Key Points
Fondaparinux seems to be an effective and safe alternative for the management of suspected HIT.
Abstract
Current guidelines for heparin-induced thrombocytopenia (HIT) management recommend heparin cessation and switching to a nonheparin anticoagulant (ie, argatroban, danaparoid) upon clinical suspicion. Fondaparinux may be effective but information supporting its use is limited. We retrospectively evaluated 239 patients who received a nonheparin anticoagulant (fondaparinux = 133, danaparoid = 59, and argatroban = 47) for suspected or confirmed HIT. A propensity score was constructed based on age, gender, creatinine, 4T scores, and comorbidity index, and used to match 133 patients to 60 controls. Outcomes were thrombosis or thrombosis-related death and major bleeding. In the matched population there were 22 (16.5%) episodes of thromboses in the fondaparinux group and 13 (21.4%) in the control group (χ2P = .424). Bleeding was observed in 28 (21.1%) patients in the fondaparinux group compared with 12 (20%) in the control group (χ2P = .867). Survival analysis, and subgroup and unmatched analyses showed similar results. In the fondaparinux group, 60% of patients received prophylactic doses. Fondaparinux has similar effectiveness and safety as argatroban and danaparoid in patients with suspected HIT. Prophylactic fondaparinux doses seem to be effective if no indication for full anticoagulation exists.
Introduction
Heparin-induced thrombocytopenia (HIT) is a severe complication of heparin therapy with potentially devastating consequences.1-4 This complication occurs as a result of antibodies binding to the highly immunogenic complex of heparin and platelet factor 4 (PF4). These antibodies subsequently activate platelets through their Fc receptors, causing the release of prothrombotic platelet-derived microparticles, which in turn promote thrombin generation and contribute to a hypercoagulable state.5-8 HIT has been reported to occur in approximately 1% to 3% of all patients receiving unfractionated heparin products, and if it is not recognized, thrombosis (arterial, venous, or both) can occur in up to 50% of patients with thrombosis-related deaths ranging from 5% to 10%.1,2,9,10
HIT is suspected clinically in the setting of recent or current heparin exposure if thrombocytopenia and/or thrombosis develop. Clinical scores such as the 4T score or the HIT Expert Probability Score are commonly used to risk stratify patients, but the diagnosis involves demonstrating the presence of the antibodies and their dependence on heparin usually through an enzyme-linked immunosorbent assay (ELISA) and a confirmatory test such as the serotonin release assay (SRA).9-12 Current recommendations are that once HIT is suspected, all heparin products must be discontinued and patients be switched to a non-heparin anticoagulant at full therapeutic doses. The 2012 edition of the American College of Chest Physician guidelines have suggested the use of either danaparoid (a factor Xa inhibitor), argatroban, or lepirudin (both direct thrombin inhibitors).2,12
Due to the rarity of the condition there is a paucity of good quality studies evaluating the use of these agents in the treatment of HIT. Support for the use of danaparoid comes from one prospective randomized controlled trial that compared danaparoid with dextran 70, demonstrating a trend toward reduced recurrent thrombosis, without increasing the risk of major bleeding.13 Evidence supporting the use of argatroban and lepirudin come from pooled-analyses of prospective cohort studies.12,14-18 Both agents demonstrate a benefit in preventing new thrombosis compared with the use of vitamin-K antagonists. However, danaparoid has been demonstrated to carry an approximate 10% in vitro cross-reactivity rate with HIT-associated antibodies, thus possibly potentiating the thrombotic complications of HIT.19 In the case of argatroban and lepirudin, both agents demonstrate no cross-reactivity with HIT antibodies in prospective cohort studies, but have been shown to have a high bleeding risk ranging from 5% to 11.5%.20-24 Most importantly, danaparoid and lepirudin are currently unavailable in many jurisdictions, leaving the use of argatroban (which must be given by continuous IV infusion) as the only treatment option with its associated costs, inconvenience, and bleeding risk.
Fondaparinux is another factor Xa inhibitor that is modeled after the critical heparin pentasaccharide sequence.25 Given its wide availability, ease of administration, low cost, and safety profile, this agent is an attractive option for the treatment of HIT. It has been used off-label to treat HIT and some evidence suggests that HIT might be indeed the most frequent indication in the United States,26 although this is different in other countries.27 The evidence supporting its use however is very limited, ranging from case reports to very small cohorts and case series. In the 1 prospective cohort study that evaluated fondaparinux for the management of HIT, the authors reported similar rates of recurrent venous thromboembolic events (VTEs) and major bleeding when comparing fondaparinux to historical controls treated with lepirudin.28,29 In our institution, fondaparinux has been used off-label for HIT for a number of years,30,31 although argatroban and danaparoid are also available. The choice of agent is left to the discretion of the treating physician based on the individual patient’s characteristics. Usually, argatroban is chosen if the patient has renal failure or there is a need to use an agent that has a shorter half-life, such as a patient requiring frequent invasive procedures in an intensive care setting or if the patient is thought to have a higher bleeding risk. Therefore, we sought to investigate the safety and efficacy of fondaparinux in the treatment of patients with suspected HIT compared with patients treated with the approved agents, danaparoid and argatroban.
Methods
Study design and participants
We conducted a retrospective cohort study, including adult men and nonpregnant, nonbreastfeeding women admitted to the London Health Sciences Centre between February 2005 and June 2011 and who received a non-heparin anticoagulant for suspected HIT. All patients who had fondaparinux, danaparoid, or argatroban prescribed during their hospital admission were identified by searching the electronic pharmacy records and crosschecked with all patients who had a HIT-ELISA ordered during the same time period. Ordering of a HIT-ELISA test was at the discretion of the admitting physician, but in our center all tests had to be authorized by a hematologist. In order to better ascertain the outcomes associated with each drug, we excluded all patients who received more than one nonheparin anticoagulant during the same hospital admission. Patients were also excluded if health records were unavailable, if they were not exposed to heparin during hospital admission, or if they were diagnosed with HIT as an outpatient. Information on demographic and clinical variables, HIT diagnostic criteria (4T score), HIT-ELISA result (including optical density when available) and SRA, drug use, and outcomes of interest were obtained by reviewing electronic and paper records by 2 reviewers and discrepancies were resolved by consensus conjointly with a third investigator. Two investigators who were unaware of the drug the patients were receiving evaluated all outcomes. Information was recorded using a standardized ad hoc electronic data capture tool.
We considered a positive diagnosis of HIT in patients who had either a positive SRA or in whom a diagnosis of HIT was documented by an attending hematologist during hospital admission, since not all patients had an SRA done. Criteria for a diagnosis of HIT were based on Greinacher and Warkentin’s definition and included a high clinical likelihood in the presence of a positive HIT-ELISA or an intermediate likelihood in the presence of a strongly positive HIT-ELISA.32
Laboratory tests
Patients’ plasma was collected in 3.2% (0.109 mol/L) sodium citrate polypropylene tubes (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ) and centrifuged at 1500g for 20 minutes at room temperature and tested usually within 24 hours. If this was not possible, aliquots were frozen at −70°C until tested. Detection of antibodies directed against the PF4/heparin complex was done using a commercial polyspecific solid phase enzyme-linked immunosorbent assay (PF4 Enhanced; GTi Diagnostics, Waukesha WI) according to the manufacturer’s instructions. Mean optical densities ≤0.300 were considered negative, whereas those ≥0.400 were considered positive. Optical densities ≥2.0 were considered strongly positive. In patients with clinical uncertainty, further testing using an SRA was requested. These assays were sent to a reference laboratory (Hamilton Health Sciences Centre, Hamilton, ON, Canada) and performed as previously reported.33
Study outcomes
The primary efficacy outcome was a composite of the following: the occurrence of an objectively documented new arterial or venous thrombotic event, amputation, gangrene, thrombosis-related death, or death in which a thrombotic event cannot be excluded. Confirmed arterial and venous thrombosis, and thrombosis-related mortality were based on objective autopsy or radiographic findings including ultrasound, computed tomography, magnetic resonance imaging, and angiography. VTEs were diagnosed using previously published criteria.34 The primary safety outcome was the occurrence of a major bleeding event (defined according to the International Society on Thrombosis and Haemostasis35 ) during the period of administration of a nonheparin anticoagulant and up to 2 days after its discontinuation. We attempted to assess nonmajor bleeding events (defined as overt bleeding that did not meet the criteria for a major bleed, but were documented by any health professional); however, due to improper documentation, we were unable to do this. Other secondary outcomes included time to platelet count recovery from nadir to at least 100 × 109/L or to baseline, if the baseline count was <100 × 109/L. Unless otherwise stated, all outcomes were measured at 30 days or until hospital discharge, whichever occurred last.
Statistical analysis
Due to the retrospective nature of the study, we performed a post hoc power analysis, which showed that our sample size achieved 74% power to detect a 10% difference in thrombosis outcomes (assuming a baseline proportion of 21% for the control group and 16% for the treatment group using a 1-sided score test [Gart and Nam] at the 0.025 level of significance).
Due to the study design, we expected potential imbalances between groups and in an effort to reduce potential bias, a propensity score–matched analysis was planned. A propensity score has been defined as the “conditional probability of being treated given the covariates.”36-39 It is used to balance observed covariates, although in contrast with randomization, it does not balance variables that were not observed. Propensity score matching was done using a software package implemented in SPSS 20.0 (IBM, Armonk NY) and R2.12.0,40 and available freely through a GNU General Public License.41 To estimate the propensity score, we used logistic regression including 5 covariates: age, gender, creatinine, 4T scores, and the Charlson comorbidity index as modified by Quan et al.42 These variables were selected because they were considered to be relevant predictors of thrombosis and bleeding.43 Matching was done using the nearest neighbor method using a 1:M (one-to-many) ratio with replacement. Analyses were conducted in the unmatched and matched cohorts as described hereafter. The primary analysis was done between the fondaparinux and the argatroban/danaparoid group with secondary analyses for individual drugs. Finally, to test for consistency across strata, the cohort was divided by propensity score quintiles and stratified group comparisons were conducted for the main outcomes. Summary statistics are presented as a median and interquartile range (IQR), mean and standard deviation (SD), or number and percentage. A 95% confidence interval (CI) for proportions was calculated using the Wilson score method44 in OpenEpi version 3.01.45 Comparisons between groups were done using χ2 or Fisher’s exact tests, as appropriate, for categorical variables. The Mantel–Haenszel common odds ratio (OR) were estimated for the primary efficacy and safety endpoints using contingency table analysis under an assumption of a common OR of 1. Imprecision was evaluated by determining the 95% CI using nonparametric bootstrapping with 1000 replications. Continuous variables were compared using Mann-Whitney or Student t tests. Survival analysis was conducted using the Kaplan-Meier method using the log-rank test for comparison between groups.46,47 P < .05 were considered as significant.
Results
Baseline patients’ characteristics
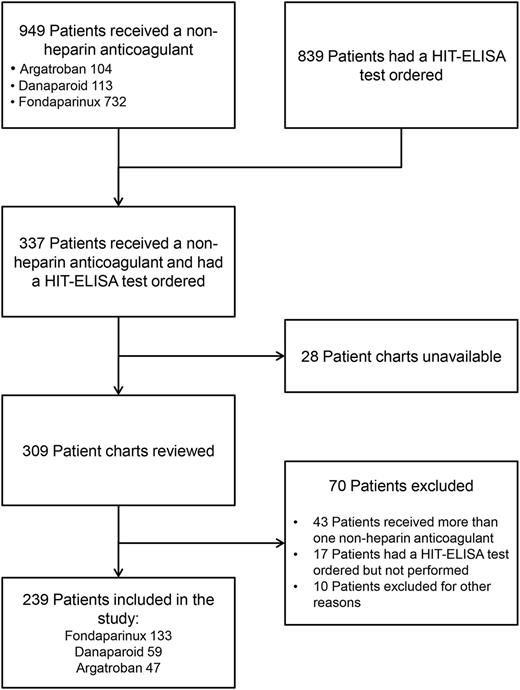
Between February 2005 and June 2011, 949 patients received a non-heparin anticoagulant (argatroban = 104, danaparoid = 113, and fondaparinux = 732), 839 patients had a HIT-ELISA test ordered, and we identified 337 patients who received a nonheparin anticoagulant and had a HIT-ELISA test ordered. The flow of patients through the study is shown in Figure 1. The final study cohort included 239 patients, 133 (55.6%) received fondaparinux, 59 (24.7%) danaparoid, and 47 (19.7%) argatroban. The baseline characteristics of the unmatched and matched cohorts are shown in Table 1 and supplemental Table 1, available on the Blood Web site (for individual anticoagulants). The mean duration of admission for all patients was 40 days, and the mean duration of follow up from heparin discontinuation to hospital discharge was 28 days. Creatinine and the Charlson comorbidity index were higher in the argatroban/danaparoid group. Among the argatroban/danaparoid group, more patients were categorized as high probability according to the 4T score; however, there was no difference in the median 4T score or in the proportion of patients with a positive HIT-ELISA assay. Approximately one-third of patients in both groups were diagnosed with HIT during their hospital stay.
Flowchart of the cohort study showing the process of cohort integration.
Baseline characteristics of the unmatched and matched cohorts
| . | Unmatched cohort . | Matched cohort . | ||||
|---|---|---|---|---|---|---|
| . | Argatroban/danaparoid (n = 106) . | Fondaparinux (n = 133) . | P* . | Argatroban/danaparoid (n = 60) . | Fondaparinux (n = 133) . | P* . |
| Age, years, median (IQR) | 68 (58-77) | 68 (61-78) | .511 | 67 (58-77) | 68 (61-78) | .537 |
| Male gender, N (%) | 50 (47.2) | 64 (48.1) | .884 | 30 (29.1) | 64 (48.1) | .809 |
| Length of hospital stay, days (mean [SD]) | 42.6 (33.1) | 39.1 (43.7) | .489 | 42.6 (35.9) | 39.1 (43.7) | .585 |
| Creatinine, mmol/L (median [IQR]) | 152 (81-250) | 90 (62-125) | <.001 | 96 (59-166) | 90 (62-125) | .238 |
| Duration of alternative anticoagulation, days (median [IQR]) | 4 (2-10) | 4 (2-10) | .637 | 4 (2-7) | 4 (2-10) | .326 |
| Indication for anticoagulation, N (%) | ||||||
| Venous thromboembolism | 24 (22.6) | 40 (30.1) | .197 | 17 (28.3) | 40 (30.1) | .806 |
| Arterial thrombosis | 7 (6.6) | 10 (7.5) | .785 | 4 (6.7) | 10 (7.5) | 1.0 |
| Prosthetic mechanical heart valve | 8 (7.5) | 7 (5.3) | .470 | 4 (6.7) | 7 (5.3) | .741 |
| Charlson comorbidity index, median (IQR) | 2 (1-3) | 1 (0−2) | .030 | 1 (0-2) | 1 (0-2) | .945 |
| 4T score, median (IQR) | 4 (3-6) | 5 (3-6) | .707 | 4 (3-6) | 5 (3-6) | .439 |
| 4T score category, N (%) | .018 | .008 | ||||
| Low probability (0-3) | 39 (36.8) | 38 (28.6) | — | 25 (41.7) | 38 (28.6) | — |
| Intermediate probability (4-5) | 29 (27.4) | 60 (45.1) | — | 13 (21.7) | 60 (45.1) | — |
| High probability (6-8) | 38 (35.8) | 35 (26.3) | — | 22 (36.7) | 35 (26.3) | — |
| HIT-ELISA test result, N (%) | .813 | .199 | ||||
| Negative | 57 (53.8) | 69 (51.9) | — | 39 (65.0) | 69 (51.9) | — |
| Equivocal | 13 (12.3) | 14 (10.5) | — | 6 (10.0) | 14 (10.5) | — |
| Positive | 36 (34.0) | 50 (37.6) | — | 15 (25.0) | 50 (37.6) | — |
| HIT diagnosis during admission, N (%) | 38 (35.8) | 44 (33.1) | .654 | 20 (33.3) | 44 (33.1) | .973 |
| . | Unmatched cohort . | Matched cohort . | ||||
|---|---|---|---|---|---|---|
| . | Argatroban/danaparoid (n = 106) . | Fondaparinux (n = 133) . | P* . | Argatroban/danaparoid (n = 60) . | Fondaparinux (n = 133) . | P* . |
| Age, years, median (IQR) | 68 (58-77) | 68 (61-78) | .511 | 67 (58-77) | 68 (61-78) | .537 |
| Male gender, N (%) | 50 (47.2) | 64 (48.1) | .884 | 30 (29.1) | 64 (48.1) | .809 |
| Length of hospital stay, days (mean [SD]) | 42.6 (33.1) | 39.1 (43.7) | .489 | 42.6 (35.9) | 39.1 (43.7) | .585 |
| Creatinine, mmol/L (median [IQR]) | 152 (81-250) | 90 (62-125) | <.001 | 96 (59-166) | 90 (62-125) | .238 |
| Duration of alternative anticoagulation, days (median [IQR]) | 4 (2-10) | 4 (2-10) | .637 | 4 (2-7) | 4 (2-10) | .326 |
| Indication for anticoagulation, N (%) | ||||||
| Venous thromboembolism | 24 (22.6) | 40 (30.1) | .197 | 17 (28.3) | 40 (30.1) | .806 |
| Arterial thrombosis | 7 (6.6) | 10 (7.5) | .785 | 4 (6.7) | 10 (7.5) | 1.0 |
| Prosthetic mechanical heart valve | 8 (7.5) | 7 (5.3) | .470 | 4 (6.7) | 7 (5.3) | .741 |
| Charlson comorbidity index, median (IQR) | 2 (1-3) | 1 (0−2) | .030 | 1 (0-2) | 1 (0-2) | .945 |
| 4T score, median (IQR) | 4 (3-6) | 5 (3-6) | .707 | 4 (3-6) | 5 (3-6) | .439 |
| 4T score category, N (%) | .018 | .008 | ||||
| Low probability (0-3) | 39 (36.8) | 38 (28.6) | — | 25 (41.7) | 38 (28.6) | — |
| Intermediate probability (4-5) | 29 (27.4) | 60 (45.1) | — | 13 (21.7) | 60 (45.1) | — |
| High probability (6-8) | 38 (35.8) | 35 (26.3) | — | 22 (36.7) | 35 (26.3) | — |
| HIT-ELISA test result, N (%) | .813 | .199 | ||||
| Negative | 57 (53.8) | 69 (51.9) | — | 39 (65.0) | 69 (51.9) | — |
| Equivocal | 13 (12.3) | 14 (10.5) | — | 6 (10.0) | 14 (10.5) | — |
| Positive | 36 (34.0) | 50 (37.6) | — | 15 (25.0) | 50 (37.6) | — |
| HIT diagnosis during admission, N (%) | 38 (35.8) | 44 (33.1) | .654 | 20 (33.3) | 44 (33.1) | .973 |
ELISA, enzyme-linked immunosorbent assay; HIT, heparin-induced thrombocytopenia; IQR, interquartile range; SD, standard deviation.
χ2 or Fisher’s exact test for categorical variables, Mann Whitney U, or Student t test for continuous variables.
Matched cohort patients’ characteristics
The matching procedure resulted in balanced groups. The dot plot of standardized mean differences between the argatroban/danaparoid group and the fondaparinux group for all covariates considered in the propensity score is shown in supplemental Figure 1. The distribution curves of propensity scores for both groups before and after matching are shown in supplemental Figure 2. After matching, 133 patients in the fondaparinux group were matched to 60 controls (20 argatroban and 40 danaparoid). No statistical differences were seen after matching for length of hospital stay, creatinine, and the Charlson comorbidity index. The proportion of patients within the high probability group category according to the 4T score was higher in the argatroban/danaparoid group, but again, there was no difference in the median 4T score or in the percentage of patients diagnosed with HIT.
Primary efficacy outcome
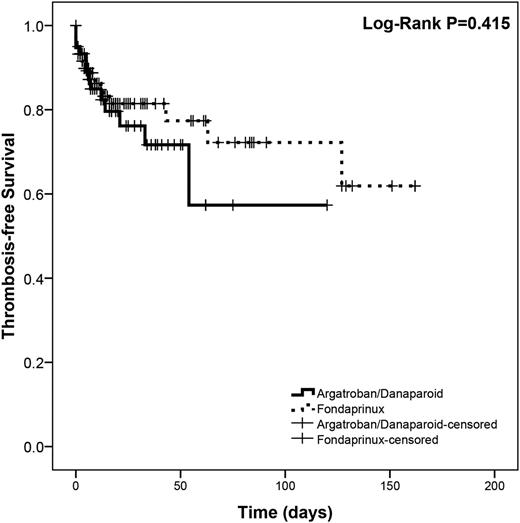
Results are summarized in Table 2. Overall, there were 43/239 (18%) and 35/193 (18.1%) thrombotic events in the unmatched and matched cohorts, respectively. There was no statistically significant difference in the efficacy composite outcome between the groups, before or after matching. A sub-analysis stratified by propensity score quintile found no differences in the proportion of events in any quintile. After matching, the Mantel-Haenszel common OR was 0.717 (95% CI, 0.333-1.541; P = .394). Survival analysis showed no difference between groups (log-rank P = .415; Figure 2). In a sub-analysis by individual anticoagulant, we did not find a statistically significant difference between individual agents (16.5% for fondaparinux, 18.6% for danaparoid, and 21.3% for argatroban; P = .759) (supplemental Table 2).
Primary study outcomes
| . | Argatroban/danaparoid n/N (%) . | Fondaparinux n/N (%) . | P* . |
|---|---|---|---|
| Primary efficacy outcome† | |||
| Unmatched cohort | 21/106 (19.8) | 22/133 (16.5) | .392 |
| Matched cohort | 13/60 (21.4) | 22/133 (16.5) | .424 |
| Primary safety outcome‡ | |||
| Unmatched cohort | 27/106 (25.5) | 28/133 (21.1) | .420 |
| Matched cohort | 12/60 (20.0) | 28/133 (21.1) | .867 |
| . | Argatroban/danaparoid n/N (%) . | Fondaparinux n/N (%) . | P* . |
|---|---|---|---|
| Primary efficacy outcome† | |||
| Unmatched cohort | 21/106 (19.8) | 22/133 (16.5) | .392 |
| Matched cohort | 13/60 (21.4) | 22/133 (16.5) | .424 |
| Primary safety outcome‡ | |||
| Unmatched cohort | 27/106 (25.5) | 28/133 (21.1) | .420 |
| Matched cohort | 12/60 (20.0) | 28/133 (21.1) | .867 |
χ2 or Fisher’s exact test.
Thrombosis and thrombosis-related mortality during nonheparin anticoagulation.
Bleeding and bleeding-related mortality during nonheparin anticoagulation.
Kaplan-Meier survival curve showing thrombosis-free survival in the control and treatment groups after propensity score matching.
Kaplan-Meier survival curve showing thrombosis-free survival in the control and treatment groups after propensity score matching.
Primary safety outcome
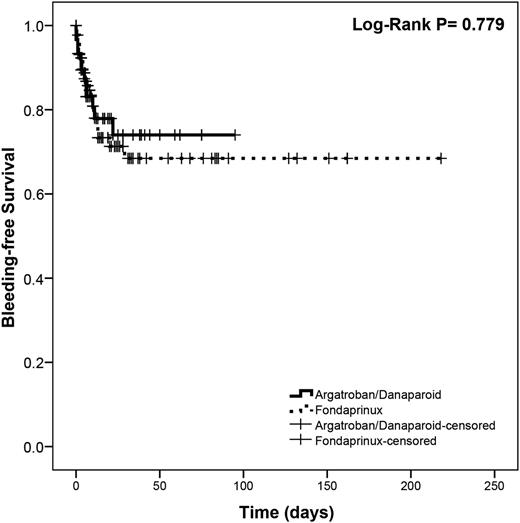
Overall, there were 55/239 (23.0%) and 40/193 (20.7%) bleeding events in the unmatched and matched cohorts, respectively. We did not find differences between groups before or after matching (Table 2). A sub-analysis stratified by propensity score quintile found no differences in the proportion of events in any quintile. In the matched cohort, the Mantel-Haenszel common OR was 1.067 (95% CI, 0.500-2.275; P = .867). Survival analysis showed no difference between groups (log-rank P = .779; Figure 3). In a sub-analysis by individual anticoagulants, a higher percentage of argatroban patients experienced bleeding, but this was not statistically significant (supplemental Table 2).
Kaplan-Meier survival curve showing bleeding-free survival in the control and treatment groups after propensity score matching.
Kaplan-Meier survival curve showing bleeding-free survival in the control and treatment groups after propensity score matching.
Additional analysis
There were 71 deaths during hospital admission (29.7%), 41 (38.7%) in the argatroban/danaparoid group and 30 (22.6%) in the fondaparinux group (P = .007). There were 48/157 (30.6%) deaths among patients not diagnosed with HIT compared with 23/82 (28%) among patients diagnosed with HIT. In the unmatched cohort, the median time to platelet recovery (baseline or >100 × 109/L) was 4 days in the argatroban/danaparoid group (interquartile range [IQR], 2-8) and 4 days (IQR, 2-6) in the fondaparinux group (P = .641). This did not change in the matched cohort.
Exploratory subgroup analyses stratified by the presence or not of a confirmed HIT diagnosis during hospitalization showed no differences for the primary efficacy outcome. However, there were more bleeding events among patients diagnosed with HIT who received fondaparinux, and the opposite was seen in fondaparinux patients without a diagnosis of HIT (Table 3). Finally, among the 133 patients who received fondaparinux, 79 (59.4%) received prophylactic doses (2.5 mg) and 54 (40.6%) had indications for full anticoagulation (eg, VTE) and therefore received therapeutic doses.
Primary study outcomes stratified by confirmed diagnosis of heparin-induced thrombocytopenia
| . | Argatroban/danaparoid n/N (%) . | Fondaparinux n/N (%) . | P* . |
|---|---|---|---|
| HIT diagnosed during hospitalization | |||
| Primary efficacy outcome† | |||
| Unmatched cohort | 10/38 (26.3) | 7/44 (15.9) | .246 |
| Matched cohort | 5/20 (25.0) | 7/44 (15.9) | .492 |
| Primary safety outcome‡ | |||
| Unmatched cohort | 7/38 (18.4) | 16/44 (36.4) | .071 |
| Matched cohort | 1/20 (5.0) | 16/44 (36.4) | .008 |
| HIT not diagnosed during hospitalization | |||
| Primary efficacy outcome† | |||
| Unmatched cohort | 11/68 (16.2) | 15/89 (16.9) | .910 |
| Matched cohort | 8/40 (20.0) | 15/89 (16.9) | .666 |
| Primary safety outcome‡ | |||
| Unmatched cohort | 20/68 (29.4) | 12/89 (13.5) | .014 |
| Matched cohort | 11/40 (27.5) | 12/89 (13.5) | .054 |
| . | Argatroban/danaparoid n/N (%) . | Fondaparinux n/N (%) . | P* . |
|---|---|---|---|
| HIT diagnosed during hospitalization | |||
| Primary efficacy outcome† | |||
| Unmatched cohort | 10/38 (26.3) | 7/44 (15.9) | .246 |
| Matched cohort | 5/20 (25.0) | 7/44 (15.9) | .492 |
| Primary safety outcome‡ | |||
| Unmatched cohort | 7/38 (18.4) | 16/44 (36.4) | .071 |
| Matched cohort | 1/20 (5.0) | 16/44 (36.4) | .008 |
| HIT not diagnosed during hospitalization | |||
| Primary efficacy outcome† | |||
| Unmatched cohort | 11/68 (16.2) | 15/89 (16.9) | .910 |
| Matched cohort | 8/40 (20.0) | 15/89 (16.9) | .666 |
| Primary safety outcome‡ | |||
| Unmatched cohort | 20/68 (29.4) | 12/89 (13.5) | .014 |
| Matched cohort | 11/40 (27.5) | 12/89 (13.5) | .054 |
χ2 or Fisher’s exact test.
Thrombosis and thrombosis-related mortality during nonheparin anticoagulation.
Bleeding and bleeding-related mortality during nonheparin anticoagulation.
Discussion
Fondaparinux is currently indicated for the prevention of VTE in patients undergoing orthopedic procedures and abdominal surgery, for the treatment of venous thromboembolic disease, the management or unstable angina, and non-ST or ST segment elevation myocardial infarction. However, studies have suggested that off-label use in patients with suspected or confirmed HIT may exceed its used for approved indications.26 The present study is the largest to date, to evaluate the use of fondaparinux for the treatment of HIT. Previous studies have been limited to small retrospective studies and case reports.28-31,48 Our findings suggest that in patients with suspected or confirmed HIT, fondaparinux is as effective as danaparoid or argatroban to prevent thrombotic events, probably with a similar safety profile.
Interestingly, when looking at the unmatched patient cohort, we found that there were a higher percentage of patients in the argatroban group with bleeding complications. We attributed this finding to the fact that this group also had a higher proportion of patients with renal dysfunction. Despite this finding, there was still no statistically significant difference identified when comparing its rates to the other two drugs.
From a clinical perspective, there are important factors to consider with our results. First, with respect to cost and convenience, fondaparinux is both less expensive and more convenient to use than the current standards. For instance, argatroban, being an infusion, requires IV access, intensive nursing monitoring, frequent blood work, and regular dose readjustments by a physician.16,22 Additionally, although danaparoid can be given either IV or by subcutaneous injection following an initial IV bolus, it is not available in all jurisdictions. Second, the benefits of fondaparinux have been demonstrated in both in vitro and in vivo studies.23,24,49,50 In vitro, fondaparinux exerts very minimal cross-reactivity to HIT antibodies.23,49 In vivo, it has been demonstrated that despite the fact that it is possible for anti–fondaparinux-PF4 antibodies to form, these antibodies have been shown to not have any clinical implications in the development of HIT.24,50 This is in contrast to danaparoid that has been shown to have up to 10% cross-reactivity to HIT antibodies, thus possibly potentiating the complications of HIT.18,19
However, there are limitations to our study that should be considered. First, given that this was a retrospective cohort study, it is susceptible to selection bias, especially if a nonrepresentative sample of patients were selected for analysis. The use of propensity score matching allowed us to balance our treatment and control groups on measured covariates. However, given the observational nature of our study, unmeasured confounders, which were not incorporated in the propensity score, may still influence the results. Second, conclusions such as causation and relative risk cannot be established, as these types of inferences would require a prospective study design at minimum, and ideally a prospective randomized controlled trial. However, despite this limitation, our results suggest that fondaparinux is noninferior to the current standards with respect to thrombosis prevention and bleeding risk. Third, although there was no statistically significant difference found between the treatment and control groups, there is the possibility of a type II error. We were unable to do a power calculation a priori, given the low prevalence of HIT. In our post hoc analysis, we found that our sample size achieved 74% power to detect a 10% difference in thrombosis between groups, with the caveats associated with this type of calculation. Fourth, given that we were unable to retrieve all potential patient files, we could not match all potentially eligible patients, which may limit the generalizability of our findings. In addition, given the retrospective nature of this study, not all patients with suspected HIT had an SRA done for confirmation. Diagnosis of HIT was made in patients who had either a positive SRA or a positive HIT-ELISA, in combination with an intermediate or high clinical likelihood. The fact that a gold standard diagnostic test (ie, an SRA) was not done on all patients would introduce a source of bias, as patients may have been misclassified as having, or not having, HIT. Fifth, the follow up period was relatively short and there was a possibility of an increased thrombotic risk beyond hospital discharge; however, our follow up was similar to previous studies evaluating argatroban and danaparoid.13,16,17 Finally, we noticed that our bleeding-related complications rates for both argatroban and danaparoid were higher than the rates reported in previous studies.11,12 The small sample size of each group (and higher margin of error) and the higher proportion of patients with renal dysfunction in the argatroban group may have contributed to this finding.
In conclusion, our study is the largest conducted to date providing evidence supporting the use of fondaparinux for the management of suspected HIT. In addition, it appears that unless there are indications for full dose anticoagulation, only prophylactic doses of fondaparinux may be needed for the treatment of suspected or confirmed HIT. Further studies are clearly needed in this area.
Presented in abstract form at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.L.-L. and M.J.K. designed the study; M.K., M.A., and S.S. abstracted data; A.L.-L. and M.K. analyzed the data; M.K. wrote the first draft of the manuscript; and A.L.-L., S.S., M.A., and M.J.K. edited the manuscript. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: A.L.-L. received honoraria from Pfizer, Leo Pharma, and Boehringer Ingelheim and has participated in studies sponsored by Pfizer, Leo Pharma, Bayer, Daiichi-Sankyo, Novartis, and Celgene. None of these entities were involved in any aspect of this study. The remaining authors declare no competing financial interests.
Correspondence: Alejandro Lazo-Langner, Hematology Division, London Health Sciences Centre, 800 Commissioners Rd E, Room E6-216A, London ON, N6A 5W9, Canada; e-mail: alejandro.lazolangner@lhsc.on.ca.