In this issue of Blood, Feng and coworkers present data suggesting a role for von Willebrand factor (VWF) in the proteolytic inactivation of complement C3b by factor I (CFI).1 Whereas smaller VWF multimers, especially dimers, tetramers, and hexamers, enhance C3b inactivation by CFI, large and unusually large VWF multimers are devoid of this cofactor activity and, therefore, they enhance complement activation by the alternative pathway C3 convertase, C3bBb.
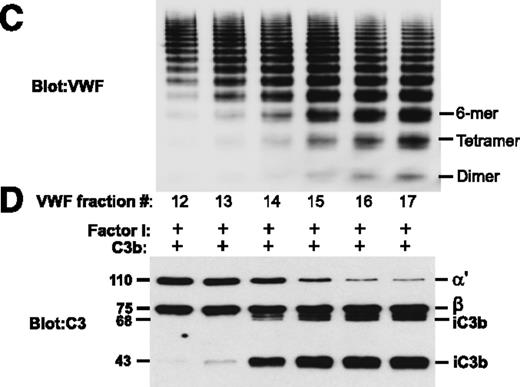
Normal plasma VWF subjected to sizing chromatography was analyzed by sodium dodecyl sulfate agarose gel electrophoresis (C). Fractions with smaller multimers (dimers, tetramers, hexamers) enhanced CFI-mediated degradation of C3b, generating inactivated C3b (iC3b) with 68-kDa and 43-kDa bands resulting from proteolysis of the 110-kDa α′ chain of C3b (D). Fractions with larger VWF multimers did not promote C3b degradation. See Figure 2 in the article by Feng et al that begins on page 1034.
Normal plasma VWF subjected to sizing chromatography was analyzed by sodium dodecyl sulfate agarose gel electrophoresis (C). Fractions with smaller multimers (dimers, tetramers, hexamers) enhanced CFI-mediated degradation of C3b, generating inactivated C3b (iC3b) with 68-kDa and 43-kDa bands resulting from proteolysis of the 110-kDa α′ chain of C3b (D). Fractions with larger VWF multimers did not promote C3b degradation. See Figure 2 in the article by Feng et al that begins on page 1034.
When a severe deficiency of VWF-cleaving protease, either caused by autoantibodies inhibiting its activity or by a constitutional defect without circulating inhibitors, was first reported in a series of patients with sporadic or familial thrombotic thrombocytopenic purpura (TTP), respectively, a differential diagnosis between TTP and atypical hemolytic uremic syndrome (HUS) seemed to become possible.2 In contrast to patients clinically diagnosed with TTP, those with a diagnosis of atypical HUS showed normal or only mildly decreased activity of the VWF-cleaving protease,2 which nowadays is known as ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 domains, number 13).3 The clinically often-difficult distinction between TTP and atypical HUS seemed to become further facilitated by finding mutations in complement components enhancing the activatability of the alternative complement pathway in at least 50% of patients with atypical HUS.4 Heterozygous hypofunctional mutations of the complement regulatory proteins factor H (CFH), CFI, membrane cofactor protein, thrombomodulin (THBD), or hyperfunctional mutations of the complement factors C3 or factor B suggested an important pathophysiological role for complement activation in patients with atypical HUS, whereas the predominant pathogenetic role in TTP was attributed to unusually large and “activated” VWF, resulting from lacking proteolytic regulation by ADAMTS13 and leading to platelet clumping in the microcirculation of various organs.
More recently, several publications highlighted the fact that complement activation via the alternative pathway (leading to generation of the anaphylatoxins C3a and C5a and the terminal membrane attack complex C5b-9) may not be restricted to atypical HUS, being also a feature of classical ADAMTS13-deficient TTP.5,6 The extent of complement activation upon admission was even found to be associated with the outcome, with fatal cases showing more extensive complement activation.6 It is less clear, however, whether complement activation in acute episodes of acquired or hereditary ADAMTS13-deficient TTP is truly a driving pathogenetic factor, or rather an epiphenomenon following ischemic organ damage by widespread VWF-platelet clumping leading to microvascular thrombosis.
Nevertheless, it is clear that the 2 defense systems, complement and hemostasis, are intrinsically linked at many levels. For instance, the endothelial transmembrane protein, thrombomodulin, supports the thrombin-induced generation of activated protein C, initiating anticoagulant and anti-inflammatory pathways. It also functions as a regulator of the complement system mediating C3b inactivation by CFI in the presence of CFH and supporting inactivation of the anaphylatoxins C3a and C5a by promoting the activation of procarboxypeptidase B (thrombin-activatable fibrinolysis inhibitor).7 Heterozygous mutations of THBD leading to impaired complement regulation are found in ∼5% of patients with atypical HUS.
In a recent Blood article (January 2014),8 the interaction of CFH with VWF was studied. It was shown that CFH colocalized with VWF to the Weibel-Palade bodies in endothelial cells, that CFH binds with high affinity to VWF, and that the cofactor function of CFH in the degradation of C3b by CFI was enhanced by increasing concentrations of VWF. On the other hand, VWF was partially protected by CFH from ADAMTS13-mediated degradation.8
Feng et al further studied the influence of plasma-derived VWF, a recombinant VWF dimer, and unusually large VWF multimers on the proteolytic inactivation of C3b by CFI.1 As seen in their Figure 2, panels C and D, the degradation of C3b (consisting of a stable 75-kDa β chain and a disulfide-linked 110-kDa α′ chain) to inactivated C3b (iC3b, displaying 68-kDa and 43-kDa fragments of the α′ chain) by CFI is strongly enhanced by VWF fractions containing smaller VWF multimers, specifically dimers, tetramers, and hexamers. The VWF fractions from normal plasma containing larger VWF multimers (10-mer and larger species) were devoid of any cofactor function (see figure). Using a recombinant VWF dimer, the authors confirmed its cofactor function in C3b degradation by CFI, which suggested that the cofactor function was indeed attributable to VWF and not to a copurified contaminant.
Feng and colleagues conclude that normal plasma VWF multimers contribute to inhibit complement activation by enhancing C3b degradation, whereas larger VWF species, including the unusually large VWF multimers found in patients lacking ADAMTS13 activity, do not have CFI cofactor function that would enhance complement activation.1
The in vivo significance of these findings, however, is unclear at present. As nicely shown by the authors with their different VWF fractions obtained by sizing chromatography of normal plasma cryoprecipitate, normal plasma obviously contains not only VWF fractions rich in dimers, tetramers, and hexamers contributing to complement regulation, but also fractions with larger multimers devoid of complement regulatory function, which rather favor complement activation. The net result of VWF in normal plasma and the in vivo role of VWF in the activation or regulation of the complement system clearly needs further study. The article by Feng et al stimulates our interest in the highly complex interactions of complement and hemostasis, both in normal physiology and in disease states such as the thrombotic microangiopathies.
Conflict-of-interest disclosure: The author has received research funding from Baxter Bioscience for setting up the hereditary TTP registry (www.ttpregistry.net, ClinicalTrials.gov NCT01257269), and is chairman of the Data Safety Monitoring Committee for the BAX930 study (rADAMTS13 in hereditary TTP patients).