Key Points
Malarial parasite growth is impeded in erythropoietic protoporphyric erythrocytes because of decreased host cell ferrochelatase activity.
A ferrochelatase competitive inhibitor prevents the growth of malarial parasites in normal red cells.
Abstract
Many red cell polymorphisms are a result of selective pressure by the malarial parasite. Here, we add another red cell disease to the panoply of erythrocytic changes that give rise to resistance to malaria. Erythrocytes from individuals with erythropoietic protoporphyria (EPP) have low levels of the final enzyme in the heme biosynthetic pathway, ferrochelatase. Cells from these patients are resistant to the growth of Plasmodium falciparum malarial parasites. This phenomenon is due to the absence of ferrochelatase and not an accumulation of substrate, as demonstrated by the normal growth of P falciparum parasites in the EPP phenocopy, X-linked dominant protoporphyria, which has elevated substrate, and normal ferrochelatase levels. This observation was replicated in a mouse strain with a hypomorphic mutation in the murine ferrochelatase gene. The parasite enzyme is not essential for parasite growth as Plasmodium berghei parasites carrying a complete deletion of the ferrochelatase gene grow normally in erythrocytes, which confirms previous studies. That ferrochelatase is essential to parasite growth was confirmed by showing that inhibition of ferrochelatase using the specific competitive inhibitor, N-methylprotoporphyrin, produced a potent growth inhibition effect against cultures of P falciparum. This raises the possibility of targeting human ferrochelatase in a host-directed antimalarial strategy.
Introduction
Malaria is a lethal disease caused by the Plasmodium parasite and affects over 200 million people every year. In immunologically naive individuals and genetically naive populations it is an extremely lethal disease, which has resulted in an evolutionary battle between the host and parasite, probably since the appearance of plasmodia in vertebrates. In humans this is manifest as a suite of red cell polymorphisms that give rise to various diseases when present in the homozygous state and protection against malaria as heterozygotes. Although the exact mechanism of protection is unclear for many of these polymorphisms, their geographic colocation with areas of malarial endemicity provides strong circumstantial evidence of their role in protection against death from malaria.1-3
Several red cell factors are scavenged by the parasite during intraerythrocytic growth, including redox enzymes, protein kinases, and heme biosynthesis enzymes.4-8 Confirmation that deficiencies in these host enzymes contribute to host resistance could pave the way for novel antimalarial therapeutics.
Heme is an essential cofactor in many proteins and enzymes. Given these essential functions, blockade of parasite heme biosynthesis has been proposed as an antimalarial strategy.9 Although the parasite possesses an 8-step canonical heme biosynthetic pathway, it is possible that the parasite also scavenges heme from the host red cell.10,11
Despite no longer synthesizing heme, erythrocytes contain residual amounts of some heme biosynthetic enzymes, including ferrochelatase (EC 4.99.1.1), δ-aminolevulinate dehydratase (EC 4.2.1.24; ALAD), and coproporphyrinogen oxidase (EC 1.3.3.3).12-14 There is evidence that the parasite may import host erythrocytic heme biosynthetic enzymes and use these to generate heme in an “extrinsic pathway.”4,8 These host enzymes have been observed in the cytosol of the parasite.11 Although parasite-encoded heme biosynthetic enzymes are present in the parasite, production of heme by these enzymes is not essential for parasite survival. The apicoplast (and hence intrinsic heme synthesis) can be removed from the erythrocytic stage of Plasmodium falciparum15 and deletion of the parasite orthologs of δ-aminolevulinic acid synthase (EC 2.3.1.37; ALAS) and ferrochelatase in Plasmodium berghei has no effect on the growth of the parasites in the blood.10
In the present study, we demonstrate that genetic deficiencies in host ferrochelatase inhibit growth of the erythrocytic stage of Plasmodium in both human erythropoietic protoporphyric red cells and in a mouse ferrochelatase knockdown mutation. We demonstrate that parasite ferrochelatase is not required for the red cell stage of the parasite cycle by genetically disabling the gene in P berghei parasites. Finally, we demonstrate that ferrochelatase, per se, is essential for parasite growth by inhibiting its activity using N-methylprotoporphyrin (N-MPP), a ferrochelatase substrate analog and competitive inhibitor of the enzyme. N-MPP totally prevented the growth of P falciparum.
Materials and methods
P falciparum culture
P falciparum strains 3D7, K1, and W2mef were cultured according to the method of Trager and Jensen.16
Collection and preparation of purified red blood cells
Blood from individuals with porphyrias was collected by venepuncture into 5-mL sodium citrate tubes. Blood was then centrifuged at 170g for 13 minutes and the plasma and white cell fractions removed. Blood was washed 2 times in RPMI and stored at 4°C, and then washed further prior to use. For some samples, blood was stored at 4°C for up to 24 hours prior to preparation and parasite infection. Matched normal control samples were stored in the same fashion as experimental samples.
P falciparum growth inhibition assays
Synchronized P falciparum ring or trophozoite-stage parasites were grown with N-MPP (Frontier Scientific) for up to 48 hours prior to analysis of growth. For the washout experiments, cultures (with or without 3 hours N-MPP treatment) were centrifuged and supernatant removed and washed 3 times in 100× cell pellet volume of cell culture medium lacking human serum (cell culture medium [CCM]-wash). The CCM was composed of RPMI 1640 (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, glucose and glutamine-free) supplemented with 1× glutamax, 0.2% Albumax (all from Life Technologies, Australia), 4% pooled AB+ human serum (Invitrogen), 10 mM D-glucose, 25 mg/ml gentamycin, 6 mM HEPES and 0.2 mM hypoxanthine (all from Sigma). “CCM-wash” lacked serum and Albumax. Protoporphyrin IX (PPIX) was added in varying concentrations to erythrocytes with either 50 μM N-MPP or PPIX alone and incubated for 48 hours prior to analysis. For growth assays in porphyric blood, parasites were purified17,18 and then added to the test blood at a final percentage parasitemia of ∼1%. Parasitemias were counted on Giemsa-stained thin blood smears after incubation for up to 72 hours. At least 1000 cells were counted on each slide. In some experiments, parasite growth was also quantified by flow cytometry analysis using YOYO-1 dye.19 Analysis involved comparing experimental and control arms.
Study subjects
We studied blood cells from 3 French Caucasian X-linked dominant protoporphyria (XLDPP) and 4 Australian erythropoietic protoporphyria (EPP) patients recruited at the Centre Français des Porphyries and the Department of Medicine, University of Melbourne, respectively (supplemental Table 1, see supplemental Data available at the Blood Web site). All patients presented with a typical history of skin photosensitivity and a high level of protoporphyrin in their erythrocytes. The collection and experimental procedures involving these samples were performed in accordance with the 1983 revision of the Declaration of Helsinki. The study was approved by the Comité de Recherche Clinique, Institut Pasteur, Paris, and the Human Research Ethics Committee of Tasmania (H0011444), Melbourne Health (2011.013), and Macquarie University (5201200356).
Experimental Plasmodium chabaudi infection
Fechm1Pas mice, originally produced from an ENU-mutagenesis screen at the Pasteur Institute (Paris), were kindly provided by X. Montagutelli (Génétique founctionnelle de la souris, Institut Pasteur, Paris). Female and male Fech+/+, Fech+/m1Pas, and Fechm1Pas/m1Pas mice (genotyped20 ) on a C57BL/6 background were infected with P chabaudi adami DS at 7 to 12 weeks of age. Female mice were infected IV with 5 × 105 infected red blood cells (RBCs)/mL and male mice with 2.5 × 105 infected RBCs/mL. Parasitemia was determined by counting cells on Giemsa-stained thin blood smears. Percentage of parasitemia was calculated on at least 500 cells per slide. The University of Tasmania Animal Ethics Committee approved the animal experiments (A0001049).
Analysis of ferrochelatase and PPIX levels in human patient red cells
Ferrochelatase activity was determined by fluorometric measurement of zinc-mesoporphyrin formation after incubating for 60 minutes at 37°C as established by Li and colleagues.21 For routine assays, a peripheral lymphocyte homogenate was prepared in 50 mM Tris-HCl (pH 7.6), 20% glycerol, and protein concentration measured using the Bradford method. The reaction consisted of a 5-minute preincubation at 37°C with 200 µL of lymphocyte homogenate, 200 µL of incubation buffer (250 mM Tris-HCl [pH 7.6], 1% [vol/vol] Triton X-100, 1.75 mM palmitic acid), and 40 µL of 0.5 mM mesoporphyrin (final concentration 43 µM). Then 20 µL of 1 mM zinc acetate solution was added and the incubation continued for a further 60 minutes. A blank was prepared without the cell homogenate. The reaction was stopped by adding DMSO/methanol mixture (30:70, vol/vol). After centrifugation, the fluorescence of the supernatant was measured at 580 nm with an excitation wavelength of 410 nm. The enzymatic activity was expressed as nanomoles of zinc-mesoporphyrin formed per hour per milligram of protein at 37°C (normal value: 4.83 ± 0.91, mean ± SD). The erythrocyte protoporphyrin levels were determined by standard methods.22
Analysis of N-MPP, PPIX, and heme levels in N-MPP–treated red cells
Compacted human red cells (1 mL) were incubated in CCM at 3% hematocrit with various concentrations of N-MPP in duplicate at 37°C for 48 hours. Cells were harvested by centrifugation at 500g for 10 minutes at 24°C. These were then washed 3 times in CCM wash to remove unabsorbed N-MPP. In each wash step, CCM wash (1 mL) was added, then vortexed for 5 seconds followed by centrifugation at 500g for 10 minutes at 24°C. Washed red cells (300 μL) were lyzed by adding 300 μL of 0.2% acetic acid in Milli-Q water, followed by gentle vortexing for ∼20 seconds. To extract free N-MPP and other porphyrins, 100% ethanol (14 mL) was added to the lysed red cells and shaken for 20 seconds. The samples were then centrifuged at 3000g for 15 minutes at 24°C. The supernatant was collected into 15 mL plastic tubes and volumes were reduced to ∼3 mL using a SpeedVac concentrator (Thermo Savant) at 45°C. The concentrated samples were resuspended in 0.2% acetic acid in Milli-Q water (12 mL) followed by gentle vortexing for 20 seconds. For the standard curve, human red cells (300 μL) were spiked with 20, 50, 100, 500, 1000, and 5000 nM concentrations of N-MPP and were extracted in the same manner as the N-MPP incubated samples. The detection limit for N-MPP was 3 nM. Porphyrin peaks for N-MPP, PPIX, and heme were all identified according to retention times relative to their corresponding standards. Only the N-MPP concentrations were calculated according to the standard curve above, whereas PPIX and heme were graphed according to their peak area.
C-18 solid-phase extraction of porphyrins
Porphyrins were semi-purified and concentrated using C-18 solid-phase extraction (SPE; Waters C-18 plus light cartridge, 130 mg). During all steps of the SPE, a flow rate of ∼1 to 2 mL × min−1 was maintained. The C-18 columns were activated with 100% methanol (2 mL) and then equilibrated in 0.2% acetic acid in Milli-Q water (2 mL). The samples (15 mL in 0.2% acetic acid, <30% ethanol) were loaded onto the column and washed with 0.2% acetic acid in Milli-Q water (2 mL). The concentrated porphyrins were eluted with 100% methanol (1.5 mL) into 1.5-mL plastic tubes and then dried using SpeedVac at 45°C. Dried samples were resuspended in 100% methanol (100 μL) by sonication for 5 minutes in an ultrasonic bath. The resuspended porphyrins were centrifuged at 16 000g for 10 minutes prior to ultra-high-pressure liquid chromatography (UHPLC) analysis.
UHPLC analysis of N-MPP, PPIX, and heme
Samples were analyzed using an Agilent 1290 Infinity Binary UHPLC and an Agilent 1260 fluorescence detector. The UHPLC method was followed as described23 with minor modifications. Buffer A (1 M ammonium acetate, 10% acetonitrile, pH 5.16), buffer B (90% methanol, 10% acetonitrile), flow rate (1 mL × min−1), injection volume (40 μL), column temperature (30°C), sample tray temperature (30°C), column (Agilent Eclipse Plus C-18, 1.8 μm, 4.6 × 75 mm, 600 bar), fluorescence photon multiplier tube (18), excitation (401 nm), and emission (627 nm), buffer running conditions: column was equilibrated with buffer A for 1 hour, 0 to 3 minutes (100% A to 65% A: 35% B), 3 to 9 minutes (65% A: 35% B to 10% A: 90% B), 9 to 14 minutes isocratic (10% A: 90% B). N-MPP and PPIX standards were used to identify the peaks. Peak areas were used to calculate porphyrin concentrations.
Genetic knock out of the P berghei ferrochelatase gene
The ferrochelatase gene24 of P berghei ANKA strain (PbFC; PBANKA_114070) encodes a protein of 349 aa localized to the mitochondrion.25 The PbFC gene in P berghei was interrupted by double crossover homologous recombination to introduce a selectable marker, human dihydrofolate reductase, deleting the catalytic site.26 To generate the knockout vector, a 589-bp region downstream of the PbFC coding sequence was amplified using the primers 5′-CTGACTCGAGATTATTGAAAAAAATCTAAGTGGCTGG and 5′-GACTCCCGGGCCTCTGTCGTTTTGATCTCTTTTGG. The resulting polymerase chain reaction (PCR) product was ligated into XhoI and XmaI sites of vector pL0006 (a kind gift from Andy Waters, University of Glasgow). A 634-bp region upstream of the PbFC coding sequence was amplified using the primers 5′-CTGAAAGCTTGATAAACTTATTTTCATTTGGCTTGG and 5′-GACTAGATCTGTATATCAAACTATAAATTCGATACAATTC. The resulting PCR was ligated into HindIII and BglII sites of the pL0006 vector already containing the PbFC 3′ flank. The resultant plasmid, pL0006(PbFC KO), was transfected into P berghei ANKA parasites as previously described.26 A clonal line was recovered by limiting dilution of pyrimethamine-resistant parasites and inoculated into 10 mice. Clonal parasites (hereafter referred to as PbFC-KO) were isolated and analyzed by PCR screening. For PCR screens, we used the primers 5′-GTTTGGACTCCTTTGTTTCG and 5′-GACGATGCAGTTTAGCGAAC, which amplifies a band of 1327 bp if the hDHFR has replaced the PbFC gene. In addition, we used the primers 5′-GATCAGATCTAAAATGGATATAGACGATTTCTTAAAATG and 5′-GATCCCTAGGCCAGCCACTTAGATTTTTTTCAATAAT, which amplifies the native PbFC gene, and therefore will only occur if the PbFC gene remains present. PCR analysis confirmed integration of the selectable marker into the intended site (supplemental Figure 2).
Statistical analysis
P values were calculated using 2-tailed t tests assuming equal variance and the Mantel-Cox log-rank test for survival.
Results
Ferrochelatase deficiencies in human red cells impair P falciparum parasite growth
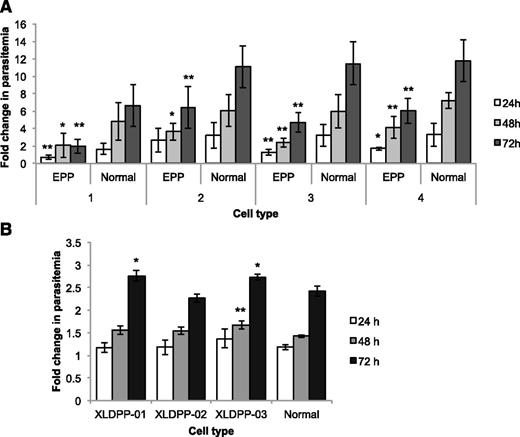
Ferrochelatase deficiency manifests in humans as EPP27 (MIM 177000). The condition is inherited in a pattern that resembles autosomal dominance with low penetrance due to the coinheritance of a common hypomorphic allele in trans to a deleterious allele in the ferrochelatase gene.28 We obtained red cells from 4 human EPP patients, all with laboratory confirmed ferrochelatase gene mutations (supplemental Table 1). In separate experiments, cells from each patient were infected in vitro with synchronized P falciparum (late-stage trophozoites and schizonts) and parasite growth kinetics examined for up to 72 hours. At each of the 3 time points examined, all 4 EPP samples showed lower rates of parasite growth compared with normal human red cells (Figure 1). Examination of the parasite growth stages at each time point, and the proportional increases in parasitized cells (supplemental Figure 1A) indicated that there was a global reduction in parasite growth. There were reduced frequencies of mature stage parasites (trophozoites and schizonts) and fewer immature ring-stage cells. These differences were significant at every time point in samples EPP01 and EPP04, and at 48 and 72 hours in EPP02 and EPP03. No obvious correlations could be made between these variable effects on parasite growth and ferrochelatase enzyme activity.
P falciparum growth in red cells from individuals with EPP and XLDPP. Comparison of P falciparum growth in EPP and normal RBCs (A) and in XLDPP and normal RBCs (B). Values are expressed as fold changes in parasitemia (relative to inocula levels) (±SD) measured after culturing for 24, 48, and 72 hours. Growth in cells from 4 individuals with EPP was tested separately against 4 different normal blood samples. Significant reductions in parasite growth are indicated (*P < .05, **P < .01). Growth in cells from 3 individuals with XLDPP was tested against a single normal blood sample. Significant increases in parasite growth are indicated (*P < .05, **P < .01). SD, standard deviation.
P falciparum growth in red cells from individuals with EPP and XLDPP. Comparison of P falciparum growth in EPP and normal RBCs (A) and in XLDPP and normal RBCs (B). Values are expressed as fold changes in parasitemia (relative to inocula levels) (±SD) measured after culturing for 24, 48, and 72 hours. Growth in cells from 4 individuals with EPP was tested separately against 4 different normal blood samples. Significant reductions in parasite growth are indicated (*P < .05, **P < .01). Growth in cells from 3 individuals with XLDPP was tested against a single normal blood sample. Significant increases in parasite growth are indicated (*P < .05, **P < .01). SD, standard deviation.
Due to the decrease in ferrochelatase activity, EPP erythrocytes contain elevated levels of the enzyme’s substrate, PPIX (supplemental Table 1) and have other changes in their biology that may affect the growth of parasites. To exclude the possibility that elevated PPIX or other changes were the cause of impaired parasite growth in EPP cells, we examined the growth of parasites in erythrocytes from patients with XLDPP (MIM 300752). Ferrochelatase activity is normal in these cells but they contain elevated levels of PPIX due to a gain-of-function mutation in the gene encoding ALAS2. The mutant enzyme upregulates the entry of succinyl-CoA and glycine precursors into the heme biosynthesis pathway,29 resulting in substrate build up at the next rate-limiting step, which is ferrochelatase.30,31 Individuals with XLDPP are clinically identical to EPP patients and their red cells contain similarly elevated levels of PPIX and are morphologically and physiologically very similar; ferrochelatase activity is, however, normal (supplemental Table 1). The growth of P falciparum in red cells from 3 XLDPP patients was virtually identical to rates of parasite growth in normal red cells (Figure 1), nor were there differences in the ratios of immature or mature parasite growth stages (supplemental Figure 1B). This fortuitous genetic control demonstrates that elevated red cell PPIX levels do not affect parasite growth, implying that the parasite has a direct requirement for the red cell ferrochelatase enzyme.
A deficiency in host ferrochelatase protects against P chabaudi blood stage infection in mice
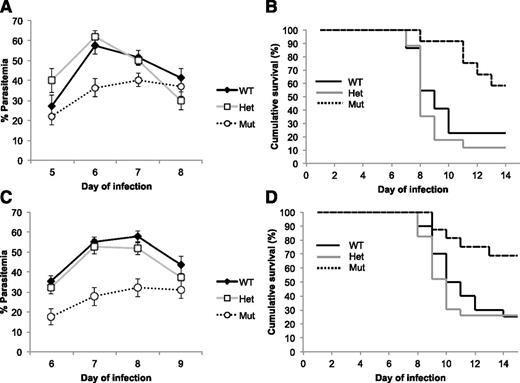
We infected mice carrying a substitution mutation (M98K) in the murine ferrochelatase gene (Fechm1Pas) with the murine malarial parasite, P chabaudi. Mice homozygous for the allele have 5% and heterozygotes 45% to 65% residual enzyme activity compared with wild-type mice.32 Homozygous Fechm1Pas mice (isogenic with C57BL/6) infected with P chabaudi were significantly more resistant to the infection than their heterozygous and wild-type littermates. There was an almost twofold reduction in peak parasitemia levels (Figure 2A,C) and 2 to 3 times greater rates of survival (Figure 2B,D). Host ferrochelatase is therefore also necessary to sustain a normal malarial infection in mice.
P chabaudi infection kinetics and survival in ferrochelatase knockdown mice. Parasitemia of P chabaudi–infected female (A) and male (C) mice. Data (±SE) represent the mean percentage of infected red cells in mice on the indicated day following inoculation with P chabaudi blood-stage parasites. The strains of mice used (all on a C57BL/6 genetic background) were (n = female and male): Fech+/+ (WT, n = 22 and 20), Fech+/m1Pas (Het, n = 17 and 23), and Fechm1Pas/m1Pas (Mut, n = 12 and 16). There was a significant difference between Fechm1Pas/m1Pas mice and both the Fech+/+ and Fech+/m1Pas strains at the day of peak infection (female, day 6, P < .01; male, day 8, P < .01). All parasitemias returned to 0 in surviving mice. Survival of P chabaudi–infected female (B) and male (D) mice. Mice were infected with P chabaudi and monitored for survival over the time period indicated. The mice used were the same as those used to study the parasitemias above. There was a significant difference in survival between Fechm1Pas/m1Pas mice and both Fech+/+ and Fech+/m1Pas strains (female, P < .01; male P < .01). SE, standard error; WT, wild type.
P chabaudi infection kinetics and survival in ferrochelatase knockdown mice. Parasitemia of P chabaudi–infected female (A) and male (C) mice. Data (±SE) represent the mean percentage of infected red cells in mice on the indicated day following inoculation with P chabaudi blood-stage parasites. The strains of mice used (all on a C57BL/6 genetic background) were (n = female and male): Fech+/+ (WT, n = 22 and 20), Fech+/m1Pas (Het, n = 17 and 23), and Fechm1Pas/m1Pas (Mut, n = 12 and 16). There was a significant difference between Fechm1Pas/m1Pas mice and both the Fech+/+ and Fech+/m1Pas strains at the day of peak infection (female, day 6, P < .01; male, day 8, P < .01). All parasitemias returned to 0 in surviving mice. Survival of P chabaudi–infected female (B) and male (D) mice. Mice were infected with P chabaudi and monitored for survival over the time period indicated. The mice used were the same as those used to study the parasitemias above. There was a significant difference in survival between Fechm1Pas/m1Pas mice and both Fech+/+ and Fech+/m1Pas strains (female, P < .01; male P < .01). SE, standard error; WT, wild type.
Parasite-encoded ferrochelatase is not essential for P berghei growth
To confirm whether parasite-encoded ferrochelatase is required for normal parasite growth,10 we interrupted the gene in P berghei that encodes ferrochelatase (PbFC; PBANKA_114070) (refer to “Materials and methods”). Correct targeting of the integration event was confirmed by PCR (supplemental Figure 2). The resulting parasite line (PbFC-KO) developed normally in mice infected by transfusion of blood stage parasites with no appreciable difference in growth rate between the PbFC-KO and the isogenic parental P berghei ANKA line.
Pharmacologic inhibition of ferrochelatase impairs P falciparum parasite growth
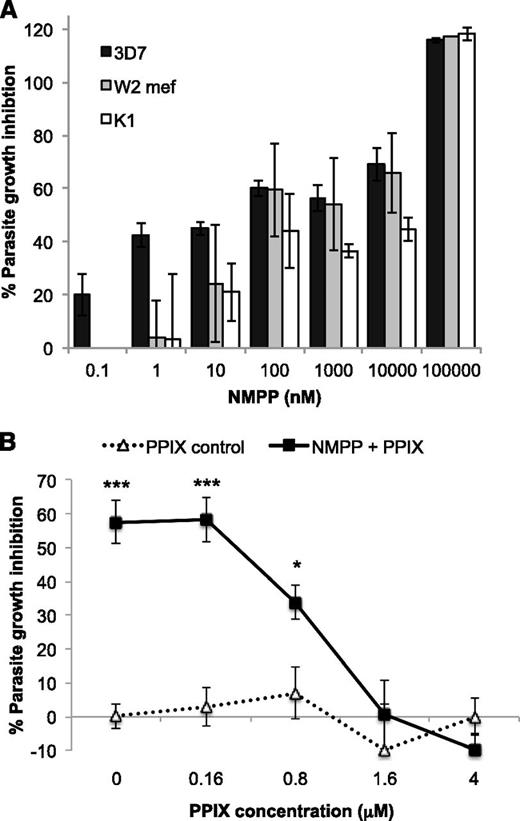
N-MPP is a potent competitive inhibitor of ferrochelatase with a Ki of ∼10 nM.33 To determine if N-MPP inhibits the growth of Plasmodium, N-MPP was titrated in cultures of various strains of P falciparum and parasite growth measured after 48 hours. A potent growth inhibitory effect was observed against all the parasite strains examined (Figure 3A), including the drug-sensitive 3D7, chloroquine-resistant K1 and multidrug-resistant W2-mef strains. The inhibition curves for each strain showed a partly nonlinear slope, with an apparent plateau between 10 and 1000 nM; this impacted on the variability of the calculated IC50 values. RBC concentrations are directly related to N-MPP treatment concentration (supplemental Figure 3A), excluding differential uptake as an explanation for this growth inhibition effect. No differences in either enzyme substrate (PPIX) or product (heme) were observed over the N-MPP dosage range, confirming that the heme biosynthetic pathway is nonfunctional in mature red cells (supplemental Figure 3B-C).
The effects of N-MPP treatment on P falciparum growth. (A) Parasite growth inhibition with increasing concentrations of N-MPP. Assays were conducted using P falciparum strains 3D7, K1, and W2-mef. Parasites, synchronized at the trophozoite stage, were treated with N-MPP at the indicated concentrations and growth determined relative to an untreated control after 48-hour incubation. Data (±SEM) represent the mean of 2 independent assays for 3D7 and 1 assay for K1 and W2-mef (each concentration was assayed in triplicate). Respective IC50 values (nM ± SE) were: 49.7 ± 2.67, 459 ± 89.2, and 199 ± 43.3. (B) Titration of PPIX against N-MPP treatment. Assays were conducted using P falciparum 3D7 parasites. Parasite cultures were treated with or without 50 μM N-MPP. Growth inhibition was determined relative to an untreated control after 48-hour incubation. Data (±SEM) represents mean of 2 independent assays (each concentration was assayed in triplicate). *P < .05, **P < .01, ***P < .001 compared with untreated cells. SEM, standard error of the mean.
The effects of N-MPP treatment on P falciparum growth. (A) Parasite growth inhibition with increasing concentrations of N-MPP. Assays were conducted using P falciparum strains 3D7, K1, and W2-mef. Parasites, synchronized at the trophozoite stage, were treated with N-MPP at the indicated concentrations and growth determined relative to an untreated control after 48-hour incubation. Data (±SEM) represent the mean of 2 independent assays for 3D7 and 1 assay for K1 and W2-mef (each concentration was assayed in triplicate). Respective IC50 values (nM ± SE) were: 49.7 ± 2.67, 459 ± 89.2, and 199 ± 43.3. (B) Titration of PPIX against N-MPP treatment. Assays were conducted using P falciparum 3D7 parasites. Parasite cultures were treated with or without 50 μM N-MPP. Growth inhibition was determined relative to an untreated control after 48-hour incubation. Data (±SEM) represents mean of 2 independent assays (each concentration was assayed in triplicate). *P < .05, **P < .01, ***P < .001 compared with untreated cells. SEM, standard error of the mean.
To assess the mode of action of N-MPP in parasitized RBCs, increasing concentrations of PPIX were added in the presence or absence of 50 μM N-MPP (Figure 3B). Alone, PPIX treatment had no effect on parasite growth at any concentration tested. However, PPIX ablated the growth-inhibitory effect of N-MPP in a dose-dependent fashion with a threshold of 100% activity at molar ratios exceeding 1:30 (PPIX:N-MPP). This demonstrates that N-MPP acts competitively with the ferrochelatase substrate PPIX and is preventing parasite growth by specifically inhibiting the ferrochelatase enzyme.
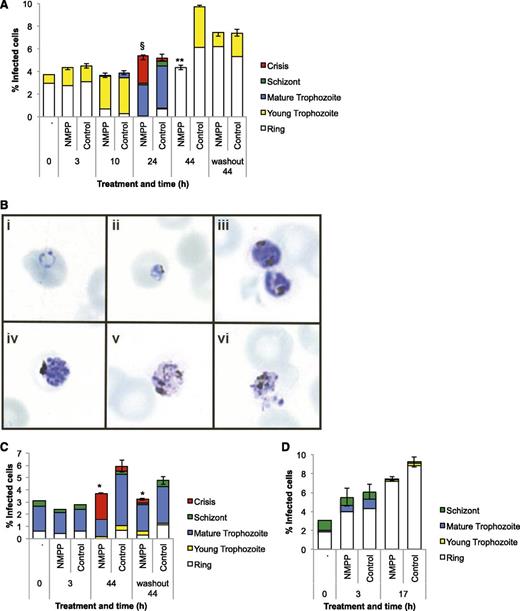
Different synchronized growth stages of P falciparum 3D7 were separately treated with N-MPP (5 µM) to determine which were most susceptible to the compound. Treatment synchronized ring-stage parasites (5-15 hours postinvasion) resulted in the appearance of distinct crisis form parasites after 24-hour incubation, whereas untreated cultures had developed into mature pigmented trophozoites by this time point (Figure 4A-B). Similar treatment of parasites synchronized at the pigmented trophozoite stage (25-35 hours postinvasion) resulted in the formation of crisis form parasites after 44 hours (Figure 4C). We also treated synchronized ring or pigmented trophozoite-stage parasites with N-MPP for a limited time (3 hours), followed by extensive washing to remove the compound, and then continued the incubation in drug-free culture medium. Significant growth inhibitory effects were observed for trophozoites, but not for rings (Figure 4A,C; washout). Treatment of synchronized schizont-stage parasites (35-45 hours postinvasion) did not affect the production of ring-stage cells after 3- and 17-hour incubation (Figure 4D). Taken together, we conclude that ferrochelatase inhibition by N-MPP is specifically toxic to late-stage (pigmented) trophozoites. Neither parasite invasion of red cells, nor the development of parasites from the ring to trophozoite stage, was affected.
The effect of N-MPP on intraerythrocytic parasite development.N-MPP (5 μM) was added to cultured P falciparum parasites synchronized at the ring (A), mature pigmented trophozoite (C), or schizont stage (D), and proportions of individual parasite stages were enumerated at the indicated time points. Cells were also treated with N-MPP for 3 hours, washed extensively, and then returned to drug-free culture medium for the remainder of the experiment (washout). Crisis-form parasites were observed at some time points. Data (±SEM) represent the mean of 1 assay performed in duplicate. *P < .05, **P < .01 comparing N-MPP vs control total percentage of infected cells; §P < .05 comparing N-MPP vs control mature trophozoites, schizonts, and crisis-form parasites. (B) Photomicrographs of different parasite stages and examples of crisis-form parasites. Healthy appearing ring (i), young trophozoite (ii), mature pigmented trophozoite (iii) and schizont (iv) stages, and crisis-form trophozoites (v and vi).
The effect of N-MPP on intraerythrocytic parasite development.N-MPP (5 μM) was added to cultured P falciparum parasites synchronized at the ring (A), mature pigmented trophozoite (C), or schizont stage (D), and proportions of individual parasite stages were enumerated at the indicated time points. Cells were also treated with N-MPP for 3 hours, washed extensively, and then returned to drug-free culture medium for the remainder of the experiment (washout). Crisis-form parasites were observed at some time points. Data (±SEM) represent the mean of 1 assay performed in duplicate. *P < .05, **P < .01 comparing N-MPP vs control total percentage of infected cells; §P < .05 comparing N-MPP vs control mature trophozoites, schizonts, and crisis-form parasites. (B) Photomicrographs of different parasite stages and examples of crisis-form parasites. Healthy appearing ring (i), young trophozoite (ii), mature pigmented trophozoite (iii) and schizont (iv) stages, and crisis-form trophozoites (v and vi).
Discussion
We demonstrate here that red cells deficient in ferrochelatase are significantly resistant to infection by the blood stage form of Plasmodium infection. Human red cells from erythropoietic protoporphyria patients, genetically deficient in ferrochelatase, poorly supported growth of P falciparum parasites. Comparison with the rare EPP phenocopy, X-linked dominant protoporphyria suggests that this effect was most likely due to the deficiency of the enzyme and not the accumulation of PPIX substrate, or other abnormality in the EPP cells. Confirmatory results were obtained with P chabaudi–infected ferrochelatase-deficient mice, which exhibited a decrease in parasitemia and an increase in survival. Several red cell abnormalities are over represented in populations residing in malaria-endemic regions, and these contribute to a relative protection against malaria.34 Most cases of EPP are caused by the inheritance of the common hypomorphic SNP IVS3-48C allele trans to a deleterious ferrochelatase gene mutation.28 Interestingly, this allele is common in southeast Asian (31%) and South American individuals but rare in West African populations suggesting positive selection.35 Based on our findings, we speculate that the benefit may be malaria protection.
An alternative explanation for the reduced growth rates in ferrochelatase-deficient cells is that these cells contain lower levels of heme, mainly as hemoglobin. It has been shown previously that the parasite can scavenge heme from the host cell and incorporate it into its own proteins.10 This would also imply that de novo heme synthesis is largely dispensable. However, there are several reasons that suggest this is not the case. First, cellular hemoglobin levels are usually normal in EPP patients.36,37 Likewise, hemoglobin levels are only slightly decreased in the Fechm1Pas mouse strain.20 Therefore, any reduction in available heme levels is modest at most, and not sufficient to explain the parasite growth inhibition effects. Second, we observed that treatment with a specific ferrochelatase inhibitor, N-MPP, inhibited the growth of cultured P falciparum. N-MPP inhibits host FECH but is also likely to inhibit parasite ferrochelatase. The cytocidal action of N-MPP occurred at submicromolar concentrations and was via specific ferrochelatase inactivation, and not other off-target effects. N-MPP treatment does not alter erythrocyte heme levels. Scavenging of host heme, which would likewise be unaffected by N-MPP, is apparently unable to compensate the loss of de novo heme biosynthesis in N-MPP-treated parasites. In addition, succinylacetone, which is a highly specific inhibitor ALAD, similarly kills P falciparum grown in culture conditions.38 Together, these findings strongly imply that de novo heme biosynthesis is essential to the parasite, and that scavenging of host heme has only a minor role in the parasite’s metabolism.
Our data also underscore the importance of host, rather than parasite, ferrochelatase for intraerythrocytic growth. Plasmodium encodes its own version of ferrochelatase, and this is active and localized to the mitochondria.25,39 However in agreement with reported findings,10 we found that parasite-encoded ferrochelatase is not necessary as a knockout of the ferrochelatase gene in P berghei had no effect on the growth of the parasite in mice infected by inoculation. Nagaraj and colleagues also showed a similar result for ALAS-deficient parasites.10 However it is evident that the parasite versions of ALAS and ferrochelatase are required for normal oocyst and sporozoite production in the mosquito.10 Part of the intrinsic heme biosynthetic pathway occurs within the parasite apicoplast.12 Interestingly, recent work has suggested that isopentenyl pyrophosphate synthesis is the only essential metabolic function of the apicoplast in erythrocytic stage P falciparum,15 lending further support to the view that the intrinsic pathway is largely dispensable. Others have reported that red cell ferrochelatase is imported into the Plasmodium cytosol during its growth in the red cell and that this accounts for up to 80% of the parasite’s ferrochelatase activity.4,8 There are also precedents for the import of other active host enzymes into the parasite,6 however the mechanisms involved are unknown. Together, these findings support the hypothesis that Plasmodium relies on host enzymes and extrinsic heme biosynthesis during the erythrocytic stage of infection. It may therefore also be concluded that the major target of the cytocidal action imparted by N-MPP is the host cell version of ferrochelatase.
Overall, we have demonstrated that host ferrochelatase is required by intraerythrocytic stage Plasmodium parasites and that ferrochelatase inhibition, either through genetic or chemical means, impedes parasite growth. We therefore propose that the host enzyme has potential as a host-directed antimalarial target.40,41 Inhibitors of an essential target would be expected to block parasite growth and avoid potential drug resistance given the target gene is not under the control of the parasite. In the case of targeting host ferrochelatase, a sporadic and temporary inhibition of the enzyme should be well tolerated in humans. EPP patients have a severe deficiency in the enzyme from birth (below 30% residual activity) and apart from skin photosensitivity, display relatively few symptoms and have a normal life expectancy; a small proportion (<5%) develop liver disease. Targeting host ferrochelatase with N-MPP would therefore provide an example of a host-directed antimalarial that may possess greater longevity than historical and current antimalarial drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank X. Schneider for genotyping; C. Flowers, B. Pedersen, M. Roberts-Thomson, O. Gorgette, F. Rodda, S. Lampkins, and P. Lawrie for technical support; the Australian Red Cross Blood Service for providing RBCs; R. Anders and L. Tilley for the P falciparum parasites; and X. Montagutelli for the Fechm1Pas mice.
This work was supported by the National Health and Medical Research Council (program grant 490037, and project grants 605524 and APP1047090), Australian Society for Parasitology, European Union Collaborative Research Grants scheme, Australian Academy of Science, Howard Hughes Medical Institute, and the Bill and Melinda Gates Foundation. This research was conducted in the frame of the Contrat de recherche de collaboration between Institut Pasteur, Paris and l’Assistance Publique-Hopitaux de Paris (ref. VAL/2013/2011-237/01).
Authorship
Contribution: C.M.S., G.v.D., G.I.M., G.B., B.J.M., and S.J.F. designed research; C.M.S., A.J., G.v.D., C.D.G., A.S., P.D., G.B., and B.J.M. performed research; H.P., I.W., J.-C.D., L.G., and H.M. contributed vital reagents; C.M.S., H.P., A.J., I.W., J.-C.D., L.G., G.v.D., G.I.M., H.M., P.D., O.M.-P., G.B., B.J.M., and S.J.F. analyzed and interpreted data; C.M.S., G.B., and B.J.M. performed statistical analysis; and C.M.S., G.I.M., O.M.-P., B.J.M., and S.J.F. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.M.S. is Department of Microbiology and Physiological Systems, University of Massachusetts Medical School, Worcester, MA.
Correspondence: Simon J. Foote, Australian National University, JCSMR, Building 131, Canberra, ACT 2601, Australia; e-mail: simon.foote@anu.edu.au; and Brendan McMorran, Australian National University, JCSMR, Building 131, Canberra, ACT 2601, Australia; e-mail: brendan.mcmorran@anu.edu.au.