Key Points
NKG2D enhances cytotoxicity and survival of CD8+ T cells, which contributes to GVHD and GVT effects after allogeneic HSCT.
The temporally distinct expression pattern of NKG2D ligands may allow separation of GVHD and GVT effects by transient NKG2D blockade.
Abstract
In allogeneic hematopoietic stem cell transplantation (HSCT), controlling graft-versus-host disease (GVHD) while maintaining graft-versus-tumor (GVT) responses is of critical importance. Using a mouse model of allogeneic HSCT, we hereby demonstrate that NKG2D expression by CD8+ T cells plays a major role in mediating GVHD and GVT effects by promoting the survival and cytotoxic function of CD8+ T cells. The expression of NKG2D ligands was not induced persistently on normal tissues of allogeneic HSCT–recipient mice treated with anti-NKG2D antibody, suggesting that transient NKG2D blockade might be sufficient to attenuate GVHD and allow CD8+ T cells to regain their GVT function. Indeed, short-term treatment with anti-NKG2D antibody restored GVT effects while maintaining an attenuated GVHD state. NKG2D expression was also detected on CD8+ T cells from allogeneic HSCT patients and trended to be higher in those with active GVHD. Together, these data support a novel role for NKG2D expression by CD8+ T cells during allogeneic HSCT, which could be potentially therapeutically exploited to separate GVHD from GVT effects.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for hematologic malignancies.1 In patients undergoing allogeneic HSCT, donor-derived T-cell activation leads to widespread host tissue damage, resulting in a clinicopathologic syndrome known as graft-versus-host disease (GVHD).2 However, T cell–mediated attack of the recipient can also be beneficial because it can eliminate malignant cells that might have escaped radiation or chemotherapy, a process known as the graft-versus-tumor (GVT) effect.3 Therefore, identifying mechanisms to control GVHD yet maintain GVT effects is of critical importance to improve the success rate of allogeneic HSCT treatment.
Allogeneic T-cell activation occurs in several phases after HSCT. The conditioning regimen contributes to priming of the immune response by inducing inflammation, leading to activation of antigen-presenting cells (APC)s, expansion and activation of donor T cells by host APCs, and, finally, trafficking of activated T cells to the GVHD target tissues where inflammation and tissue destruction occur.4,5 In addition to T-cell receptor (TCR) stimulation by allogeneic major histocompatibility complex (MHC), costimulation provided by APCs (B7 family, the tumor necrosis factor [TNF] receptor family, and adhesion molecules such as lymphocyte function-associated antigen 1)6-9 induce full T-cell activation, proliferation, and cytokine production.10 Costimulatory signal blockade during allogeneic HSCT has shown that these signals play an important role in the pathophysiology of acute GVHD.3,11
Costimulatory signals received from non-APCs could also promote the function of T cells. For example, the engagement of NKG2D with NKG2D ligands expressed on a variety of cells can promote the activity of CD8+ T cells under specific settings.12 NKG2D is expressed by subsets of natural killer (NK) cells, NK T cells, γδ T cells, and CD8+ T cells.12-15 NKG2D recognizes several MHC-related proteins with limited constitutive expression, which are upregulated on transformed, infected, and “stressed” cells.12,15-17 These ligands include the RAE-1, H60, and Mult1 proteins in rodents, and the MIC and ULBP/RAET families in humans.18-21 Because many neoplastic cells constitutively express NKG2D ligands, NKG2D expression by NK cells and T cells plays an important role in antitumor responses.17 NKG2D also facilitates TCR-mediated CD8+ T-cell activation in inflammatory states where NKG2D ligands are induced on normal tissue, such as in type 1 diabetes and in solid organ transplantation.22-24
Although NKG2D ligands are upregulated upon myeloablative conditioning before allogeneic HSCT treatment,25-29 the role that NKG2D expression on CD8+ T cells plays after allogeneic HSCT is unknown. Because activated CD8+ T cells express NKG2D, we sought to investigate how NKG2D expression might affect CD8+ T cell responses in allogeneic HSCT. Given that NKG2D ligands are expressed on both stressed normal tissue and malignant cells, we hypothesized that NKG2D plays an important role in mediating GVHD and GVT effects during allogeneic HSCT. In this manuscript, we provide data supporting a role for NKG2D on CD8+ T cells in mediating GVHD and GVT effects, which could be therapeutically exploited to separate the 2 processes.
Methods
Mice
NKG2D knockout (KO) mice were described previously30 and bred in our institution. C57BL/6, C57BL/6.SJL, C3H.SW, and Balb/c were purchased from the National Cancer institute or Charles River Laboratories. Perforin KO and P14 TCR transgenic mice were a gift from Drs Edward Behrens and John Wherry, respectively (University of Pennsylvania). Mice of 8 to 12 weeks of age were used and all experiments were performed with age- and sex-matched mice. Animal maintenance and experimentation were performed in accordance with the Institutional Animal Care and Use Committee at the University of Pennsylvania. Peripheral blood samples from patients undergoing allogeneic HSCT were collected using a protocol approved by our Institutional Review Board, in accordance with the Declaration of Helsinki.
Reagents, cell lines, flow cytometry, and statistical analysis
Monoclonal antibodies were purchased from e-biosciences (San Diego, CA): anti-CD3-FITC, anti-NKG2D-APC, anti-RAE-1-biotin; BD Biosciences (San Diego, CA): anti-CD8-FITC, anti-CD3, anti-CD28, anti-BrdU-APC, anti-interferon (IFN)-γ-APC, anti-TNF-α-PE, anti-CD45.1-PerCPCy5.5; Biolegend (San Diego, CA): anti-CD45.1-Pacific Blue, anti-H-2Kb-Pacific Blue; BioXcell: anti-NKG2D, rat IgG1 isotype control antibody; Molecular Probes, Invitrogen (Carlsbad, CA): LIVE/DEAD Fixable Aqua Dead Cell Stain Kit. All cell culture reagents and chemicals were purchased from Invitrogen (Grand Island, NY) and Sigma Aldrich (St. Louis, MO), respectively, unless otherwise specified. The A20 cell line was transduced with luciferase and cultured as described previously.31 Flow cytometry was performed by LSR-II or FACS Canto (BD Biosciences). Dead cells were excluded from analysis with LIVE/DEAD Fixable Aqua Dead Cell staining. Data were analyzed with FlowJo software (TreeStar, Ashland, OR). For cell sorting, T cells were purified with CD8 magnetic beads using magnetic-activated cell sorting (MACS) columns (Miltenyi Biotec, Auburn, CA) before cell-surface staining. Fluorescence-activated cell sorting (FACS) sorting was performed with a FACSAria cell sorter (BD Biosciences) at the University of Pennsylvania Flow Cytometry and Cell Sorting Core. FACS-sorted populations were typically of >95% purity. Statistical analysis was performed by Student t test or log-rank test using Prism computer software (GraphPad, San Diego, CA).
Bone marrow transplantation and GVT studies
Balb/c mice were lethally irradiated with 800 cGy split in 2 equal doses separated by 12 hours. The mice were then injected IV with 5 × 106 wild-type (WT) T cell–depleted (TCD) bone marrow (BM) cells with or without 1 × 106 FACS-sorted CD8+ T cells (from B6, B6.SJL, Perforin KO, or NKG2D KO mice). In some experiments, FACS-sorted CD8+ T cells from B6.SJL and NKG2D KO mice were mixed at a 1:1 ratio and injected into mice (1 × 106 FACS-sorted CD8+ T cells total). Host mice were left untreated or were injected intraperitoneally with rat IgG1 isotype control antibody (0.2 mg/mouse; BioXCell, West Lebanon, NH) or with anti-NKG2D antibody (clone BE0111; 0.2 mg/mouse; BioXCell) as indicated. For the minor mismatch GVHD model, B6 mice were lethally irradiated with 1000 cGy split in 2 equal doses separated by 12 hours. The mice were then injected IV with 5 × 106 WT TCD BM cells with or without 1 × 106 FACS-sorted CD8+ T cells from C3H.SW mice. Mice were monitored every day for survival. Clinical presentation of the mice was assessed 2 to 3 times per week by a scoring system that sums changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity.32 Mice were killed when their weight dropped to <70% of their initial body weight. For GVT experiments, 0.5 to 1 × 105 luciferase-expressing A20 cells were injected IV together with the BM cells with or without CD8+ T cells at the time of transplant. Mice were evaluated twice a week from the time of tumor injection for 30 to 50 days by bioluminescence imaging using the IVIS 200 Imaging System (Xenogen) as previously described.33
T-cell functional assays
MACS-sorted CD8+ T cells from spleens of WT (B6) or P14 mice were cultured in combinations of IL-2 (50 U/mL), anti-CD3/CD28 (2.5 μg/mL), and gp33 peptide (1 μM) in the presence of irradiated B6 splenocytes. For allostimulations, the T cells were cultured with irradiated allogeneic Balb/c splenocytes. NKG2D expression was measured on CD8+ T cells after 3 days of culture. For cytokine detection, splenocytes were stimulated with phorbol 12-myristate 13-acetate (100 ng/mL) and ionomycin (1 μg/mL) for 4 to 6 hours in the presence of Brefeldin A (10 μM). Cells were stained for cell-surface markers and for cytokines (IFN-γ and TNF-α) according to the manufacturer’s protocol (BD Biosciences). For detection of BrdU, spleen cells were resuspended in freezing media (90% fetal calf serum, 10% dimethyl sulfoxide), stored overnight at −80°C, thawed, and stained for BrdU according to the manufacturer’s directions (BD Biosciences). To test for cytotoxicity, we used bioluminescence measurements of luciferase-expressing A20 cells as previously described.34,35 Effector cells (MACS-sorted or FACS-sorted CD8+ T cells from BM-transplanted mice) were added at 40:1, 20:1, 10:1, and 5:1 effector-to-target ratios and incubated at 37°C for 4 hours with A20 cells. Anti-NKG2D antibody (10 μg/mL) or rat IgG1 isotype control antibody (10 μg/mL) was added and incubated for 30 minutes before washing and plating. Triplicate wells were averaged and percent lysis was calculated from the data using the following equation: % specific lysis = 100 × (spontaneous death bioluminescence – test bioluminescence)/(spontaneous death bioluminescence – maximal killing bioluminescence).
Fluorescence microscopy and histologic analysis
The colon was harvested from experimental and control mice, washed in phosphate-buffered saline (PBS), flash frozen in O.C.T. (Tissue-Tek) media and sectioned at 10 µm by the Cancer Histology Core (University of Pennsylvania Abramson Cancer Center). Frozen sections were fixed with acetone for 5 minutes at room temperature and washed in PBS. Endogenous biotin was blocked with the Avidin/Biotin Blocking Kit (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. Sections were stained with anti-RAE-1-biotin or rat IgG2b κ-biotin isotype control (BD Biosciences) overnight at 4°C, and washed in PBS, followed by incubation with Pan-Keratin AlexaFluor488 Conjugate (Millipore, Billerica, MA) and Streptavidin-AlexaFluor594 (Jackson Immunoresearch, West Grove, PA) for 1 hour. After washing in PBS, slides were mounted with Fluoromount-G and incubated with 4′,6-diamidino-2-phenylindole (DAPI) to stain nuclei. Fluorescence images were collected using the Axioplan microscope (Zeiss, Jena, Germany) and AxioVision 4.8 software (Zeiss).
Histologic sections were obtained from gut from mice treated with either anti-NKG2D antibody or Rat IgG1 isotype control antibody at day 7 or day 14 post-HSCT. Tissue was embedded in paraffin and sections were stained with hematoxylin and eosin. The tissue was examined by a pathologist who was blinded to the treatment condition of the animals. Tissue sections were examined for evidence of GHVD including intraepithelial lymphocytes associated with epithelial sloughing, crypt loss, villous atrophy, and epithelial apoptotic bodies.
Results
NKG2D is induced on CD8+ T cells upon TCR stimulation and augments CD8+ T cell–mediated GVHD
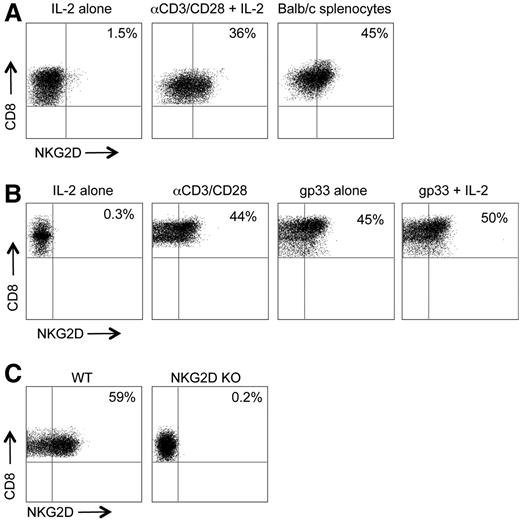
Although naïve murine CD8+ T cells do not express NKG2D,13 NKG2D was detected on CD8+ T cells after TCR stimulation by either anti-CD3/CD28, cognate antigen, or allogeneic MHC (Figure 1A-B). Similarly, we found that a large fraction of donor-derived WT B6 but not NKG2D KO CD8+ T cells expressed NKG2D 7 days after transfer into irradiated Balb/c hosts (Figure 1C). Activated CD8+ T cells from day 7 post–allo-bone marrow transplant (BMT) mice upregulated expression of DAP10, which coimmunoprecipitated with NKG2D (supplemental Figure 1). Thus, TCR stimulation through allogeneic MHC induces a bona fide NKG2D/DAP10 signaling complex on murine CD8+ T cells.
NKG2D is induced on CD8+ T cells after TCR stimulation in vitro and in vivo. (A) WT CD8+ T cells were activated with IL-2 alone, anti-CD3/CD28, and IL-2, or with irradiated Balb/c splenocytes for 3 days. (B) CD8+ T cells from P14 mice were stimulated with IL-2 alone, anti-CD3/CD28, gp33 peptide, or gp33 peptide + IL-2 for 3 days. Representative flow cytometric plots of NKG2D expression on CD8+ T cells are shown. (C) Irradiated Balb/c mice were transplanted with B6 TCD BM cells and B6 or NKG2D KO-derived FACS-sorted CD8+ T cells. On day 7, donor-derived CD8+ T cells were analyzed for expression of NKG2D by flow cytometry. One representative of 2 to 3 independent experiments is shown.
NKG2D is induced on CD8+ T cells after TCR stimulation in vitro and in vivo. (A) WT CD8+ T cells were activated with IL-2 alone, anti-CD3/CD28, and IL-2, or with irradiated Balb/c splenocytes for 3 days. (B) CD8+ T cells from P14 mice were stimulated with IL-2 alone, anti-CD3/CD28, gp33 peptide, or gp33 peptide + IL-2 for 3 days. Representative flow cytometric plots of NKG2D expression on CD8+ T cells are shown. (C) Irradiated Balb/c mice were transplanted with B6 TCD BM cells and B6 or NKG2D KO-derived FACS-sorted CD8+ T cells. On day 7, donor-derived CD8+ T cells were analyzed for expression of NKG2D by flow cytometry. One representative of 2 to 3 independent experiments is shown.
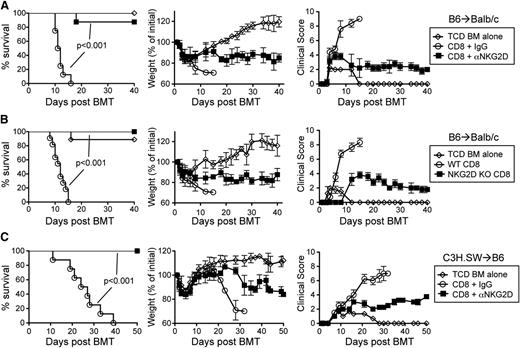
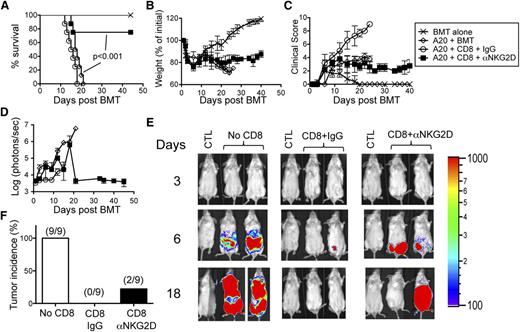
To test the role of NKG2D in CD8+ T cell–mediated GVHD, we used a major mismatch model of GVHD (B6→Balb/c) and treated the mice with either isotype control or anti-NKG2D antibody. All mice treated with isotype-control antibody died within 2 weeks post-BMT (Figure 2A). In contrast, 90% of mice treated with anti-NKG2D antibody survived for >40 days and displayed attenuated weight loss and clinical score (Figure 2A). Similarly, Balb/c mice transplanted with NKG2D KO CD8+ T cells displayed decreased mortality, weight loss, and clinical scores compared with mice transplanted with WT CD8+ T cells (Figure 2B). Furthermore, NKG2D blockade significantly reduced mortality, weight loss, and clinical scores in a minor mismatch model (C3H.SW→B6) of GVHD (Figure 2C).
NKG2D blockade attenuates CD8+ T cell–mediated GVHD. (A) Irradiated Balb/c mice were transplanted with B6-derived TCD BM alone or with B6-derived TCD BM cells and FACS-sorted CD8+ T cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 2 weeks. (B) Irradiated Balb/c mice were transplanted with B6-derived TCD BM alone or with B6-derived TCD BM cells and B6-derived or NKG2D KO-derived FACS-sorted CD8+ T cells. (C) Irradiated B6 mice were transplanted with C3H.SW-derived TCD BM alone or with B6-derived TCD BM cells and FACS-sorted CD8+ T cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 2 weeks. The mice were monitored for survival (left), body weight changes (middle), and clinical score (right) for 40 days post-BMT. The survival graph was generated using all mice from 2 or 3 independent experiments (n = 3-5 mice/group per experiment). One representative of 2 or 3 independent experiments is shown for weight changes and clinical score (n = 3-4 mice/group). Statistical analysis for survival was performed using the log-rank test.
NKG2D blockade attenuates CD8+ T cell–mediated GVHD. (A) Irradiated Balb/c mice were transplanted with B6-derived TCD BM alone or with B6-derived TCD BM cells and FACS-sorted CD8+ T cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 2 weeks. (B) Irradiated Balb/c mice were transplanted with B6-derived TCD BM alone or with B6-derived TCD BM cells and B6-derived or NKG2D KO-derived FACS-sorted CD8+ T cells. (C) Irradiated B6 mice were transplanted with C3H.SW-derived TCD BM alone or with B6-derived TCD BM cells and FACS-sorted CD8+ T cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 2 weeks. The mice were monitored for survival (left), body weight changes (middle), and clinical score (right) for 40 days post-BMT. The survival graph was generated using all mice from 2 or 3 independent experiments (n = 3-5 mice/group per experiment). One representative of 2 or 3 independent experiments is shown for weight changes and clinical score (n = 3-4 mice/group). Statistical analysis for survival was performed using the log-rank test.
We next tested whether human CD8+ T cells from allogeneic HSCT patients also express NKG2D. CD8+ T cells from 35 blood samples of allogeneic HSCT patients were analyzed for NKG2D expression. The CD8+ T cells from all patients expressed NKG2D. Interestingly, although not statistically significant, patients with active GVHD at the time of the blood draw trended toward higher levels of NKG2D compared with patients without GVHD (supplemental Figure 2). These data suggest that our findings that NKG2D expression by CD8+ T cells augments GVHD pathogenesis in mice may be clinically relevant.
NKG2D KO CD8+ T cells exhibit reduced survival and cytotoxicity in a cell-intrinsic manner
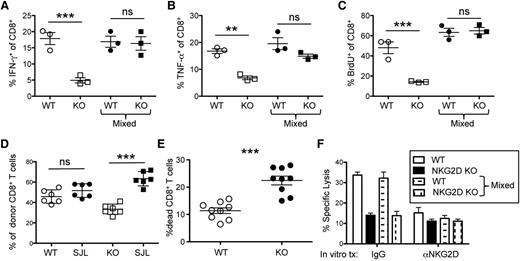
To test the function of NKG2D KO CD8+ T cells in a cell-intrinsic manner, we performed BMT using FACS-sorted WT or NKG2D KO CD8+ T cells and compared the response with mice transplanted with WT and NKG2D KO CD8+ T cells mixed together. The production of cytokines (IFN-γ and TNF-α) and BrdU incorporation rates were significantly reduced in NKG2D KO compared with WT CD8+ T cells on day 7 post-BMT but not when the WT and NKG2D KO CD8+ cells were mixed in the same recipient (Figure 3A-C). Despite similar BrdU incorporation, a significant enrichment in the fraction of WT CD8+ T cells compared with NKG2D KO CD8+ T cells was seen on day 7 post-BMT (Figure 3D), suggesting that WT CD8+ T cells exhibit a survival advantage over NKG2D KO CD8+ T cells. Indeed, a larger fraction of dead cells was detected in CD8+ T cells of NKG2D KO origin compared with WT (Figure 3E). NKG2D KO CD8+ T cells also showed reduced cytotoxicity against A20 leukemia targets when removed from mice either transplanted singly or with a mixture of WT and NKG2D KO CD8+ T cells (Figure 3F). The cytotoxicity of WT CD8+ T cells against A20 cells was reduced in vitro by the addition of anti-NKG2D antibody, suggesting that NKG2D augments killing of A20 cells (Figure 3F). Together, these data suggest that, although decreased survival and cytotoxicity is the primary defect that is cell intrinsic to NKG2D KO CD8+ T cells, the reduction in cytokine production and proliferation in the absence of NKG2D are cell-extrinsic effects.
NKG2D KO CD8+ T cells display a survival disadvantage and reduced cytotoxicity in a cell-intrinsic manner. Irradiated Balb/c mice were transplanted with B6-derived TCD BM cells and FACS-sorted WT CD8+ T cells (CD45.1+), NKG2D KO CD8+ T cells (CD45.2+), or a mix of WT CD8+ T cells and NKG2D KO CD8+ T cells. (A) On day 7, spleen cells were harvested from the transplanted mice and stimulated with phorbol 12-myristate 13-acetate/ionomycin for 4 hours. Scatter plots of the fraction of IFN-γ and (B) TNF-α–producing WT and NKG2D KO CD8+ T cells in the individually transplanted setting and in the mixed setting are shown. (C) Transplanted mice were treated with BrdU on day 6 post-BMT and harvested on day 7. The fraction of BrdU-incorporated WT and NKG2D KO CD8+ T cells in the individually transplanted setting and in the mixed setting is shown. (D) Irradiated Balb/c mice were transplanted with B6-derived TCD BM cells and WT B6.SJL CD8+ T cells mixed with either WT B6 CD8+ T cells (CD45.2+) or NKG2D KO CD8+ T cells (CD45.2). The percentage of B6.SJL WT, WT B6, or NKG2D KO CD8+ T cells out of all donor-derived CD8+ T cells is represented as a scatter plot. (E) The percent of dead (live/dead stain+) CD8+ T cells of WT or NKG2D KO origin from mice receiving a mix of WT and NKG2D KO CD8+ T cells is plotted. (F) WT or NKG2D KO CD8+ T cells from individually transplanted or mixed transplanted mice were pooled, FACS-sorted, and cultured with A20 cells with (right bars) or without (left bars) anti-NKG2D antibody treatment in vitro at a 1:20 effector-to-target ratio. Cytotoxicity of the A20 cells was measured 4 hours later. ns, **, and *** indicate that the groups compared are not significantly different or display statistical significance of P < .01 and P < .001, respectively by Student t test.
NKG2D KO CD8+ T cells display a survival disadvantage and reduced cytotoxicity in a cell-intrinsic manner. Irradiated Balb/c mice were transplanted with B6-derived TCD BM cells and FACS-sorted WT CD8+ T cells (CD45.1+), NKG2D KO CD8+ T cells (CD45.2+), or a mix of WT CD8+ T cells and NKG2D KO CD8+ T cells. (A) On day 7, spleen cells were harvested from the transplanted mice and stimulated with phorbol 12-myristate 13-acetate/ionomycin for 4 hours. Scatter plots of the fraction of IFN-γ and (B) TNF-α–producing WT and NKG2D KO CD8+ T cells in the individually transplanted setting and in the mixed setting are shown. (C) Transplanted mice were treated with BrdU on day 6 post-BMT and harvested on day 7. The fraction of BrdU-incorporated WT and NKG2D KO CD8+ T cells in the individually transplanted setting and in the mixed setting is shown. (D) Irradiated Balb/c mice were transplanted with B6-derived TCD BM cells and WT B6.SJL CD8+ T cells mixed with either WT B6 CD8+ T cells (CD45.2+) or NKG2D KO CD8+ T cells (CD45.2). The percentage of B6.SJL WT, WT B6, or NKG2D KO CD8+ T cells out of all donor-derived CD8+ T cells is represented as a scatter plot. (E) The percent of dead (live/dead stain+) CD8+ T cells of WT or NKG2D KO origin from mice receiving a mix of WT and NKG2D KO CD8+ T cells is plotted. (F) WT or NKG2D KO CD8+ T cells from individually transplanted or mixed transplanted mice were pooled, FACS-sorted, and cultured with A20 cells with (right bars) or without (left bars) anti-NKG2D antibody treatment in vitro at a 1:20 effector-to-target ratio. Cytotoxicity of the A20 cells was measured 4 hours later. ns, **, and *** indicate that the groups compared are not significantly different or display statistical significance of P < .01 and P < .001, respectively by Student t test.
To test whether the attenuated GVHD state induced by NKG2D blockade was related to the suppression of cytotoxic activity of CD8+ T cells, we tested the effects of anti-NKG2D antibody on GVHD induced by perforin KO CD8+ T cells. Perforin KO CD8+ T cells display defective cytotoxic responses and hence mediate reduced GVHD in some but not all GVHD models.36-38 Importantly, we found that in the B6→Balb/c model, GVHD-induced weight loss, clinical score, and mortality mediated by CD8+ T cells from perforin KO mice were partially reduced compared with CD8+ T cells from WT mice (supplemental Figure 3). If NKG2D-mediated protection was caused by reduced cytotoxic activity of CD8+ T cells in allogeneic BMT mice, we reasoned that perforin deficiency would be unable to further enhance the protective effects of NKG2D blockade. Indeed, the combination of perforin deficiency and NKG2D blockade was not additive in attenuating GVHD-induced weight loss or clinical score (supplemental Figure 3). These data suggest that NKG2D blockade, at least in part, mediates its protective effect by suppressing the cytotoxic activity of CD8+ T cells in allogeneic HSCT mice.
Prolonged NKG2D blockade attenuates both GVHD and GVL responses
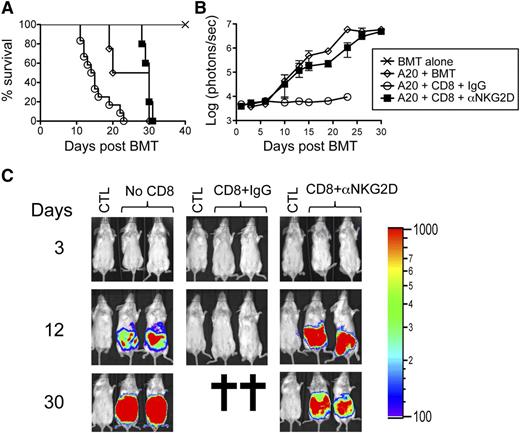
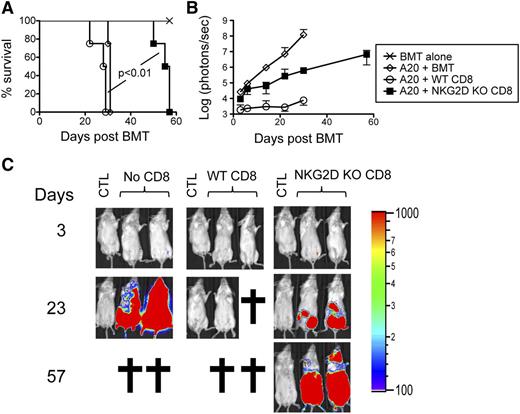
Given its role in cytotoxic responses, we predicted that NKG2D would be required for optimal GVT responses. Lethally-irradiated Balb/c mice were transplanted with BM and FACS-sorted CD8+ T cells from B6 donors together with luciferase-expressing A20 cells and treated with isotype control or anti-NKG2D antibody. A20 cells grew in mice transplanted with BM alone but not in isotype control antibody-treated mice transplanted with BM and CD8+ T cells, suggesting that donor-derived CD8+ T cells eradicated the A20 tumor cells. Although reduced GVHD-induced mortality was seen, anti–NKG2D-treated mice transplanted with BM and CD8+ T cells failed to clear the tumor cells (Figure 4A-C). To corroborate these findings, similar experiments were performed with NKG2D KO CD8+ T cells. Recipients of NKG2D KO CD8+ T cells displayed decreased GVHD-related mortality but ultimately failed to control leukemic growth (Figure 5A-C). Together, these data suggest that although the absence of NKG2D on CD8+ T cells is beneficial in attenuating GVHD, it is detrimental for preserving GVT effects against NKG2D ligand–expressing tumor cells such as A20.
Prolonged anti-NKG2D antibody treatment attenuates GVT responses. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted CD8+ T cells with or without luciferase-expressing A20 cells (A20luc). The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 30 days. (A) A survival graph using all mice from 2 independent experiments (n = 6 mice/group) is shown. (B) The tumor burden measured by bioluminescence is shown as compiled data from 1 representative of 2 independent experiments (n = 3 mice/group). (C) Representative bioluminescence images of tumor-bearing mice on days 3, 12, and 30 are shown. CTL represents non–tumor-bearing mice used for measuring background bioluminescence for comparison purposes.
Prolonged anti-NKG2D antibody treatment attenuates GVT responses. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted CD8+ T cells with or without luciferase-expressing A20 cells (A20luc). The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 30 days. (A) A survival graph using all mice from 2 independent experiments (n = 6 mice/group) is shown. (B) The tumor burden measured by bioluminescence is shown as compiled data from 1 representative of 2 independent experiments (n = 3 mice/group). (C) Representative bioluminescence images of tumor-bearing mice on days 3, 12, and 30 are shown. CTL represents non–tumor-bearing mice used for measuring background bioluminescence for comparison purposes.
NKG2D KO CD8+ T cells display defective GVT responses. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted WT or NKG2D KO CD8+ T cells with or without luciferase-expressing A20 cells. (A) A survival graph using all mice from 2 independent experiments (n = 8 mice/group) is shown. (B) The tumor burden measured by bioluminescence is shown as compiled data from 1 representative of 2 independent experiments (n = 4 mice/group). (C) Representative bioluminescence images of tumor-bearing mice on days 3, 23, and 57 are shown. CTL represents non–tumor-bearing mice used for measuring background bioluminescence for comparison purposes.
NKG2D KO CD8+ T cells display defective GVT responses. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted WT or NKG2D KO CD8+ T cells with or without luciferase-expressing A20 cells. (A) A survival graph using all mice from 2 independent experiments (n = 8 mice/group) is shown. (B) The tumor burden measured by bioluminescence is shown as compiled data from 1 representative of 2 independent experiments (n = 4 mice/group). (C) Representative bioluminescence images of tumor-bearing mice on days 3, 23, and 57 are shown. CTL represents non–tumor-bearing mice used for measuring background bioluminescence for comparison purposes.
The expression of NKG2D ligand RAE-1 is downregulated in mice treated with anti-NKG2D antibody at day 14 posttransplantation
NKG2D ligands such as RAE-1γ are not expressed on normal tissue but are upregulated in neoplasia, inflammation, and states involving tissue damage. To examine the expression of RAE-1γ during GVHD, frozen sections were obtained from the colons of allogeneic BM–transplanted mice treated with isotype control or anti-NKG2D antibody. Compared with mice transplanted with BM alone, RAE-1γ expression was increased in the colons of mice treated with either isotype control or anti-NKG2D antibody on day 7 posttransplantation (Figure 6A-D). At day 14 posttransplantation, mice treated with isotype control but not with anti-NKG2D antibody showed a further increase in RAE-1γ expression by cells in the colon (Figure 6E-F). The reduction in RAE-1γ expression by anti-NKG2D treatment correlated with attenuated tissue damage as indicated by histologic analysis (Figure 6G-H). Histologic analysis revealed numerous intraepithelial lymphocytes with mild tissue damage in both isotype control and anti–NKG2D-treated mice at day 7 posttransplantation (Figure 6G-H). However, at day 14, extensive tissue damage was noted in isotype control-treated mice, which included numerous intraepithelial lymphocytes associated with epithelial sloughing, crypt loss, villous atrophy, and epithelial apoptotic bodies. In contrast, anti-NKG2D antibody-treated mice continued to display a lymphocytic infiltrate with minimal tissue injury (Figure 6G-H). Thus, the expression of RAE-1γ, which correlated with tissue damage, was not sustained when mice were treated with anti-NKG2D antibody.
RAE-1 is persistently induced on colonic epithelial cells in GVHD-inflicted mice treated with isotype control but not anti-NKG2D antibody. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted CD8+ T cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody. Frozen sections of colonic tissue were obtained from the mice at the indicated time points. Tissues were stained with fluorochrome-labeled Pan Keratin antibody and DAPI, and with (A) fluorochrome-labeled secondary antibody only or with RAE-1/secondary antibody in (B) TCD BM–alone mice on day 7, (C) IgG-treated mice on day 7, (D) anti-NKG2D antibody-treated mice on day 7, (E) IgG-treated mice on day 14, and (F) anti-NKG2D antibody-treated mice on day 14. Panels from left to right represent images showing fluorescence with Pan Keratin alone, RAE-1 alone, and RAE-1/Pan Keratin/DAPI. Pan Keratin is shown as green, RAE-1 as red, and DAPI as blue. (G) Sections from paraffin-embedded colons obtained on days 7 and 14 post-BMT were stained with hematoxylin and eosin from mice treated with IgG or (H) anti-NKG2D antibody. One representative of 3 independent experiments is shown.
RAE-1 is persistently induced on colonic epithelial cells in GVHD-inflicted mice treated with isotype control but not anti-NKG2D antibody. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted CD8+ T cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody. Frozen sections of colonic tissue were obtained from the mice at the indicated time points. Tissues were stained with fluorochrome-labeled Pan Keratin antibody and DAPI, and with (A) fluorochrome-labeled secondary antibody only or with RAE-1/secondary antibody in (B) TCD BM–alone mice on day 7, (C) IgG-treated mice on day 7, (D) anti-NKG2D antibody-treated mice on day 7, (E) IgG-treated mice on day 14, and (F) anti-NKG2D antibody-treated mice on day 14. Panels from left to right represent images showing fluorescence with Pan Keratin alone, RAE-1 alone, and RAE-1/Pan Keratin/DAPI. Pan Keratin is shown as green, RAE-1 as red, and DAPI as blue. (G) Sections from paraffin-embedded colons obtained on days 7 and 14 post-BMT were stained with hematoxylin and eosin from mice treated with IgG or (H) anti-NKG2D antibody. One representative of 3 independent experiments is shown.
Transient NKG2D blockade attenuates GVHD while preserving GVL effects
Given that RAE-1γ expression was reduced with short-term NKG2D blockade compared with isotype control, we reasoned that prolonged anti-NKG2D antibody treatment would not be necessary for protection against GVHD. However, early cessation of anti-NKG2D antibody treatment might allow NKG2D-mediated GVT effects to resume, because RAE-1 is constitutively expressed on many transformed cells, including A20 cells. To test this possibility, lethally irradiated Balb/c mice were transplanted with BM and FACS-sorted CD8+ T cells from B6 donors together with A20 cells and treated transiently (6 days, every other day) with isotype control or anti-NKG2D antibody. Mice transiently treated with anti-NKG2D antibody showed significantly improved survival, weight loss, and clinical score compared with isotype control-treated mice (Figure 7A-C). Moreover, a large fraction of anti-NKG2D antibody-treated mice cleared the A20 cells (Figure 7D-F). This resulted in ∼75% tumor-free survival of the anti–NKG2D-treated mice. These data suggest that transient NKG2D blockade might be an effective strategy in attenuating GVHD while preserving GVT effects.
Transient NKG2D blockade attenuates GVHD while preserving GVT effects. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted CD8+ T cells with or without luciferase-expressing A20 cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 1 week. Mice were monitored for (A) survival, (B) weight changes, and (C) clinical score. (D) The tumor burden measured by bioluminescence is shown as compiled data from 1 representative of 2 independent experiments (n = 4-5 mice/group). (E) Representative bioluminescence images of tumor-bearing mice on days 3, 6, and 18 are shown. CTL represents non–tumor-bearing mice used for measuring background bioluminescence for comparison purposes. (F) The tumor incidence of mice in each group at day 40 post-BMT is shown. The number represents the actual fraction of mice with tumors. The survival graph was generated using all mice from 2 independent experiments (n = 9 mice/group). One representative of 2 independent experiments is shown for weight changes and clinical score (n = 4-5 mice/group). Statistical analysis for survival was performed using the log-rank test.
Transient NKG2D blockade attenuates GVHD while preserving GVT effects. Irradiated Balb/c mice were transplanted with B6-derived BM alone or with B6-derived BM cells and FACS-sorted CD8+ T cells with or without luciferase-expressing A20 cells. The mice were treated 3 times per week with either isotype control antibody (IgG) or anti-NKG2D antibody for 1 week. Mice were monitored for (A) survival, (B) weight changes, and (C) clinical score. (D) The tumor burden measured by bioluminescence is shown as compiled data from 1 representative of 2 independent experiments (n = 4-5 mice/group). (E) Representative bioluminescence images of tumor-bearing mice on days 3, 6, and 18 are shown. CTL represents non–tumor-bearing mice used for measuring background bioluminescence for comparison purposes. (F) The tumor incidence of mice in each group at day 40 post-BMT is shown. The number represents the actual fraction of mice with tumors. The survival graph was generated using all mice from 2 independent experiments (n = 9 mice/group). One representative of 2 independent experiments is shown for weight changes and clinical score (n = 4-5 mice/group). Statistical analysis for survival was performed using the log-rank test.
Discussion
In this manuscript, we have provided evidence supporting an important role for NKG2D expression by CD8+ T cells in mediating GVHD and GVT effects in a mouse model of allogeneic HSCT. Blocking NKG2D either through anti-NKG2D antibody or using NKG2D KO cells demonstrated that NKG2D expression promotes GVHD pathogenesis by enhancing survival and the cytotoxic function of CD8+ T cells. Moreover, NKG2D expression was also required for optimal GVT responses. However, differences in the timing of NKG2D ligand expression by normal tissue and tumor cells provided an opportunity whereby NKG2D blockade attenuated GVHD while preserving GVT effects. Thus, short-term NKG2D blockade potentially represents a novel therapeutic strategy for separating GVHD and GVT effects after allogeneic HSCT.
To test the impact of NKG2D deficiency in a CD8+ T cell–intrinsic manner, the function of NKG2D KO CD8+ T cells was examined in a setting where both WT and NKG2D KO CD8+ T cells were mixed before transplant. NKG2D KO CD8+ T cells removed at day 7 post-BMT showed equal capacity to incorporate BrdU and to produce proinflammatory cytokines compared with their WT counterparts in the same mouse, suggesting that proliferation and cytokine production were not affected by NKG2D deficiency. However, despite the equal BrdU incorporation rate, a significant skewing of the WT:NKG2D KO CD8+ T-cell ratio was observed. This suggested that NKG2D might provide a survival advantage for proliferating CD8+ T cells. Although how NKG2D promotes survival of CD8+ T-cells is unclear at this time, it is possible that NKG2D is required for optimal IL-15 signaling. A recent study showed that NKG2D enhances IL-15–mediated PI3K activation and promotes the survival of CD8+ T cells.39 Given that IL-15 signaling is critical in GVHD pathogenesis,40 NKG2D expression by CD8+ T cells might provide a survival boost by potentiating the effects of IL-15 in this setting.
In addition to offering a survival advantage, NKG2D also promoted the cytotoxic function of CD8+ T cells in a cell-intrinsic manner. As shown with the cytotoxicity assay against the A20 cell line, in vitro blockade by anti-NKG2D antibodies significantly diminished target cell lysis. Thus, although allogeneic MHC recognition by T cells alone can induce the cytotoxic function of donor CD8+ T cells, the coengagement of NKG2D ligands augments this effect. This is consistent with data demonstrating that the acute induction of NKG2D ligands can promote cytotoxic effects by T cells.41 Given that blockade of perforin-mediated killing activity significantly improved GVHD-induced mortality in our model, the decrease in cytotoxic function by NKG2D blockade likely contributed to the alleviation of GVHD severity. Although mortality could not be used as a parameter to test the additive nature of NKG2D blockade and perforin deficiency, this notion is consistent with our data demonstrating that perforin deficiency does not provide further benefit to anti-NKG2D antibody treatment in the alleviation of GVHD.
NKG2D may enhance CD8+ T cell–mediated cytotoxic function through its costimulatory activity. Although a short splice variant of NKG2D has been reported to be associated with DAP12 in IL-2–activated murine NK cells,42 in mouse and human CD8+ T cells, NKG2D signals though DAP10,43 which contains a YXXM motif similar to that of CD28. Thus, costimulatory signals provided by NKG2D can augment TCR signaling44-46 potentially leading to enhanced cytotoxicity of NKG2D ligand–expressing epithelial cells during GVHD. Alternatively, the cytotoxicity of NKG2D+ CD8+ T cells could occur in a TCR-independent manner. For example, IL-15 has been shown to upregulate NKG2D and DAP10 expression and to augment ERK phosphorylation, which allows NKG2D to mediate cytotoxicity in a TCR-independent manner in celiac disease.47 Moreover, culturing human CD8+ T cells with high doses of IL-2, IFN-γ, and CD3 enables these cells to kill in an NKG2D-dependent but TCR-independent manner.48,49
Given the important role of NKG2D in augmenting the cytotoxic function of CD8+ T cells during GVHD, we predicted that NKG2D blockade would be counterproductive for GVT effects. CD8+ T cells that express the NKG2D receptor have been shown to acquire potent cytolytic ability against leukemia cells.24,48,49 Consistent with these findings, genetic deficiency of NKG2D or prolonged NKG2D blockade resulted in an attenuated ability of CD8+ T cells to control A20 tumor growth. These data suggest that NKG2D not only plays a role in GVHD but also in GVT effects against NKG2D ligand–expressing tumors.
Among costimulatory molecules studied in GVHD, NKG2D is unique because of the expression pattern of its ligands. Ligands of other costimulatory molecules are mainly expressed by APCs and thus are involved in the initial priming and activation of T cells. Hence, separation of GVHD and GVT effects might be difficult when manipulating the expression of these costimulatory molecules. In contrast, NKG2D ligands are inducibly expressed by multiple cell types of both hematopoietic and nonhematopoietic origin. Although the expression of NKG2D ligands is often constitutive on tumor cells, NKG2D ligands are only transiently induced after tissue damage such as that caused by myeloablative conditioning regimens.50,51 In allogeneic HSCT, the expression of NKG2D ligands is further maintained by ongoing inflammation caused by GVHD. This was apparent in our tissue sections, whereby RAE-1 expression was detected in mice transplanted with BM and CD8+ T cells, but not with BM alone on day 7 posttransplantation, which is in line with published data.52 Although no dramatic difference was seen between mice treated with isotype control antibody and anti-NKG2D antibody at this time point, RAE-1 expression was markedly increased by day 14 posttransplantation in the isotype control-treated, but not the anti-NKG2D antibody-treated, group. This finding suggested that a window of opportunity might exist, where NKG2D is no longer important for GVHD because of the lack of ligand expression by normal tissue but is critical for enabling donor CD8+ T cells to eliminate NKG2D ligand–expressing tumors. Indeed, transient blockade with anti-NKG2D antibody maintained the attenuated GVHD state but allowed a large fraction of mice (∼75%) to completely eliminate their tumors. Thus, short-term treatment with anti-NKG2D blocking antibodies might be an effective strategy in separating GVHD and GVT effects in allogeneic HSCT. Our preliminary data from a small cohort of allogeneic HSCT patients with active GVHD show that their CD8+ T cells tend to express higher levels of NKG2D compared with HSCT patients without GVHD (supplemental Figure 3), providing clinical relevance to our study.
In summary, our data provided here demonstrate a novel role for NKG2D on CD8+ T cells in mediating GVHD and GVT effects, which is therapeutically exploitable. In addition to being directly applicable to the clinical setting, our data yield insight into the nature of the events underlying immune recognition of GVHD target cells by allogeneic CD8+ T cells. A greater understanding of the mechanisms that control NKG2D receptor and NKG2D ligand expression might lead to improved therapeutic interventions capable of augmenting NKG2D-mediated immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Avinash Bhandoola for careful reading of this manuscript and members of the Kambayashi, Behrens, Koretzky, Nichols, and Bassiri laboratory for advice; and Mariko Okumura and Wen Lu for technical assistance.
This work was supported by grants from the Translational Center of Excellence in Hematological Malignancies of the Abramson Cancer Center and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL107589, R01HL111501, T32HL007775) and National Cancer Institute (P30CA016520).
Authorship
Contribution: M.A.K., J.L.B., A.D.F., T.M.L., A.S., and L.P.R. performed experiments; R.R., R.H.V., and D.H.R. provided valuable reagents and intellectual input; and M.A.K. and T.K. designed research, analyzed data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taku Kambayashi, Department of Pathology and Laboratory Medicine, 288 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA, 19104; e-mail: taku.kambayashi@uphs.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal