Key Points
PTEN phosphatase activity, independent of other PTEN functions, is required to prevent T-cell lymphoma.
Abstract
Mice with T-cell–specific loss of the tumor suppressor gene PTEN early in T-cell ontogeny develop thymic lymphomas that invariably harbor a reciprocal translocation involving the T-cell receptor α/δ locus and c-myc, t(14;15). In addition to its known function as a lipid phosphatase opposing PI3K signaling, PTEN has also been described as playing a prominent role in promoting genomic stability. As a result, it has been uncertain which one(s) of these 2 separable features were required to block the development of lymphoma. Here, using a conditional model in which T cells selectively express 1 phosphatase-dead PTEN mutant (C124S) and maintain 1 null allele, we show that PTEN phosphatase activity is required for preventing the emergence of a malignant T-cell population harboring t(14;15), thus constituting a critical function of PTEN in preventing lymphomagenesis.
Introduction
The tumor suppressor gene phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was initially characterized as a lipid phosphatase that directly opposes PI3K signaling.1,2 PTEN was subsequently shown additionally to possess noncatalytic nuclear functions in its C-terminal domain that help maintain genomic stability.3-5
Mutations in PTEN are associated with multiple cancers in humans, including T-cell malignancies,6-12 and targeted deletion of PTEN in murine T cells results in the development of a T-cell malignancy with characteristics of human T-cell acute lymphoblastic leukemia.13-16 Interestingly, the most common PTEN-specific mutations that have been detected in primary T-cell acute lymphoblastic leukemia samples are mutational disruptions within the C-terminal region that do not alter phosphatase activity.7 Moreover, a study reported spontaneous tumor development, including T-cell lymphomas, in mice harboring a knock-in mutation that causes loss of the PTEN C-terminal region.17 This has led to the suggestion that the role of PTEN as a tumor suppressor in T-cell leukemia is independent of its phosphatase function.
In this study, we address this question using a conditional mouse model that maintains T-cell–specific expression of a phosphatase-dead PTEN mutant (C124S) in the absence of wild-type (WT) PTEN. We find that loss of PTEN phosphatase activity alone is sufficient to enable lymphomagenesis. Furthermore we show that these lymphomas phenocopy those that develop following complete loss of PTEN in T cells,14,18 including the presence of the characteristic t(14;15) translocation. The fact that the loss of PTEN phosphatase activity alone is sufficient for this specific genetic lesion to occur suggests that it requires hyperactive PI3K/Akt signaling and is independent of PTEN’s nuclear role in promoting genomic stability. These findings delineate a clear functional role for PTEN phosphatase activity in the prevention of T-cell lymphoma.
Study design
Mice and bone marrow chimeras
The generation of PTENC124S/+ and CD4-Cre Ptenfl/fl (PTEN-ΔT) mice has been previously described.19,20 All animals were cared for according to the guidelines of Beth Israel Deaconess Medical Center and Massachusetts General Hospital, and the Institutional Animal Care and Use Committee approved all experimental protocols. For bone marrow chimeras, mice were lethally irradiated (10 Gy) before reconstitution with 2 × 106 total bone marrow cells.
Western blotting, SKY analysis, and chromosome painting
Cell lysate preparation, SDS-PAGE, immunoblotting, and development using enhanced chemiluminescence were performed as previously described.14 T cells were fractionated using EasySep (StemCell Technologies). All Abs were purchased from Cell Signaling Technology. Metaphases were prepared and spectral karyotyping (SKY) was analyzed as previously described.14 Single-chromosome paints specific for mouse chr14 and chr15 were performed and analyzed as previously described.21
Flow cytometry and phospho-flow
Monoclonal antibodies were purchased from BD. Cells were stained by standard methods, and events were collected on an LSRII (BD) and were analyzed by FlowJo software (Tree Star, Inc). Phospho-flow was performed as previously described.20
Results and discussion
As previously reported by our laboratory and by others,13,14,16 mice with a T-cell–specific deletion of PTEN (PTENfl/fl CD4-Cre, or PTEN-ΔT mice) uniformly develop lymphoma by 10 to 20 weeks of age. To study the role of T-cell PTEN phosphatase activity in preventing lymphoma, we generated mice in which all cells express 1 phosphatase-dead PTEN mutant allele, C124S (PTENC124S/+ mice).20 PTENC124S/+ mice, similar to both PTEN heterozygous mice (PTEN+/−) and T-cell–specific PTEN heterozygous mice (CD4-Cre PTENfl/+), do not develop lymphoma before 20 weeks of age20,22,23 (and data not shown). We next crossed PTENC124S/+ mice to CD4-Cre mice, and further crossed these mice to PTENfl/fl animals, to generate CD4-Cre PTENC124S/fl mice (PTENC124S/ΔT). These mice carry 1 copy of the phosphatase-dead PTEN mutant in every cell, including T cells, but they are null for the other PTEN allele only in T cells.
We analyzed PI3K/Akt signaling in both thymocytes and peripheral mature CD4+ T cells of PTENC124S/ΔT mice vs PTEN-ΔT mice at basal levels and following T-cell receptor (TCR) crosslinking +/− costimulation. We found enhanced PI3K/Akt signaling in, and little differences between, PTEN-ΔT and PTENC124S/ΔT in both thymocytes (Figure 1A) and mature CD4+ T cells (Figure 1C-D). Substantial signaling differences were noted in Akt and its direct targets Foxo1/3a and GSK3β. Other molecules in the PI3K pathway that are not direct targets of Akt, such as mTOR and S6, were less affected, and differences in 4E-BP1 phosphorylation were more variable. Phospho-flow was used to evaluate signaling differences of specific populations within the thymus (Figure 1B). Phospho-Akt Ser473 was slightly higher in activated PTEN-ΔT and PTENC124S/ΔT CD4SP cells compared with WT, with little differences seen between PTEN-ΔT and PTENC124S/ΔT CD4SP cells. WT DP thymocytes showed little increase in phospho-Akt levels on activation relative to PTEN-ΔT and PTENC124S/ΔT DP cells, although there were no differences seen between PTEN-ΔT and PTENC124S/ΔT DP cells. Together these data demonstrate similar kinetics and degree of PI3K/Akt activation in T cells and in thymocytes that completely lack PTEN vs T cells that lack only PTEN phosphatase activity.
T-cell and thymocyte signaling in T-cell–specific PTEN-null and PTEN–phosphatase-dead mice. (A) Western blot analysis of PI3K/Akt signaling in total thymocytes of 4- to 5-week-old WT, PTEN-ΔT, and PTENC124S/ΔT mice, representative of >6 experiments. Crosslinking of anti-CD3 (3 μg, clone 2C11) was achieved using anti-hamster IgG, with or without the addition of 1 μg soluble anti-CD28. (B) Phospho-flow analysis of PI3K/Akt signaling in total thymocytes, gated on either CD4 single-positive (CD4SP) or CD4/CD8 double-positive (DP) cells, representative of >4 experiments. The same activation conditions as western blot experiments, with anti-CD28, were used for phospho-flow. (C) Western blot analysis of PI3K/Akt signaling in purified CD4+ premalignant T cells from spleen and lymph nodes of 4- to 5-week-old WT, PTEN-ΔT, and PTENC124S/ΔT mice, representative of >6 experiments. Crosslinking of anti-CD3 (3 μg, clone 2C11) was achieved using anti-hamster IgG, and 1 μg soluble anti-CD28 was added on the addition of anti-CD3. (D) Quantitative analysis of western blots of peripheral CD4+ T cells stimulated for 15′ with anti-CD3 +/− anti-CD28. WT stimulated with anti-CD3 alone (without anti-CD28) is set to a relative unit of 1, to which all genotypes and stimulation with anti-CD28 are compared, for each phosphoprotein/total protein. Results are for 3 independent experiments.
T-cell and thymocyte signaling in T-cell–specific PTEN-null and PTEN–phosphatase-dead mice. (A) Western blot analysis of PI3K/Akt signaling in total thymocytes of 4- to 5-week-old WT, PTEN-ΔT, and PTENC124S/ΔT mice, representative of >6 experiments. Crosslinking of anti-CD3 (3 μg, clone 2C11) was achieved using anti-hamster IgG, with or without the addition of 1 μg soluble anti-CD28. (B) Phospho-flow analysis of PI3K/Akt signaling in total thymocytes, gated on either CD4 single-positive (CD4SP) or CD4/CD8 double-positive (DP) cells, representative of >4 experiments. The same activation conditions as western blot experiments, with anti-CD28, were used for phospho-flow. (C) Western blot analysis of PI3K/Akt signaling in purified CD4+ premalignant T cells from spleen and lymph nodes of 4- to 5-week-old WT, PTEN-ΔT, and PTENC124S/ΔT mice, representative of >6 experiments. Crosslinking of anti-CD3 (3 μg, clone 2C11) was achieved using anti-hamster IgG, and 1 μg soluble anti-CD28 was added on the addition of anti-CD3. (D) Quantitative analysis of western blots of peripheral CD4+ T cells stimulated for 15′ with anti-CD3 +/− anti-CD28. WT stimulated with anti-CD3 alone (without anti-CD28) is set to a relative unit of 1, to which all genotypes and stimulation with anti-CD28 are compared, for each phosphoprotein/total protein. Results are for 3 independent experiments.
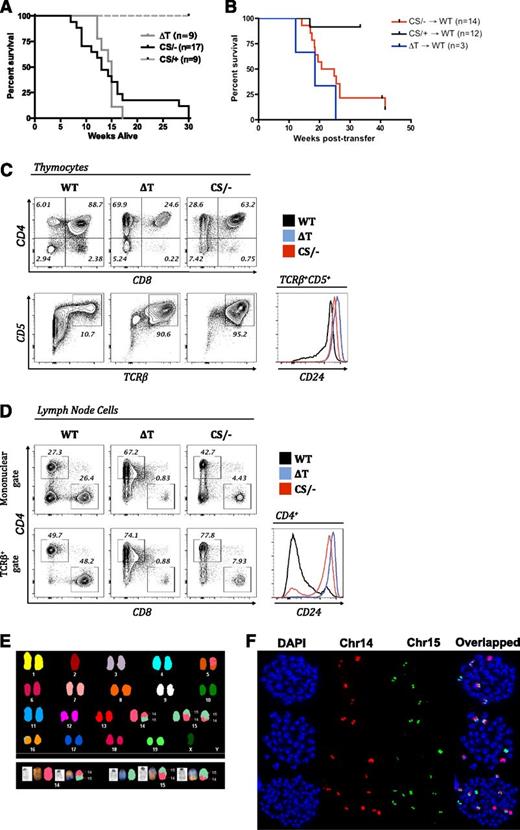
Despite the isolated loss of only PTEN phosphatase activity, similar to PTEN-ΔT mice, 100% of PTENC124S/ΔT mice developed lymphoma early in life (Figure 2A). Bone marrow chimera experiments, in which lethally irradiated WT recipients were reconstituted with bone marrow from either PTEN-ΔT, PTENC124S/ΔT, or PTENC124S/+ mice, indicated that lymphoma was intrinsic to hematopoietic cells of either PTEN-ΔT or PTENC124S/ΔT origin (Figure 2B) and was not the result of the altered environment of the C124S knock-in itself. Malignant cells from PTENC124S/ΔT mice were predominantly semimature CD4 single positive cells with high expression of TCRβ, CD5, and CD24 in the thymus (Figure 2C), although CD4/CD8 DP cells were observed in some tumors, demonstrating a range of immunophenotypes, and these malignant cells maintained expression of CD24 in the periphery (Figure 2D), similar to what has been observed in PTEN-ΔT mice. It is important to note that malignant cells from PTENC124S/ΔT exhibited the same t(14;15) translocation event characteristic of PTEN-ΔT malignant T cells (Figure 2E-F and supplemental Table 1), indicating that PTEN phosphatase activity is critical for the prevention of lymphomas harboring this typical translocation event.
Comparison of survival and characterization of lymphoma in T-cell–specific PTEN-null and PTEN–phosphatase-dead mice. (A) Survival of PTEN-ΔT (ΔT) and PTENC124S/ΔT (CS/−) mice. (B) Bone marrow chimeras were prepared by transplanting bone marrow from PTEN-ΔT, PTENC124S/ΔT, or PTENC124S/+ (CS/+) mice into lethally irradiated WT congenic recipients. Survival of chimeric mice is measured in weeks after reconstitution. (C) Immunophenotyping of lymphoma cells from thymi of 10- to 16-week-old WT, PTEN-ΔT (ΔT), and PTENC124S/ΔT (CS/−) mice, representative of >10 mice. Cells were stained with antibodies to CD4, CD8, CD5, CD24, and TCRβ and were analyzed by flow cytometry. (D) Immunophenotyping of lymphoma cells from lymph nodes of 10- to 16-week-old WT, PTEN-ΔT, and PTENC124S/ΔT mice, representative of >10 mice. Cells were stained with antibodies to CD4, CD8, CD24, and TCRβ and were analyzed by flow cytometry. The bottom panel is gated on TCRβ+ cells. (E) Detection of t(14;15) by SKY analysis in metaphases from lymph nodes in PTENC124S/ΔT mice with lymphoma (>10-weeks-old). Note that distinct colors within a chromosome are indicative of a translocation. (F) Representative single chromosome painting specific for mouse chr14 (red) and chr15 (green) of lymphomas from PTENC124S/ΔT mice. Quantitation of chromosome painting is shown in supplemental Table 1 (see supplemental Data available on the Blood Web site).
Comparison of survival and characterization of lymphoma in T-cell–specific PTEN-null and PTEN–phosphatase-dead mice. (A) Survival of PTEN-ΔT (ΔT) and PTENC124S/ΔT (CS/−) mice. (B) Bone marrow chimeras were prepared by transplanting bone marrow from PTEN-ΔT, PTENC124S/ΔT, or PTENC124S/+ (CS/+) mice into lethally irradiated WT congenic recipients. Survival of chimeric mice is measured in weeks after reconstitution. (C) Immunophenotyping of lymphoma cells from thymi of 10- to 16-week-old WT, PTEN-ΔT (ΔT), and PTENC124S/ΔT (CS/−) mice, representative of >10 mice. Cells were stained with antibodies to CD4, CD8, CD5, CD24, and TCRβ and were analyzed by flow cytometry. (D) Immunophenotyping of lymphoma cells from lymph nodes of 10- to 16-week-old WT, PTEN-ΔT, and PTENC124S/ΔT mice, representative of >10 mice. Cells were stained with antibodies to CD4, CD8, CD24, and TCRβ and were analyzed by flow cytometry. The bottom panel is gated on TCRβ+ cells. (E) Detection of t(14;15) by SKY analysis in metaphases from lymph nodes in PTENC124S/ΔT mice with lymphoma (>10-weeks-old). Note that distinct colors within a chromosome are indicative of a translocation. (F) Representative single chromosome painting specific for mouse chr14 (red) and chr15 (green) of lymphomas from PTENC124S/ΔT mice. Quantitation of chromosome painting is shown in supplemental Table 1 (see supplemental Data available on the Blood Web site).
We have shown that cancer-associated PTEN mutants heterodimerize with WT PTEN protein to form an inactive dimer, and a dominant-negative effect over WT PTEN protein has been demonstrated in vivo.20 That study provided mechanistic evidence to explain why single-allele PTEN mutants trigger more severe phenotypes when compared with single-allele deletion of PTEN. Our model makes use of the conditional expression of a cancer-associated phosphatase-dead mutant in the absence of WT PTEN, thus enabling the role of phosphatase activity in lymphomagenesis to be tested. Our finding that t(14;15) occurs in the absence of PTEN phosphatase activity alone suggests that PI3K downregulation is a critical feature of PTEN’s tumor-suppressive function in developing T cells.
Interestingly, mice heterozygous for a PTEN C-terminal truncation mutant that maintains phosphatase activity develop multiple cancers, including lymphomas of both B- and T-cell origin.17 As deletion of PTEN during B-cell development does not result in B-cell lymphomas,24 this suggests a gain-of-function effect of this mutation (although cell extrinsic events have not been ruled out), and it indicates that specific features required for PTEN tumor-suppressive activity vary in cells of different lineages. In our model of T-cell lymphoma, it is possible that the loss of PTEN allows the survival of cells that, through other mechanisms, have acquired genetic aberrations such as translocation of c-myc. Indeed, mice in which β-catenin activity is constitutively maintained during T-cell development, resulting in increased survival of thymocytes and impaired repair of double-stranded DNA breaks, develop lymphoma exhibiting the same recurrent translocation exhibited in our model.25 Finally, we did not find any evidence that PTEN phosphatase activity was linked to the maintenance of genomic stability per se, as we observed comparable basal and radiation-induced DNA damage in WT, PTEN-ΔT, and PTENC124S/ΔT thymocytes and CD4+ T cells (data not shown). PTEN phosphatase activity, which has now been shown to be crippled even in the presence of a single copy of mutants such as C124S,20 is likely upstream of events required for lymphomagenesis in this model. Our model indicates that PTEN phosphatase activity is indispensable for the prevention of T-cell lymphomagenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.H.N. designed and performed research and wrote the paper; A.P. designed and performed research, created the C124S mutant mouse, and edited the paper; Y.L. and G.H.W. designed and performed research; C.Y. and Y.-J.K. performed research and analyzed data; and P.P.P. and L.A.T. designed research and edited the paper.
Conflict-of-interest disclosure: A family member of L.A.T. is employed by and owns stock in Novartis. The remaining authors declare no competing financial interests.
The current affiliation for A.P. is Department of Biochemistry and Molecular Biology, School of Biomedical Sciences, Monash University, VIC 3800, Australia.
Correspondence: Laurence A. Turka, Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital, 149 13th St, Room 5101, Boston, MA 02129; e-mail: lturka@partners.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal