after 4 hours by lung lavage, as were neutrophil numbers in peripheral blood (iii). Data are mean ± SEM of 3 experiments with 5 to 8 mice/group; statistics unpaired Student t test between sham and LPS treatment and Kruskal-Wallis test with Dunn’s multiple comparisons (i,ii) or 2-way ANOVA with Bonferroni multiple comparisons (iii) for genotypes. (B) Reduced LPS-induced neutrophil infiltration into lung tissue of P1V1 and P1V3 mice. H&E-stained mouse lung histology slides prepared after LPS challenge as in (A). Black arrows indicate blood vessels, white arrows airway walls, and the red arrow infiltrated neutrophils. Photographs represent 4 to 9 mice per group. The size bar represents 50 μm. The grey-scale insert in the PVWT/LPS panel is a ×2.5 magnification of the red frame. Neutrophils are identified by their characteristic donut- or horseshoe-shaped nuclei and are marked with dots. (C,D) Reduced leukocyte transmigration and airway infiltration in lung tissue of P1V1 and P1V3 mice. Leukocyte transmigration into lung tissue (C) and airway infiltration (D) in response to LPS challenge quantitated from histology slides, as in (B), as leukocytes within a radius of 50 μm of blood vessels or 50 μm of the airway wall, respectively, with multiple measurements per slide. Data are mean ± SEM of n = 4 to 9 mice per genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons.
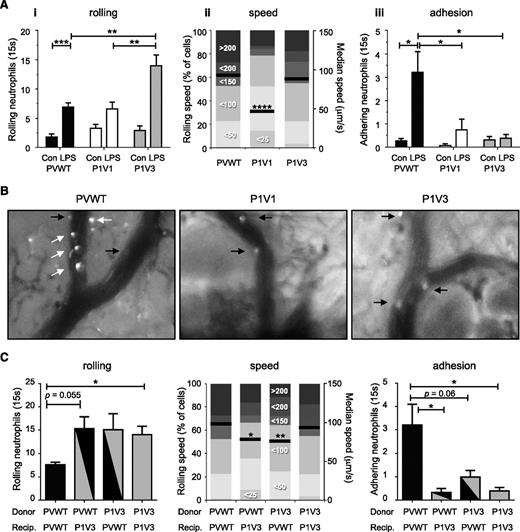
Intravital microscopy of airway postcapillary venules in the tracheal microvasculature7,34 enabled us to monitor LPS-induced neutrophil recruitment under similar conditions. LPS-challenge stimulated neutrophil rolling in PVWT postcapillary venules fourfold and adhesion 11-fold. In P1V1 and P1V3 mice, intravascular adhesion was reduced by 72% and 88%, respectively (Figure 3A-B and supplemental Videos 3 and 4), which seems sufficiently profound to explain the loss of transmigration and airway infiltration. In addition, rolling was altered, with a twofold reduction in the median velocity (from 94 to 46 μm/s) in P1V1 mice and a twofold increase in the number of rolling cells in P1V3 mice (Figure 3A and supplemental Videos 2-4). The latter could be a consequence of lost adhesion, as rolling cells accumulate when unable to arrest, but reduced velocity suggested additional rolling-intrinsic defects.
Altered neutrophil rolling and impaired adhesion in airway postcapillary venules of P1V1 and P1V3 mice during acute LPS-induced lung inflammation. (A) Altered neutrophil rolling and impaired adhesion in airway postcapillary venules of P1V1 and P1V3 mice during LPS-induced lung inflammation. Mice were challenged with LPS as in Figure 3 and intravital microscopy was performed by taking 15-second videos of 5 to 10 of the airway postcapillary venules/mouse. Numbers of rolling neutrophils (i), rolling velocity (ii; mean velocity and speed categories), and firm adhesion (iii) were assessed by video analysis. Data are mean ± SEM of 4 to 5 mice/group for numbers of rolling and adhering cells. Rolling velocity is median speed of ≥90 cells/genotype; statistics unpaired Student t test between sham- and LPS-treatment or Kruskal-Wallis test with Dunn’s multiple comparisons for genotypes. (B) Representative images of LPS-induced neutrophil recruitment in airway postcapillary venules. Inflammation was induced with LPS and was monitored by airway intravital microscopy as in (A). Images are stills from representative videos. White arrows denote firmly adhering neutrophils (stationary throughout recording), and black arrows show cells rolling along the vessel wall. (C) Neutrophil-intrinsic and neutrophil-extrinsic factors are sufficient to impair LPS-induced pulmonary neutrophil recruitment in P1V3 mice. Mice were lethally irradiated, their hematopoietic system was reconstituted with bone marrow, as indicated, LPS-dependent lung inflammation was induced after reconstitution, and pulmonary neutrophil recruitment was assessed by intravital microscopy as in (A). Data are mean ± SEM of 4 to 5 mice/group; statistics as in (A).
Altered neutrophil rolling and impaired adhesion in airway postcapillary venules of P1V1 and P1V3 mice during acute LPS-induced lung inflammation. (A) Altered neutrophil rolling and impaired adhesion in airway postcapillary venules of P1V1 and P1V3 mice during LPS-induced lung inflammation. Mice were challenged with LPS as in Figure 3 and intravital microscopy was performed by taking 15-second videos of 5 to 10 of the airway postcapillary venules/mouse. Numbers of rolling neutrophils (i), rolling velocity (ii; mean velocity and speed categories), and firm adhesion (iii) were assessed by video analysis. Data are mean ± SEM of 4 to 5 mice/group for numbers of rolling and adhering cells. Rolling velocity is median speed of ≥90 cells/genotype; statistics unpaired Student t test between sham- and LPS-treatment or Kruskal-Wallis test with Dunn’s multiple comparisons for genotypes. (B) Representative images of LPS-induced neutrophil recruitment in airway postcapillary venules. Inflammation was induced with LPS and was monitored by airway intravital microscopy as in (A). Images are stills from representative videos. White arrows denote firmly adhering neutrophils (stationary throughout recording), and black arrows show cells rolling along the vessel wall. (C) Neutrophil-intrinsic and neutrophil-extrinsic factors are sufficient to impair LPS-induced pulmonary neutrophil recruitment in P1V3 mice. Mice were lethally irradiated, their hematopoietic system was reconstituted with bone marrow, as indicated, LPS-dependent lung inflammation was induced after reconstitution, and pulmonary neutrophil recruitment was assessed by intravital microscopy as in (A). Data are mean ± SEM of 4 to 5 mice/group; statistics as in (A).
Adoptive transfer in P1V3 mice had shown hematopoietic-extrinsic defects during peritoneal recruitment. To investigate whether the same is true during pulmonary neutrophil recruitment, we combined bone marrow transplants with intravital microscopy. Either a P1V3 hematopoietic system or P1V3 tissue environment was sufficient to impair LPS-stimulated neutrophil adhesion and alter rolling as observed in P1V3 mice (Figure 3C). This emphasizes both the different requirements for recruitment to various organs and that P-Rex and Vav are important throughout.
Impaired L- and E-selectin–dependent adhesion and ICAM1-dependent slow rolling in LPS-inflamed airway postcapillary venules of P1V1 and P1V3 mice
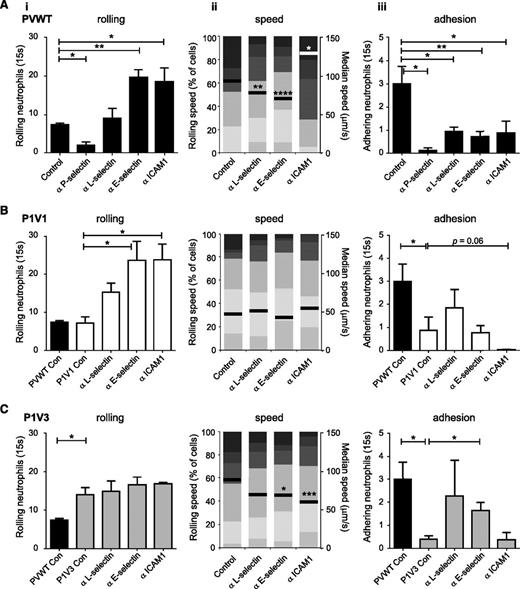
Rolling and adhesion are conferred by selectins and integrins, but the importance of individual molecules varies between tissues. To determine which are required in the LPS-inflamed airway microvasculature, we used blocking antibodies (Figure 4 and supplemental Figure 5; these show identical data sets sorted by genotype and adhesion molecules, respectively). Blockade of P-, L-, E-selectin or ICAM1 inhibited LPS-induced neutrophil adhesion in PVWT mice (Figure 4A), as expected from the cremaster muscle. P-selectin blockade also inhibited rolling, as expected, ruling out P-selectin as causing the recruitment defect in P1V1 and P1V3 mice. Surprisingly, blockade of L- or E-selectin failed to reduce rolling despite efficiently inhibiting adhesion, suggesting that these selectins are required for adhesion in the airway vasculature, whereas they confer rolling and slow rolling, respectively, in the cremaster muscle.35 Slow rolling in the airway vasculature is instead mediated by ICAM1, as its blockade caused PVWT cells to roll faster. These data identify the adhesion molecules important for neutrophil recruitment in the airway microvasculature and further emphasize the distinct molecular requirements in different organs.
Impaired LPS-induced ICAM1-dependent slow rolling and L- and E-selectin–dependent adhesion of neutrophils in airway postcapillary venules of P1V1 and P1V3 mice. (A) Neutrophil rolling in airway postcapillary venules of PVWT mice depends on P-selectin, slow rolling on ICAM1, and adhesion on these plus L- and E-selectin. Blocking ABs against the indicated molecules were injected into PVWT mice 15 minutes before induction of acute lung inflammation with LPS, and intravital microscopy of airway postcapillary venules was performed and assessed for rolling neutrophils (i), rolling velocity (ii), and firm adhesion (iii) from multiple videos per mouse as in Figure 3. Data are mean ± SEM of 3 to 4 mice/group. Rolling velocity is from ≥140 cells/genotype; statistics Mann-Whitney U test for genotypes with AB treatment. (B,C) LPS-induced ICAM1-dependent slow rolling and L- and E-selectin–dependent adhesion in airway postcapillary venules are impaired in P1V1 and P1V3 mice. Blocking ABs against the indicated molecules were injected into P1V1 (B) or P1V3 (C) mice before the induction of acute lung inflammation with LPS, and intravital microscopy is as in (A). Data are mean ± SEM of 3 to 4 mice/condition. Rolling velocity is from ≥140 cells/genotype; statistics unpaired Student t test for genotypes with AB treatment. Note: supplemental Figure 5 shows the same data arranged by adhesion molecule.
Impaired LPS-induced ICAM1-dependent slow rolling and L- and E-selectin–dependent adhesion of neutrophils in airway postcapillary venules of P1V1 and P1V3 mice. (A) Neutrophil rolling in airway postcapillary venules of PVWT mice depends on P-selectin, slow rolling on ICAM1, and adhesion on these plus L- and E-selectin. Blocking ABs against the indicated molecules were injected into PVWT mice 15 minutes before induction of acute lung inflammation with LPS, and intravital microscopy of airway postcapillary venules was performed and assessed for rolling neutrophils (i), rolling velocity (ii), and firm adhesion (iii) from multiple videos per mouse as in Figure 3. Data are mean ± SEM of 3 to 4 mice/group. Rolling velocity is from ≥140 cells/genotype; statistics Mann-Whitney U test for genotypes with AB treatment. (B,C) LPS-induced ICAM1-dependent slow rolling and L- and E-selectin–dependent adhesion in airway postcapillary venules are impaired in P1V1 and P1V3 mice. Blocking ABs against the indicated molecules were injected into P1V1 (B) or P1V3 (C) mice before the induction of acute lung inflammation with LPS, and intravital microscopy is as in (A). Data are mean ± SEM of 3 to 4 mice/condition. Rolling velocity is from ≥140 cells/genotype; statistics unpaired Student t test for genotypes with AB treatment. Note: supplemental Figure 5 shows the same data arranged by adhesion molecule.
We reasoned that responses sensitive to blocking antibodies (ABs) in PVWT but not P1V1 or P1V3 mice should identify selectins and integrins affected by P-Rex/Vav deficiency (Figure 4B-C and supplemental Figure 5). ICAM1-blockade showed a tendency to suppress residual neutrophil adhesion in P1V1, but not P1V3 mice (although the latter was hard to interpret as residual adhesion was minimal), suggesting that ICAM1-dependent adhesion may retain partial functionality in P1V1, but possibly not P1V3 mice. In contrast, blockade of L- or E-selectin in P1V1 or P1V3 mice failed to decrease neutrophil adhesion further, suggesting that P-Rex/Vav deficiency causes L- and E-selectin–dependent adhesion defects. Finally, while increased rolling upon blockade of L-, E-selectin or ICAM1 in P1V1 mice could be a consequence of lost adhesion, ICAM1-blockade greatly accelerated rolling velocity in PVWT, but not P1V1 or P1V3 mice, suggesting that ICAM1-dependent slow neutrophil rolling is impaired by P-Rex/Vav deficiency. In summary, ICAM1-dependent slow rolling and L- and E-selectin–dependent adhesion are impaired in P1V1 and P1V3 mice, and ICAM1-mediated adhesion may be affected in P1V3 mice.
P1V1 and P1V3 mouse neutrophils have reduced integrin surface levels
Next, we assessed the expression of selectins, integrins and their ligands on the surface of P1V1 and P1V3 neutrophils and lung vascular endothelial cells. P1V1 and P1V3 neutrophils showed normal or elevated surface levels of PSGL1 or L-selectin under control conditions or upon priming with TNFα or GM-CSF, and underwent normal L-selectin shedding (supplemental Figure 6A), so these did not account for the impaired adhesion. In contrast, P1V1 and P1V3 neutrophils showed significantly reduced LFA1 surface levels, except after GM-CSF priming. As expected from previous findings, P1V1 neutrophils also had reduced Mac1 levels21 (supplemental Figure 6B). These reduced integrin cell surface levels undoubtedly contribute to the impaired ICAM1-dependent slow rolling in P1V1 and P1V3 mice.
Surface levels of PSGL1, P-selectin, E-selectin and ICAM1 were either normal or increased in lung vascular endothelial cells isolated from P1V1 and P1V3 mice, and the cells responded normally to TNFα− or LPS-stimulation by upregulating ICAM1 (supplemental Figure 7). Only thrombin induced significant loss of P- and E-selectin from the surface of P1V1 cells, but this is unlikely to reflect the defect in pulmonary recruitment. Therefore, the neutrophil adhesion defects in P1V1 and P1V3 mice were not obviously caused by adhesion molecule levels in lung vascular endothelial cells.
P-Rex/Vav deficiency in platelets recapitulates the impaired neutrophil recruitment during LPS-induced acute lung inflammation
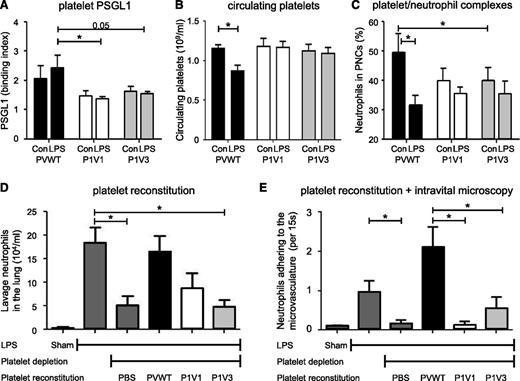
As adhesion molecule levels insufficiently explained the recruitment defects in P1V1 and P1V3 mice, we considered platelet involvement, as platelets promote leukocyte recruitment.36,37 P-Rex1, Vav1 and Vav3 are known to be expressed in platelets but play minor roles, if any, in platelet aggregation.38-40 Western blotting confirmed the expression of P-Rex1 and Vav1 in PVWT platelets, Rac1 and Vav2 were unaffected by P-Rex/Vav-deficiency, P-Rex2 and Rac2 remained undetectable, as expected,21,41 and Vav1 levels were normal in P1V3 platelets (supplemental Figure 8A; isoform-specific ABs for endogenous Vav3 were unavailable). As platelet PSGL1 is the major counterligand for P-, L-, and E-selectins, we measured its surface levels in platelets isolated on pulmonary LPS-challenge. PSGL1 was reduced in P1V1 and P1V3 platelets, whereas P-selectin was not (Figure 5A and supplemental Figure 8B-C). Furthermore, peripheral platelet counts decreased in PVWT mice, a known consequence of platelet sequestration at sites of inflammation, but not in P1V1 or P1V3 mice (Figure 5B). Similarly, the occurrence of platelet/neutrophil complexes (PNCs) in peripheral blood was reduced in P1V1 and P1V3 mice and was unresponsive to LPS treatment, unlike in PVWT mice where LPS challenge presumably induced pulmonary sequestration (Figure 5C). In contrast, P1V1 and P1V3 platelets underwent normal thrombin-or ADP-stimulated aggregation (supplemental Figure 8D), confirming that P-Rex and Vav were dispensable for hemostatic platelet functions.
LPS-induced pulmonary neutrophil recruitment can be reconstituted with PVWT but not P1V1 or P1V3 platelets. (A) P1V1 and P1V3 platelets show reduced surface expression of PSGL1. Mice were challenged with LPS or mock-challenged as in Figure 2, and the surface expression of PSGL1 on platelets in peripheral blood was assessed after 4 hours. Data are mean ± SEM of n = 5 to 8 mice/genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons test. (B) P1V1 and P1V3 platelets are not sequestered during LPS-induced acute lung inflammation. Mice were challenged with LPS as in (A) and platelet numbers in peripheral blood assessed 4 hours after challenge. Data are from 2 experiments and are mean ± SEM of n = 4 to 8 mice/genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons test for genotypes. (C) P1V1 and P1V3 mice show reduced PNC formation. Mice were challenged with LPS as in (A) and the percentage of neutrophils that were attached to platelets in peripheral blood assessed 4 hours after challenge. Data are mean ± SEM of n = 6 to 8 mice/genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons test. (D) Reconstitution of LPS-induced pulmonary neutrophil recruitment in platelet-depleted PVWT mice with PVWT but not P1V1 and P1V3 platelets. Isolated platelets were transfused into thrombocytopenic PVWT mice and neutrophil recruitment into LPS-inflamed lungs assessed as in Figure 2A. Data are mean ± SEM of n = 3 to 5 mice; statistics 1-way ANOVA with Bonferroni multiple comparisons. (E) Reconstitution of LPS-induced neutrophil adhesion to the airway postcapillary venule wall with PVWT but not P1V1 and P1V3 platelets. Isolated platelets were transfused into thrombocytopenic PVWT mice, lung inflammation was induced as in (A), and neutrophil adhesion to airway postcapillary venule walls was assessed by intravital microscopy 4 hours after LPS challenge as in Figure 3A. Data are mean ± SEM of n = 3 mice/group from 5 to 10 videos/animal; statistics 1-way ANOVA with Bonferroni multiple comparisons.
LPS-induced pulmonary neutrophil recruitment can be reconstituted with PVWT but not P1V1 or P1V3 platelets. (A) P1V1 and P1V3 platelets show reduced surface expression of PSGL1. Mice were challenged with LPS or mock-challenged as in Figure 2, and the surface expression of PSGL1 on platelets in peripheral blood was assessed after 4 hours. Data are mean ± SEM of n = 5 to 8 mice/genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons test. (B) P1V1 and P1V3 platelets are not sequestered during LPS-induced acute lung inflammation. Mice were challenged with LPS as in (A) and platelet numbers in peripheral blood assessed 4 hours after challenge. Data are from 2 experiments and are mean ± SEM of n = 4 to 8 mice/genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons test for genotypes. (C) P1V1 and P1V3 mice show reduced PNC formation. Mice were challenged with LPS as in (A) and the percentage of neutrophils that were attached to platelets in peripheral blood assessed 4 hours after challenge. Data are mean ± SEM of n = 6 to 8 mice/genotype; statistics 2-way ANOVA with Bonferroni multiple comparisons test. (D) Reconstitution of LPS-induced pulmonary neutrophil recruitment in platelet-depleted PVWT mice with PVWT but not P1V1 and P1V3 platelets. Isolated platelets were transfused into thrombocytopenic PVWT mice and neutrophil recruitment into LPS-inflamed lungs assessed as in Figure 2A. Data are mean ± SEM of n = 3 to 5 mice; statistics 1-way ANOVA with Bonferroni multiple comparisons. (E) Reconstitution of LPS-induced neutrophil adhesion to the airway postcapillary venule wall with PVWT but not P1V1 and P1V3 platelets. Isolated platelets were transfused into thrombocytopenic PVWT mice, lung inflammation was induced as in (A), and neutrophil adhesion to airway postcapillary venule walls was assessed by intravital microscopy 4 hours after LPS challenge as in Figure 3A. Data are mean ± SEM of n = 3 mice/group from 5 to 10 videos/animal; statistics 1-way ANOVA with Bonferroni multiple comparisons.
To evaluate directly whether platelet Rac-GEF expression contributes to neutrophil recruitment into the LPS-inflamed lung, we depleted PVWT mice of platelets using busulfan treatment42,43 and reconstituted them with platelets isolated from PVWT, P1V1, or P1V3 mice. Platelet depletion substantially reduced neutrophil recruitment into the LPS-inflamed lung (and into the TGC-inflamed peritoneum; supplemental Figure 8E), and the LPS response could be successfully reconstituted with PVWT platelets, as expected. In contrast, P1V1 or P1V3 platelets failed to rescue neutrophil recruitment (Figure 5D), suggesting that P-Rex/Vav-deficiency in platelets is a major cause of impaired neutrophil recruitment in P1V1 and P1V3 mice, thus showing an unexpected and important role of P-Rex and Vav in the proinflammatory functions of platelets.
To assess whether P-Rex/Vav expression in platelets affects neutrophil adhesion to the vascular endothelium, we combined platelet reconstitution with intravital microscopy. Platelet depletion blocked neutrophil adhesion to the LPS-inflamed airway postcapillary venule wall, and the response could be reconstituted with PVWT but not P1V1 or P1V3 platelets (Figure 5E). This shows that P-Rex and Vav in platelets are required for the intravascular adhesion of neutrophils during inflammatory cell recruitment into the lung.
P-Rex and Vav are required for leukocyte recruitment during Ova-induced allergic lung inflammation and associated loss of airway hyperresponsiveness
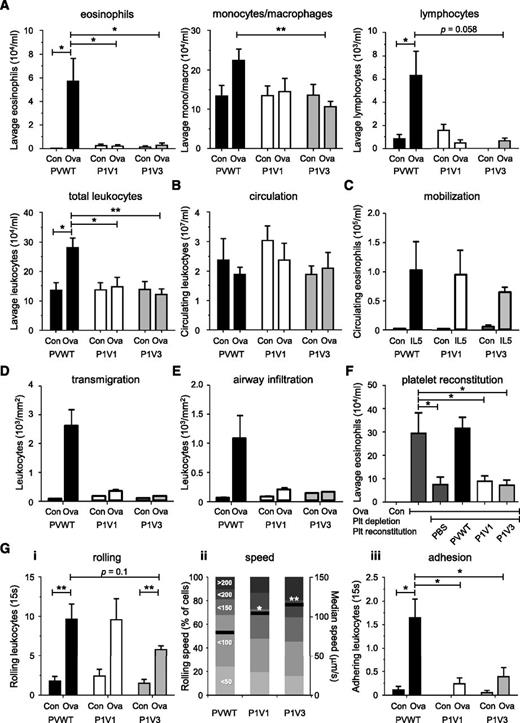
To test whether the importance of P-Rex and Vav in leukocyte recruitment is restricted to neutrophils, we assessed Ova-induced allergic lung inflammation, characterized by pulmonary recruitment of eosinophils, lymphocytes, and monocytes. Ova challenge induced robust recruitment of all leukocytes typical for this inflammation in PVWT, but not P1V1 and P1V3 mice (Figure 6A). Therefore, P-Rex and Vav are required for the inflammatory recruitment of a whole range of leukocyte types.
P-Rex and Vav control the pulmonary recruitment of eosinophils, monocytes/macrophages, and lymphocytes during Ova-induced lung allergy in a platelet- and leukocyte-adhesion–dependent manner. (A-B) Absence of pulmonary leukocyte recruitment in P1V1 and P1V3 mice during Ova-induced lung allergy. Mice were sensitized to Ova, challenged with aerosolized Ova to induce allergy, or were mock treated, and pulmonary recruitment of eosinophils, monocytes/macrophages, lymphocytes, or total leukocytes, as indicated, was assessed by lung lavage 24 hours after Ova challenge (A), as were total leukocyte numbers in peripheral blood (B). Data are mean ± SEM of 3 experiments with 4 to 14 mice/group; statistics in (A) unpaired Student t test between sham and Ova treatment, and 1-way ANOVA with Tukey’s multiple comparisons or Kruskal-Wallis with Dunn’s multiple comparisons for genotypes; statistics in (B) are 2-way ANOVA with Bonferroni multiple comparisons. (C) Normal IL-5–dependent eosinophil mobilization. Mice were IV injected with IL-5 to induce eosinophil mobilization from the bone marrow into the blood stream or were sham treated, and eosinophil numbers in the peripheral blood were assessed after 24 hours by enzyme-linked immunosorbent assay. Data are mean ± SEM of 3 mice/group; statistics as in (B). (D-E) Reduced Ova-induced leukocyte transmigration and airway infiltration in lungs of P1V1 and P1V3 mice. Quantitative histologic analysis of leukocyte transmigration into lung tissue (D) and airway infiltration (E) during Ova-dependent lung inflammation, quantitated as in Figure 2B-D. Data are mean ± SEM of 4 to 9 mice/group; statistics 2-way ANOVA with Bonferroni multiple comparisons. (F) Reconstitution of Ova-induced pulmonary eosinophil recruitment in platelet-depleted PVWT mice with PVWT but not P1V1 or P1V3 platelets. Platelets were transfused into thrombocytopenic PVWT mice as in Figure 5 and eosinophil recruitment into the Ova-inflamed lung was assessed as in (A). Data are mean ± SEM of n = 4 to 8 mice/group; statistics 1-way ANOVA with Bonferroni multiple comparisons. (G) Altered eosinophil rolling and impaired adhesion in postcapillary venules of the Ova-inflamed allergic lung. Lung allergy was induced as in (A) and intravital microscopy of the tracheal postcapillary venules was performed and analyzed as in Figure 3 for numbers of rolling eosinophils (i), rolling velocity (ii; both mean velocity and categories), and firm adhesion (iii). Data are mean ± SEM of 2 to 4 sham and 4 to 6 Ova-treated mice/group for numbers of rolling and adhering cells. Rolling velocity is from ≥120 cells; statistics as in (D).
P-Rex and Vav control the pulmonary recruitment of eosinophils, monocytes/macrophages, and lymphocytes during Ova-induced lung allergy in a platelet- and leukocyte-adhesion–dependent manner. (A-B) Absence of pulmonary leukocyte recruitment in P1V1 and P1V3 mice during Ova-induced lung allergy. Mice were sensitized to Ova, challenged with aerosolized Ova to induce allergy, or were mock treated, and pulmonary recruitment of eosinophils, monocytes/macrophages, lymphocytes, or total leukocytes, as indicated, was assessed by lung lavage 24 hours after Ova challenge (A), as were total leukocyte numbers in peripheral blood (B). Data are mean ± SEM of 3 experiments with 4 to 14 mice/group; statistics in (A) unpaired Student t test between sham and Ova treatment, and 1-way ANOVA with Tukey’s multiple comparisons or Kruskal-Wallis with Dunn’s multiple comparisons for genotypes; statistics in (B) are 2-way ANOVA with Bonferroni multiple comparisons. (C) Normal IL-5–dependent eosinophil mobilization. Mice were IV injected with IL-5 to induce eosinophil mobilization from the bone marrow into the blood stream or were sham treated, and eosinophil numbers in the peripheral blood were assessed after 24 hours by enzyme-linked immunosorbent assay. Data are mean ± SEM of 3 mice/group; statistics as in (B). (D-E) Reduced Ova-induced leukocyte transmigration and airway infiltration in lungs of P1V1 and P1V3 mice. Quantitative histologic analysis of leukocyte transmigration into lung tissue (D) and airway infiltration (E) during Ova-dependent lung inflammation, quantitated as in Figure 2B-D. Data are mean ± SEM of 4 to 9 mice/group; statistics 2-way ANOVA with Bonferroni multiple comparisons. (F) Reconstitution of Ova-induced pulmonary eosinophil recruitment in platelet-depleted PVWT mice with PVWT but not P1V1 or P1V3 platelets. Platelets were transfused into thrombocytopenic PVWT mice as in Figure 5 and eosinophil recruitment into the Ova-inflamed lung was assessed as in (A). Data are mean ± SEM of n = 4 to 8 mice/group; statistics 1-way ANOVA with Bonferroni multiple comparisons. (G) Altered eosinophil rolling and impaired adhesion in postcapillary venules of the Ova-inflamed allergic lung. Lung allergy was induced as in (A) and intravital microscopy of the tracheal postcapillary venules was performed and analyzed as in Figure 3 for numbers of rolling eosinophils (i), rolling velocity (ii; both mean velocity and categories), and firm adhesion (iii). Data are mean ± SEM of 2 to 4 sham and 4 to 6 Ova-treated mice/group for numbers of rolling and adhering cells. Rolling velocity is from ≥120 cells; statistics as in (D).
Peripheral leukocyte levels were normal in Ova-challenged P1V1 and P1V3 mice, as were IL-5–dependent eosinophil mobilization and pulmonary IL-4 and IL-5 levels (Figure 6B and supplemental Figure 9A-B). Serum IgE levels were normal in P1V3 mice, but were reduced in P1V1 mice, likely because of the role of Vav1 in AB-class switching, but both strains responded proportionally by producing serum IgE (supplemental Figure 9C). Therefore, cytokine and IgE production or leukocyte mobilization did not cause the defect in Ova-induced pulmonary leukocyte recruitment in P1V1 and P1V3 mice.
Histology showed complete loss of Ova-induced leukocyte transmigration into the lung tissue and infiltration around the airway walls (Figure 6D-E), so that allergic lung inflammation was effectively abolished in P1V1 and P1V3 mice. Platelet reconstitution showed that platelet P-Rex/Vav are essential for eosinophil recruitment to the Ova-inflamed lung (Figure 6F), and intravital airway microscopy showed reduced eosinophil adhesion to the postcapillary venule wall sufficient to explain the loss of transmigration and airway infiltration, as well as abnormal rolling, with reduced numbers of rolling cells in P1V3 mice and significantly increased rolling velocities in both strains (Figure 6G). Therefore, P-Rex and Vav family Rac-GEFs are required for the recruitment of eosinophils and other types of leukocytes during allergic pulmonary inflammation in a platelet-dependent manner by facilitating leukocyte adhesion to the postcapillary venule wall.
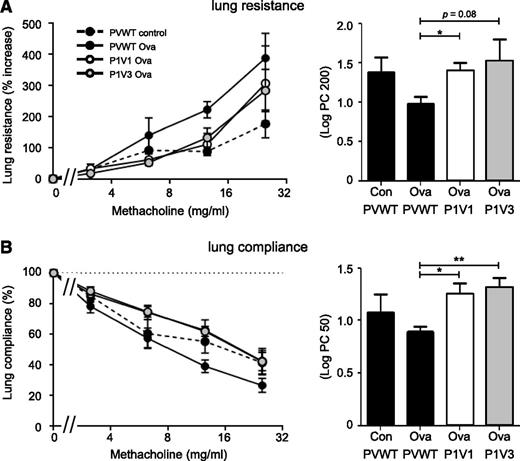
Finally, allergic inflammation can lead to reduced lung function, characterized by bronchial hyperresponsiveness. As P1V1 and P1V3 mice were protected from lung inflammation during Ova-induced allergy, we tested whether they were also protected from the associated bronchial hyperresponsiveness. We induced allergic inflammation of the airways with Ova as described earlier and we tested bronchial responsiveness to an increasing dose of the muscarinic receptor agonist methacholine, which causes bronchoconstriction. Allergic PVWT animals showed dose-dependent decrease in lung function, as seen by increased lung resistance and decreased lung compliance, whereas lung function was preserved in P1V1 and P1V3 mice (Figure 7). Therefore, P-Rex/Vav deficiency protects mice from allergen-induced bronchial hyperresponsiveness.
P1V1 and P1V3 mice are protected from impairments in lung function in Ova-induced allergy. P1V1 and P1V3 mice are protected from methacholine-induced increase in lung resistance (A) and loss of lung compliance (B) during Ova-induced lung allergy. Lung allergy was induced with Ova or mock-induced in P1V1, P1V3, and PVWT mice as in Figure 6, and lung function was assessed by challenge with rising concentrations of methacholine, measuring lung resistance (A) and compliance (B). Bar graphs show the dose of methacholine required to increase lung resistance by 200% over basal (A) or to reduce lung compliance by 50% compared with basal (B). Data are mean ± SEM of n = 4 to 6 mice/group; statistics are 1-way ANOVA with Bonferroni multiple comparisons test between Ova-sensitized groups.
P1V1 and P1V3 mice are protected from impairments in lung function in Ova-induced allergy. P1V1 and P1V3 mice are protected from methacholine-induced increase in lung resistance (A) and loss of lung compliance (B) during Ova-induced lung allergy. Lung allergy was induced with Ova or mock-induced in P1V1, P1V3, and PVWT mice as in Figure 6, and lung function was assessed by challenge with rising concentrations of methacholine, measuring lung resistance (A) and compliance (B). Bar graphs show the dose of methacholine required to increase lung resistance by 200% over basal (A) or to reduce lung compliance by 50% compared with basal (B). Data are mean ± SEM of n = 4 to 6 mice/group; statistics are 1-way ANOVA with Bonferroni multiple comparisons test between Ova-sensitized groups.
Discussion
We have shown that Rac-GEFs from the P-Rex and Vav families cooperate in leukocyte recruitment during allergic and nonallergic inflammation, and we have identified key underlying roles of these Rac-GEFs in platelet-dependent leukocyte/endothelial adhesion.
Our most surprising finding was that P1V1 or P1V3 platelets were sufficient to induce leukocyte recruitment defects similar to those seen in P1V1 and P1V3 mice, showing that the expression of P-Rex/Vav Rac-GEFs in platelets is critical for leukocyte recruitment. Platelets promote leukocyte recruitment in multiple inflammatory conditions, independently of their role in hemostasis.36 This proinflammatory platelet function involves both complex formation with circulating leukocytes (PNCs) and the secretion of soluble factors.36,37 Platelet P-selectin binding to leukocyte PSGL1 is important,42,44 as are neutrophil L-selectin and endothelial E-selectin interactions with platelet PSGL1.42,43,45 The former is unlikely to be affected in P1V1 and P1V3 mice, as P-selectin expression was normal, and P1V1 and P1V3 neutrophils could roll on the vascular endothelium. In contrast, the L- and E-selectin–dependent adhesion defects are likely to be caused by impaired PNC formation resulting from reduced surface levels of platelet PSGL1.43,45
Whereas the physical interactions among proinflammatory platelets, leukocytes, and endothelial cells are relatively well understood, the signaling pathways that render platelets proinflammatory remain largely unknown, although they are clearly distinct from those promoting aggregation. Our results identify P-Rex and Vav as key mediators of proinflammatory platelet function. In contrast, even the combined P-Rex and Vav deficiencies failed to show roles for these proteins in platelet aggregation. Although the underlying P-Rex/Vav-dependent platelet signaling mechanisms and relative contributions of individual Rac-GEF proteins remain to be fully elucidated, their dichotomous role in proinflammatory but not aggregatory platelet functions has profound conceptual implications and could lead to novel therapeutic approaches for the treatment of inflammatory diseases.
Peritoneal neutrophil recruitment was more impaired in P1V1 and P1V3 mice than in P-Rex null or Vav null mice, suggesting that individual Rac-GEFs from the P-Rex and Vav families cooperate in recruitment. We had first demonstrated cooperation between P-Rex1 and Vav1 in isolated P1V1 neutrophils, where several GPCR-dependent responses were affected more-than-additively over P-Rex null and Vav null cells.21 We had proposed this as a specialized form of cooperation where P-Rex1 and Vav1 are activated through different mechanisms before converging on the same molecular target.21 Surprisingly, this cooperation was between P-Rex1 and Vav1, despite higher expression of Vav3.23 Here we showed similarly that chemotaxis in EZ-Taxiscan chambers is impaired in P1V1 but not P1V3 neutrophils, although transwell assays had previously showed defects in both strains.21 The latter assay tests cell deformability as well as locomotion, thereby seeming to provide the more stringent conditions required to identify impairment in P1V3 neutrophils. However, the different chemotactic abilities of P1V1 and P1V3 neutrophils are unlikely to significantly come into play in vivo, because platelet-dependent intravascular adhesion precedes any steps in the recruitment cascade that would require chemotaxis. Further differences between P1V1 and P1V3 mice include KC-induced neutrophil mobilization (likely resulting from Vav1 retaining neutrophils in the bone marrow, as seen on systemic administration of fMLP27 ), basal IgE levels, and mildly increased surface levels of some adhesion molecules. Nevertheless, both strains showed similar recruitment defects in various types of inflammation, suggesting that these differences are modulatory rather than critical, and that instead features common to both strains, including platelet-activation and neutrophil LFA1 surface levels, are overridingly more important.
Neither their roles in proinflammatory platelets (which are derived from hematopoietic cells) nor their neutrophil-intrinsic functions account for the hematopoietic-extrinsic effects of P-Rex/Vav on neutrophil recruitment identified by our bone marrow transplantation experiments. Furthermore, as Vav1 expression is restricted to hematopoietic cells, the hematopoietic-extrinsic defects in P1V1 mice must be caused by loss of P-Rex1. As our investigation of adhesion molecules on vascular endothelial cells failed to show obvious defects, the hematopoietic-extrinsic functions of P-Rex1 and/or Vav3 in recruitment remain to be identified.
Firm leukocyte adhesion to the vascular endothelium is a prerequisite for transmigration and is severely impaired in P1V1 and P1V3 mice, whereas P-Rex deficiency or Vav deficiency alone cause mild adhesion defects at best.21,23,46 Isolated P1V1 and P1V3 neutrophils retained considerable ability to adhere to endothelial cells, despite reduced integrin surface levels. Adhesion defects are often more apparent under flow, for example in Vav1−/−, but not P-Rex1−/− neutrophils,26,28 so flow within the airway microvasculature may contribute to the neutrophil-intrinsic component of the adhesion defect. However, our combined platelet reconstitution and intravital microscopy experiments showed that P-Rex/Vav expression in platelets is the crucial factor conferring neutrophil intravascular adhesion.
Vav family Rac-GEFs promote integrin-dependent responses (outside-in signaling) or integrin affinity (inside-out signaling) in many cell types, but in myeloid cells, they seem to control mainly outside-in signaling,23,47 whereas P-Rex1 knockdown impairs inside-out signaling.26 Combined P-Rex/Vav deficiency might therefore affect both. The reduced platelet PSGL1 levels in P1V1 and P1V3 mice likely affect L-selectin signaling in neutrophils and E-selectin signaling in endothelial cells, thereby leading to reduced integrin affinity and leukocyte vascular adhesion. The consequences of P-Rex/Vav deficiency for the selectin- and integrin-dependent activation of Rac should be assessed in all cell types involved, and in combinations of these. In addition, the TLR4 pathway may be affected during LPS-induced inflammation, as both P-Rex and Vav mediate LPS responses.25,48 Finally, we have previously shown that Mac1 levels on the surface of P1V1 neutrophils are reduced, whereas total cellular Mac1 levels were normal.21 It remains to be seen whether the same applies for platelet PSGL1 and other adhesion molecules in the relevant cell types, perhaps through an underlying role of these Rac-GEFs in vesicle trafficking.
Peritoneal neutrophil recruitment occurred to different extents in strains of different genetic backgrounds, as expected.49 In PVWT mice, it occurred in 2 phases, with a marked dip at the commonly used time point of 4.5 hours likely reflecting a net shift in neutrophil transmigration, mobilization, and clearance. In Vav null mice, recruitment was normal at 1.5 hours but increased at 4.5 hours. Perhaps, as Vav null neutrophils cannot spread despite normal adhesion,21,23 this increases their ability to transmigrate under certain conditions. Recruitment in P-Rex null mice was comparable to that seen in P-Rex1−/− mice,24,25 showing that P-Rex2 is not required. Furthermore, although vascular permeability and transmigration are separate processes, both involve transient remodeling of endothelial cell junctions. One P-Rex1−/− mouse study reported defects in TNFα-dependent vascular permeability during pulmonary neutrophil recruitment,50 whereas another equally convincingly showed normal permeability during neutrophil recruitment to the kidney.26 Different P-Rex1−/− strains were used, but both included otherwise comparable neutrophil phenotypes, so the reason for this discrepancy remains unclear.24-26,50 Here we report normal TGC-induced vascular permeability in P1V1 or P1V3 mice, but impaired leukocyte intravascular adhesion, which is a prerequisite step to transmigration.
Finally, the inflammatory response to allergen that ensues from pulmonary leukocyte recruitment contributes to altered lung function in patients with asthma.51 Bronchial hyperresponsiveness is a characteristic feature of this disease, with airways being more sensitive to a range of stimuli, including contractile agents. Rodent models have shown that this is both treatable with antiinflammatory drugs52 and is platelet dependent.53 We show that P1V1 and P1V3 mice are protected from allergen-induced leukocyte recruitment into the lung and from the associated development of bronchial hyperresponsiveness. Our results suggest that P-Rex and Vav family Rac-GEFs may be excellent targets for the development of novel antiinflammatory drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tyrone Fuller for technical assistance and the animal facility staff at the Babraham Institute and King's College London for their passionate care in looking after our mice.
This work was supported by a graduate studentship from the Cambridge Overseas Trust (D.P.). The work at the Babraham Institute was supported by a Core grant to the Signalling Programme, and the work at King's College London was supported by the Sackler Foundation and the King's College London School of Biomedical Sciences.
Authorship
Contribution: D.P. and S.C.P. designed, performed, and analyzed experiments and wrote the paper; C.P.P. and H.C.E.W. designed and analyzed experiments and wrote the paper; Y.R.-V. and D.S. designed, performed, and analyzed experiments; R.T.A. and S.J.C. performed and analyzed experiments; and M.J.W. designed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heidi Welch, Signalling Programme, Babraham Institute, Babraham Research Campus, Cambridge CB22 3AT, United Kingdom; e-mail: heidi.welch@babraham.ac.uk; and Simon Pitchford, Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King's College London, 5th Floor, Franklin-Wilkins Building, 150 Stamford St, London SE1 9NH, United Kingdom; e-mail: simon.pitchford@kcl.ac.uk.