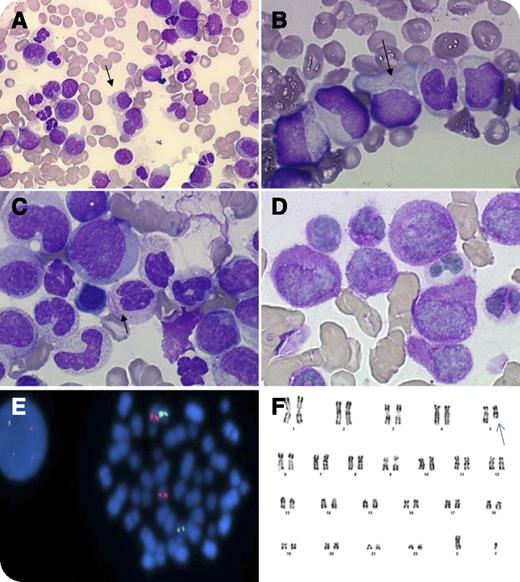
An 85-year-old man was admitted with a 4-week history of weakness, vertigo, weight loss, and pallor. Blood cell count showed 3 × 109/L leukocytes, 9 g/dL hemoglobin, 49 × 109/L platelets, and no disseminated intravascular coagulation. Cytological examination evidenced 8% blast cells in the peripheral blood and 77% abnormal promyelocytes in the bone marrow. The blasts’ cytoplasm had azurophilic granules and bundles of Auer rods (faggot cells). The nuclei were regular, irregular, or bi-lobed (panels A-C), typical of acute promyelocytic leukemia (APL). Cytochemical stains showed that blast cells strongly expresssed myeloperoxidase (100%) (panel D). The diagnosis of APL of French-American-British subtype M3 was made, and the patient received tretinoin. No response was observed, and the treatment was stopped. Fluorescence in situ hybridization excluded a promyelocyte leukemia–retinoic acid receptor alpha (RARA) fusion gene, another variant RARA translocation (panel E), and MLL gene rearrangement. Molecular analysis excluded the presence of promyelocytic leukemia zinc finger–RARA and signal transducer and activator of transcription 5b-RARA fusion transcripts. The karyotype showed a 5q deletion (panel F) suggestive of acute myeloblastic leukemia (AML) related to myelodysplasia.
In the context of severe heart disease and advanced age, the patient received only supportive care. This reports that the presence of bundles of Auer rods and typical APL morphology may occur in other forms of AML. The retinoid resistance and lack of RARA rearrangement led to further evaluation in this case, which provided an alternative diagnosis.
An 85-year-old man was admitted with a 4-week history of weakness, vertigo, weight loss, and pallor. Blood cell count showed 3 × 109/L leukocytes, 9 g/dL hemoglobin, 49 × 109/L platelets, and no disseminated intravascular coagulation. Cytological examination evidenced 8% blast cells in the peripheral blood and 77% abnormal promyelocytes in the bone marrow. The blasts’ cytoplasm had azurophilic granules and bundles of Auer rods (faggot cells). The nuclei were regular, irregular, or bi-lobed (panels A-C), typical of acute promyelocytic leukemia (APL). Cytochemical stains showed that blast cells strongly expresssed myeloperoxidase (100%) (panel D). The diagnosis of APL of French-American-British subtype M3 was made, and the patient received tretinoin. No response was observed, and the treatment was stopped. Fluorescence in situ hybridization excluded a promyelocyte leukemia–retinoic acid receptor alpha (RARA) fusion gene, another variant RARA translocation (panel E), and MLL gene rearrangement. Molecular analysis excluded the presence of promyelocytic leukemia zinc finger–RARA and signal transducer and activator of transcription 5b-RARA fusion transcripts. The karyotype showed a 5q deletion (panel F) suggestive of acute myeloblastic leukemia (AML) related to myelodysplasia.
In the context of severe heart disease and advanced age, the patient received only supportive care. This reports that the presence of bundles of Auer rods and typical APL morphology may occur in other forms of AML. The retinoid resistance and lack of RARA rearrangement led to further evaluation in this case, which provided an alternative diagnosis.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal