Key Points
Normal plasma VWF multimers act as a cofactor in the factor I–mediated cleavage of C3b to iC3b and inhibit complement activation.
Large VWF multimers, including ultra-large VWF multimers, do not have factor I cofactor activity and permit complement activation.
Abstract
Several complement proteins interact with hemostatic factors. We discovered that von Willebrand factor (VWF) acts as a cofactor for factor I–mediated cleavage of complement C3b, thereby shutting down complement activation. The complement regulatory function of VWF multimers depends on their size. Smaller VWF multimers enhance cleavage of C3b but large and ultra-large VWF (ULVWF) multimers have no effect on C3b cleavage and permit default complement activation. We conclude that normal plasma VWF multimers prevent complement activation and steer the complement pathway toward generation of inactivated C3b (iC3b). ULVWF multimers, as are present in patients with thrombotic microangiopathy, lack an inhibitory effect on complement and permit complement activation.
Introduction
The complement and coagulation systems interact as exemplified by proteins with dual coagulation and complement functions, such as thrombomodulin, which enhances activation of protein C and degradation of anaphylatoxins,1 or factor H (fH), which acts as a cofactor for cleavage of both C3b and von Willebrand factor (VWF).2,3
We have shown that fH binds to VWF and enhances cleavage of VWF by ADAMTS13.3 This raises the possibility that VWF binding might also alter complement regulation by fH. VWF mutations have been identified in patients with atypical hemolytic uremic syndrome,4 raising the possibility that VWF mutations have contributed to complement dysregulation. To examine the role of VWF in complement regulation, we compared the cleavage of C3b by factor I (fI) in the presence or absence of VWF.
Study design
All of the studies were approved by the institutional review board of the University of Texas MD Anderson Cancer Center.
Reagents
Purified C3, C3b, inactivated C3b (iC3b), fI, fH, factor B, factor D, anti-C3, and anti-C3a antibodies (Complement Technology Inc., Tyler, TX), plasma-purified VWF (pVWF; EMD Millipore Corporation, Billerica, MA), anti-VWF antibody (Dako, Carpinteria, CA), and secondary peroxidase-conjugated antibodies (GE Healthcare, Piscataway, NJ) were purchased from the relevant commercial sources. Ultra-large VWF (ULVWF)5 and recombinant VWF dimers (Δpro, encoded by a mutant VWF complementary DNA with deleted propeptide coding sequence)6 were provided by Dr Joel Moake (Rice University, Houston, TX).
In vitro cleavage of C3b, C3, or iC3b
C3b, C3, or iC3b was diluted separately in a cleavage buffer (100 mM tris(hydroxymethyl)aminomethane-HCl [pH 8.5], 4 mM CaCl2, 5 mM MgCl2, 0.02% polyoxyethyleneglycol dodecyl ether)7 and incubated with fI, fH, factor B, factor D, or VWF in different combinations at 37°C for 60 minutes. The cleaved products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by using reducing conditions and detected by Coomassie blue staining or transferred to immobilon-P membrane (Millipore) and immunoblotted by using anti-C3 or anti-C3a antibodies.
Immunoprecipitation and western blotting
Plasma-purified C3b (5 µg) and VWF (5 µg) were incubated with anti-VWF antibody (10 µL) or serum immunoglobulin G, protease inhibitor cocktail, and Sepharose-conjugated protein A/G at 4°C overnight. After 3 washes with phosphate-buffered saline buffer containing 0.01% Triton-100, precipitated proteins were separated by SDS-PAGE under reducing conditions, transferred to membranes, and immunoblotted by using anti-C3 or anti-VWF antibodies. VWF multimers were separated by nonreducing 1.7% SDS agarose gel electrophoresis before immunoblotting with anti-VWF antibody.
Results and discussion
There are 3 complement activation pathways converging on the formation of C3 convertase, which is the main engine for complement propagation. In the alternative complement pathway, C3 convertase (BbC3b) is composed of a proteolytic fragment of factor B (Bb) and C3b. Complement activation is tightly regulated at several steps, including fI/fH-mediated C3b cleavage, in which fH acts as a cofactor for fI that degrades C3b to inactive iC3b. iC3b cannot participate in further complement activation.
We investigated whether VWF functions as a cofactor for fI-mediated C3b cleavage and found that in the presence of pVWF, fI cleaved the 110-kDa α′ chain of C3b to 68 kDa and 43 kDa degradation products (iC3b) (Figure 1A). VWF alone, in the absence of fI, did not have any effect on C3b cleavage (supplemental Figure 1A available on the Blood Web site). C3b, but not C3 or iC3b, was the main substrate for fI/VWF proteolysis (Figure 1B). Increasing VWF concentration (supplemental Figure 1A) or prolonging the incubation time (supplemental Figure 1B) enhanced fI-mediated C3b cleavage. The cofactor activity of VWF in fI-mediated C3b cleavage progressively decreased in the presence of increasing concentrations of anti-VWF antibody. Although factor VIII and glycocalicin did not affect the cofactor activity of VWF, high concentrations of ADAMTS13 reduced fI/VWF-mediated C3b cleavage (supplemental Figure 2).
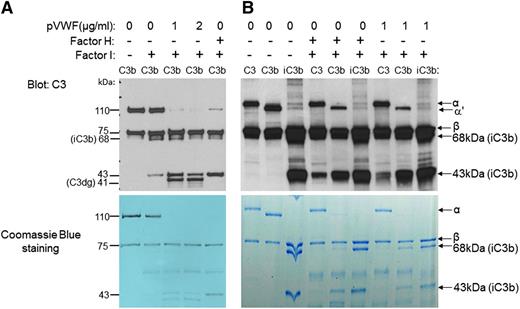
VWF is a cofactor for fI-mediated cleavage of C3b. (A) C3b (1 μg) was incubated with either fI (Factor I; 0.5 μg) and fH (Factor H; 1 μg) or with fI and different concentrations of pVWF (1 or 2 μg/mL). After western blotting with anti-C3 antibody (upper panel), α′ and β chains of C3b (110 and 75 kDa) and iC3b (68 and 43 kDa) were detected by their molecular weight. The 68- and 43-kDa bands were the products of cleavage of the α′ chain of C3b by fI. C3dg (41 kDa) is the result of cleavage of the 68-kDa band of iC3b by fI. Lower panel shows Coomassie blue staining of the same polyacrylamide gel. (B) To identify whether VWF changes the substrate specificity of fI, C3 (1 μg), C3b (1 μg), or iC3b (1 μg) was incubated with VWF and fI. Immunoblotting by using anti-C3 antibody (upper panel) showed that similar to the fI/fH-mediated cleavage, fI/VWF cleaves mainly C3b. α and β chains of C3 (120 and 75 kDa), α′ and β chains of C3b (110 and 75 kDa), and iC3b (68 and 43 kDa) were detected by their molecular weights. Lower panel shows Coomassie blue staining of the same polyacrylamide gel (n = 3).
VWF is a cofactor for fI-mediated cleavage of C3b. (A) C3b (1 μg) was incubated with either fI (Factor I; 0.5 μg) and fH (Factor H; 1 μg) or with fI and different concentrations of pVWF (1 or 2 μg/mL). After western blotting with anti-C3 antibody (upper panel), α′ and β chains of C3b (110 and 75 kDa) and iC3b (68 and 43 kDa) were detected by their molecular weight. The 68- and 43-kDa bands were the products of cleavage of the α′ chain of C3b by fI. C3dg (41 kDa) is the result of cleavage of the 68-kDa band of iC3b by fI. Lower panel shows Coomassie blue staining of the same polyacrylamide gel. (B) To identify whether VWF changes the substrate specificity of fI, C3 (1 μg), C3b (1 μg), or iC3b (1 μg) was incubated with VWF and fI. Immunoblotting by using anti-C3 antibody (upper panel) showed that similar to the fI/fH-mediated cleavage, fI/VWF cleaves mainly C3b. α and β chains of C3 (120 and 75 kDa), α′ and β chains of C3b (110 and 75 kDa), and iC3b (68 and 43 kDa) were detected by their molecular weights. Lower panel shows Coomassie blue staining of the same polyacrylamide gel (n = 3).
To investigate the effect of VWF multimeric size on C3b cleavage, we compared the cofactor activity of pVWF, recombinant VWF dimers (VWF-Δpro), and ULVWF. Although pVWF multimers enhanced C3b cleavage by fI, ULVWF did not have any effect on C3b cleavage (Figure 2A). To rule out the possibility that another plasma protein copurified with pVWF affected our results, we used VWF-Δpro and detected a similar cofactor activity (Figure 2A). To further study the effect of VWF multimeric composition on cofactor activity, we fractionated plasma VWF, collected VWF fractions from high to low molecular weight (Figure 2C), and measured the activity of each VWF fraction on fI-mediated C3b cleavage. Although VWF fractions with higher-molecular-weight multimers did not enhance C3b cleavage, VWF fractions with increasing abundance of lower-molecular-weight multimers (more specifically dimers, tetramers, and 6-mers) showed increasing cofactor activity (Figure 2D).
VWF cofactor activity in fI-mediated C3b cleavage depends on the multimeric size of VWF. (A) C3b (1 μg) was incubated with fI (0.5 μg) and pVWF (1 μg), recombinant VWF dimers (VWF-Δpro) (0.5 or 1 μg), or ULVWF (1 μg). Western blotting of the degradation products using anti-C3 antibody (upper panel) showed that pVWF and VWF-Δpro acted as cofactors for fI-mediated C3b cleavage, but ULVWF did not function as a cofactor. Lower panel shows the results of 1.7% sodium dodecyl sulfate-agarose gel electrophoresis and immunoblotting with anti-VWF antibody confirming the multimeric distribution of pVWF, ULVWF, and VWF-Δpro used in (A). (B) C3 (1 μg), proactivation complement proteins (factor B and factor D, 0.5 μg each), and fI (0.5 μg) were incubated with VWF-Δpro (1 μg) or ULVWF (1 μg). Degradation products were immunoblotted using anti-C3a, which detects C3a and any C3 fragments containing C3a (upper panel) or anti-C3 antibodies (lower panel). Although factor B in the presence of factor D and a small amount of spontaneously generated C3b (tick-over) promoted complement activation and production of C3a (upper panel) and C3b (lower panel), addition of VWF dimers helped fI to shift the balance toward complement inactivation (iC3b in the lower panel and no C3a in the upper panel). A 76-kDa band in the upper panel detected by anti-C3a antibody after incubation of C3 with VWF dimers, fI, factor B, and factor D was probably a degradation product of the α chain of C3 that was not cleaved by C3 convertase and still contained C3a but was partially cleaved by fI and lost its 43-kDa fragment. ULVWF did not enhance fI-mediated complement inhibition, and as a default pathway, C3 was directed toward generation of C3a and C3b (activation) (n = 3). (C) Plasma VWF in 0.3 mL of cryoprecipitate was fractionated on a Sephacryl S500 column (1 × 10 cm; volume, 8 mL) by using a fast protein liquid chromatograpy system. Eluted fractions (0.3 mL total) from high to low molecular weight (fractions 12 to 17) were collected in a buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 25 nM NaCl [pH 7.4]). VWF fractions were analyzed by 1.7% agarose gel electrophoresis (22 hours at 3 mA) and immunoblotted with anti-VWF antibody (1:1000 dilution). (D) Each eluted VWF fraction was used in a C3b cleavage assay. After co-incubation of C3b, fI, and eluted VWF fractions, the cleavage products of C3b were separated by SDS-PAGE and detected by immunoblotting with anti-C3 antibody (n = 2).
VWF cofactor activity in fI-mediated C3b cleavage depends on the multimeric size of VWF. (A) C3b (1 μg) was incubated with fI (0.5 μg) and pVWF (1 μg), recombinant VWF dimers (VWF-Δpro) (0.5 or 1 μg), or ULVWF (1 μg). Western blotting of the degradation products using anti-C3 antibody (upper panel) showed that pVWF and VWF-Δpro acted as cofactors for fI-mediated C3b cleavage, but ULVWF did not function as a cofactor. Lower panel shows the results of 1.7% sodium dodecyl sulfate-agarose gel electrophoresis and immunoblotting with anti-VWF antibody confirming the multimeric distribution of pVWF, ULVWF, and VWF-Δpro used in (A). (B) C3 (1 μg), proactivation complement proteins (factor B and factor D, 0.5 μg each), and fI (0.5 μg) were incubated with VWF-Δpro (1 μg) or ULVWF (1 μg). Degradation products were immunoblotted using anti-C3a, which detects C3a and any C3 fragments containing C3a (upper panel) or anti-C3 antibodies (lower panel). Although factor B in the presence of factor D and a small amount of spontaneously generated C3b (tick-over) promoted complement activation and production of C3a (upper panel) and C3b (lower panel), addition of VWF dimers helped fI to shift the balance toward complement inactivation (iC3b in the lower panel and no C3a in the upper panel). A 76-kDa band in the upper panel detected by anti-C3a antibody after incubation of C3 with VWF dimers, fI, factor B, and factor D was probably a degradation product of the α chain of C3 that was not cleaved by C3 convertase and still contained C3a but was partially cleaved by fI and lost its 43-kDa fragment. ULVWF did not enhance fI-mediated complement inhibition, and as a default pathway, C3 was directed toward generation of C3a and C3b (activation) (n = 3). (C) Plasma VWF in 0.3 mL of cryoprecipitate was fractionated on a Sephacryl S500 column (1 × 10 cm; volume, 8 mL) by using a fast protein liquid chromatograpy system. Eluted fractions (0.3 mL total) from high to low molecular weight (fractions 12 to 17) were collected in a buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 25 nM NaCl [pH 7.4]). VWF fractions were analyzed by 1.7% agarose gel electrophoresis (22 hours at 3 mA) and immunoblotted with anti-VWF antibody (1:1000 dilution). (D) Each eluted VWF fraction was used in a C3b cleavage assay. After co-incubation of C3b, fI, and eluted VWF fractions, the cleavage products of C3b were separated by SDS-PAGE and detected by immunoblotting with anti-C3 antibody (n = 2).
A small amount of C3 undergoes spontaneous hydrolysis in plasma to generate C3b, which can be inactivated by fI-mediated cleavage or become a part of C3 convertase and participate in propagation of complement. In plasma, proactivation complement proteins (factor B and factor D) coexist with the inhibitory proteins fI and fH. To investigate the effect of multimeric size of VWF on complement activity in plasma, we incubated C3, factor B, factor D, and fI with either VWF-Δpro or ULVWF. C3 autolyzed to C3b which, in the presence of factor B, resulted in formation of C3 convertase and generation of C3a and C3b (Figure 2B). Addition of VWF-Δpro significantly reduced complement activation and shifted C3 toward generation of iC3b. But ULVWF multimers did not inhibit generation of C3b and C3a from C3 and had a permissive effect on complement activation. These results suggested that in normal plasma, VWF multimers enhance degradation of C3b. Conversely, ULVWF permits default complement activation. Our results are based on biochemical studies in vitro, and their relevance to physiologic complement regulation needs to be further investigated in a cell system or in an animal model.
Binding of C3 to endothelial cell–secreted or anchored ULVWF was previously shown by Turner and Moake8 ; in this study, we found that C3b also binds to plasma VWF, as was detected by co-immunoprecipitation and surface plasmon resonance assays (supplemental Figure 3). We calculated a dissociation constant (KD) of 0.22 μM (218 nM) for the interaction between C3b and VWF, which was consistent with a high affinity as compared with the previously reported KD for the binding of C3b to fH (KD, 0.59 to 1.6 μM).9,10 Exposure to shear forces in a cone-plate viscometer increased the amount of C3b co-immunoprecipitated with VWF (supplemental Figure 4). A recent study showed that VWF enhances cofactor activity of fH in fI-mediated cleavage of C3b; however, VWF by itself did not show any fI-cofactor activity.11 Our results showed that VWF alone had fI-cofactor activity, depending on its multimeric size.
The concentration of VWF in plasma (10 μg/mL) is much lower than that of fH (500 μg/mL), and one might conclude that the complement regulatory role of VWF is negligible compared with that of fH. We hypothesize that circulating VWF could regulate complement in the area of vascular injury or on the surface of activated platelets, because VWF can bind to the subendothelium and platelets and thereby achieve high local concentrations and participate in complement regulation on cell membranes.
Our results are consistent with recent reports of complement activation in patients with thrombotic thrombocytopenia purpura.12-15 Mild to moderately reduced ADAMTS13 activity is also detected in other thrombotic microangiopathies, including hemolytic uremic syndrome 7 and sepsis,16-18 suggesting that larger VWF multimers in these conditions may increase complement activation.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Joel Moake and Leticia Nolasco for providing reagents, discussing the results, and reviewing the manuscript; Dr Magnus Höök for providing access to the Biacore instrument; and Caleb Kroll for his technical assistance.
This work was supported in part by National Institutes of Health National Cancer Institute grant CA177909 (V.A.-K.) and an Ovarian Cancer Research Fund Program Project Development grant 258813 (V.A.-K.).
Authorship
Contribution: S.F. and X.L. performed the research; M.H.K. interpreted the data and wrote the paper; D.W.C. contributed a vital reagent, interpreted the data, and wrote the paper; and V.A.-K. designed the research study, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vahid Afshar-Kharghan, Department of Benign Hematology, University of Texas MD Anderson Cancer Center, Unit 1464, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: vakharghan@mdanderson.org.

![Figure 2. VWF cofactor activity in fI-mediated C3b cleavage depends on the multimeric size of VWF. (A) C3b (1 μg) was incubated with fI (0.5 μg) and pVWF (1 μg), recombinant VWF dimers (VWF-Δpro) (0.5 or 1 μg), or ULVWF (1 μg). Western blotting of the degradation products using anti-C3 antibody (upper panel) showed that pVWF and VWF-Δpro acted as cofactors for fI-mediated C3b cleavage, but ULVWF did not function as a cofactor. Lower panel shows the results of 1.7% sodium dodecyl sulfate-agarose gel electrophoresis and immunoblotting with anti-VWF antibody confirming the multimeric distribution of pVWF, ULVWF, and VWF-Δpro used in (A). (B) C3 (1 μg), proactivation complement proteins (factor B and factor D, 0.5 μg each), and fI (0.5 μg) were incubated with VWF-Δpro (1 μg) or ULVWF (1 μg). Degradation products were immunoblotted using anti-C3a, which detects C3a and any C3 fragments containing C3a (upper panel) or anti-C3 antibodies (lower panel). Although factor B in the presence of factor D and a small amount of spontaneously generated C3b (tick-over) promoted complement activation and production of C3a (upper panel) and C3b (lower panel), addition of VWF dimers helped fI to shift the balance toward complement inactivation (iC3b in the lower panel and no C3a in the upper panel). A 76-kDa band in the upper panel detected by anti-C3a antibody after incubation of C3 with VWF dimers, fI, factor B, and factor D was probably a degradation product of the α chain of C3 that was not cleaved by C3 convertase and still contained C3a but was partially cleaved by fI and lost its 43-kDa fragment. ULVWF did not enhance fI-mediated complement inhibition, and as a default pathway, C3 was directed toward generation of C3a and C3b (activation) (n = 3). (C) Plasma VWF in 0.3 mL of cryoprecipitate was fractionated on a Sephacryl S500 column (1 × 10 cm; volume, 8 mL) by using a fast protein liquid chromatograpy system. Eluted fractions (0.3 mL total) from high to low molecular weight (fractions 12 to 17) were collected in a buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 25 nM NaCl [pH 7.4]). VWF fractions were analyzed by 1.7% agarose gel electrophoresis (22 hours at 3 mA) and immunoblotted with anti-VWF antibody (1:1000 dilution). (D) Each eluted VWF fraction was used in a C3b cleavage assay. After co-incubation of C3b, fI, and eluted VWF fractions, the cleavage products of C3b were separated by SDS-PAGE and detected by immunoblotting with anti-C3 antibody (n = 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/6/10.1182_blood-2014-06-585430/4/m_1034f2.jpeg?Expires=1767795404&Signature=WJohDQL86ysw5zenuhs56umrhhq1dZukL1HeQJsV9anSY1nsTSKGtbeg-SglvYND6VgXcrQ-aULR5-~k4qRD7BCDpuTb6bNldWWWJTl8hRgyByGSXfjksx-VRxmdOhCLL4~mewT3hQyQZDR3ZsTmAYLjfElXYOCWnxzaiWYNsVvLEa5tBxtw3Tkdy8BC7PfQFb33qLyLS2e~CyV8MpUAnP7TUsFcTrmjcaU-FzelmsY6M6fXnUThdgTIXnCm0gY1~v1St0KrVlGocxWdoReixx5IOwy-GjtbsxF3osZ8Byh2w-x6QTB4HU2BqZd8gyoc9hJaKSPqF9NHxwRp2mhseQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal