Key Points
SLC29A1 encoding the equilibrative nucleoside transporter 1 (ENT1) specifies a novel blood group system that includes the Ata antigen.
Although At(a−) people of African ancestry have functional ENT1, 3 siblings of European ancestry were identified who do not express ENT1.
Abstract
The Augustine-negative alias At(a−) blood type, which seems to be restricted to people of African ancestry, was identified half a century ago but remains one of the last blood types with no known genetic basis. Here we report that a nonsynonymous single nucleotide polymorphism in SLC29A1 (rs45458701) is responsible for the At(a−) blood type. The resulting p.Glu391Lys variation in the last extracellular loop of the equilibrative nucleoside transporter 1 (ENT1; also called SLC29a1) is known not to alter its ability to transport nucleosides and nucleoside analog drugs. Furthermore, we identified 3 individuals of European ancestry who are homozygous for a null mutation in SLC29A1 (c.589+1G>C) and thus have the Augustine-null blood type. These individuals lacking ENT1 exhibit periarticular and ectopic mineralization, which confirms an important role for ENT1/SLC29A1 in human bone homeostasis as recently suggested by the skeletal phenotype of aging Slc29a1−/− mice. Our results establish Augustine as a new blood group system and place SLC29A1 as a new candidate gene for idiopathic disorders characterized with ectopic calcification/mineralization.
Introduction
The first paper referring to the Ata blood group antigen, which can be responsible for severe hemolytic transfusion reactions1 and mild hemolytic disease of the newborn,2 dates back to the 1960s. Applewhaite et al3 identified an antibody with a novel specificity in the serum of Mrs Augustine, when the red cells of her third child gave a positive direct antiglobulin reaction at birth. Her alloantibody, abbreviated as anti-Ata after her name, reacted with >6600 blood donors tested at that time, indicating that Ata was a high-frequency antigen. Since then, many other At(a−) individuals have been identified,4,5 usually after they (or one of their family members) produced an anti-Ata. All anti-Ata producers reported thus far were of African ancestry like Mrs Augustine.
Twenty years ago, the red cells of a French woman of European ancestry were found to be negative for the Ata antigen (G.D., unpublished data, 1994; supplemental Methods available on the Blood Web site). Stimulated by pregnancy, this woman had developed a potent alloantibody directed against an antigen that was present on all tested red cells but those of her siblings (Figure 1A). However, her alloantibody was not anti-Ata because it reacted with At(a−) red cells also. Although its specificity remained unsolved at that time, G.D. suggested that this woman might have the Augustine-null phenotype, in which her red cells lack all the antigens belonging to the yet-to-be discovered Augustine blood group system, including the Ata antigen. Encouraged by our recent success in elucidating the molecular bases of 3 other long-sought-after blood group antigens,6-8 we decided to reinvestigate the case of this French woman.
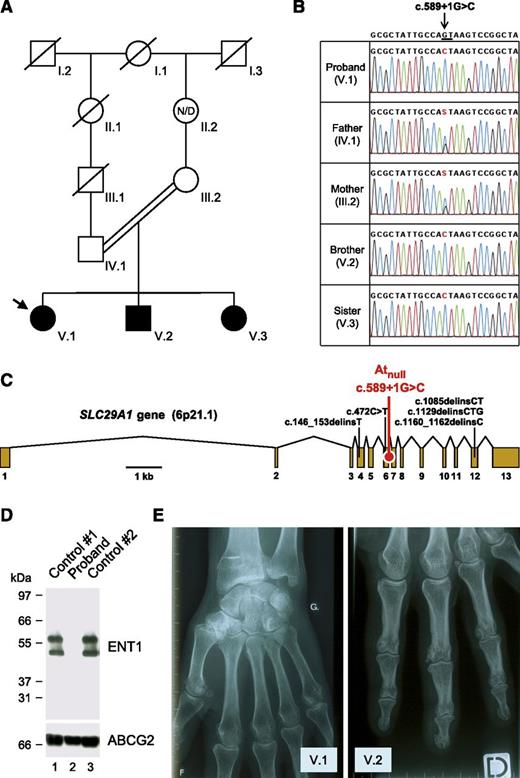
Identification of a SLC29A1-null mutation responsible for the Atnull blood type in a family of European ancestry. (A) Pedigree of the family of the proband (arrow); black-filled symbols represent individuals with the Atnull blood type; N/D, no data. (B) Detail of SLC29A1 sequencing in the proband, her parents, and siblings, showing the segregation of the c.589+1G>C mutation in her family; reference sequence (NC_000006) is indicated at the top; the highly conserved dinucleotide GT at the 5′ end of intron 6-7 is underlined; and of note, the c.589+1G>C mutation is associated with the major allele of rs45458701 and the minor allele of rs45573936 in the proband’s family. (C) Exon-intron diagram of human SLC29A1 gene (based on NM_004955) highlighting the location of the splice mutation c.589+1G>C found in the proband’s family (red) and 5 other loss-of-function mutations found in the heterozygous state in the Exome Aggregation Consortium data (black); exons are numbered 1 to 13. (D) The SLC29A1-encoded nucleoside transporter ENT1 is absent in the red cells of the proband. Red cells membranes were prepared from the proband (lane 2) and 2 controls (lanes 1 and 3), resolved by polyacrylamide gel electrophoresis under reducing conditions without heat denaturation, and immunoblotted with an antibody raised against a peptide from the extreme N terminus of the ENT1 transporter (upper); as a control, the western blot membrane was reprobed with an antibody to the ABCG2 transporter (lower). (E) X-ray views of the left hand of the proband (V.1; 49 y) and the right hand of her brother (V.2, 37 y), showing the presence of small calcifications around the metacarpo- and interphalangeal joints.
Identification of a SLC29A1-null mutation responsible for the Atnull blood type in a family of European ancestry. (A) Pedigree of the family of the proband (arrow); black-filled symbols represent individuals with the Atnull blood type; N/D, no data. (B) Detail of SLC29A1 sequencing in the proband, her parents, and siblings, showing the segregation of the c.589+1G>C mutation in her family; reference sequence (NC_000006) is indicated at the top; the highly conserved dinucleotide GT at the 5′ end of intron 6-7 is underlined; and of note, the c.589+1G>C mutation is associated with the major allele of rs45458701 and the minor allele of rs45573936 in the proband’s family. (C) Exon-intron diagram of human SLC29A1 gene (based on NM_004955) highlighting the location of the splice mutation c.589+1G>C found in the proband’s family (red) and 5 other loss-of-function mutations found in the heterozygous state in the Exome Aggregation Consortium data (black); exons are numbered 1 to 13. (D) The SLC29A1-encoded nucleoside transporter ENT1 is absent in the red cells of the proband. Red cells membranes were prepared from the proband (lane 2) and 2 controls (lanes 1 and 3), resolved by polyacrylamide gel electrophoresis under reducing conditions without heat denaturation, and immunoblotted with an antibody raised against a peptide from the extreme N terminus of the ENT1 transporter (upper); as a control, the western blot membrane was reprobed with an antibody to the ABCG2 transporter (lower). (E) X-ray views of the left hand of the proband (V.1; 49 y) and the right hand of her brother (V.2, 37 y), showing the presence of small calcifications around the metacarpo- and interphalangeal joints.
Study design
The 1994 study was performed within the ethics guidelines of the International Blood Group Reference Laboratory (Bristol, United Kingdom) and the study performed since 2012 was approved by the Ethical Committee of the French National Institute of Blood Transfusion (Paris, France).
Protein immunoprecipitation
Red cells of blood donors were incubated with the proband’s serum in low-ionic strength solution. Membranes of sensitized red cells were prepared by hypotonic lysis and then solubilized in a triple detergent buffer. Immune complexes were isolated using immobilized protein A/G, resolved by polyacrylamide gel electrophoresis, and studied by silver staining before mass spectrometry-based protein identification. Details can be found in supplemental Methods.
Protein immunoblotting
Red cell membrane extracts were probed with a rabbit affinity-purified anti-ENT1 (Applied Biological Materials, Richmond, BC, Canada) and the mouse monoclonal anti-ABCG2 BXP21 (Santa Cruz Biotechnology, Dallas, TX). Details can be found in supplemental Methods.
Genomic DNA sequencing
The primers used to amplify and sequence SLC29A1 are described in supplemental Table 1. Details can be found in supplemental Methods.
Results and discussion
To identify the potential protein targeted by the alloantibody of the aforementioned French proband, the immune complex that is formed with the red cells of random blood donors was purified and analyzed by mass spectrometry as previously described.6 Three tryptic peptides encoded by SLC29A1 were unambiguously identified (LEGPGEQETK, WLPSLVLAR, and YYQQLK). To understand why the proband developed an alloantibody to the product of SLC29A1, we first analyzed this gene in the proband and controls. Sequencing of SLC29A1 revealed that the proband was homozygous for a G-to-C transversion at the +1 position of intron 6-7 (c.589+1G>C in NM_004955; Figure 1B-C). This splice mutation was absent from all sequenced controls and public databases (National Center for Biotechnology Information Single Nucleotide Polymorphism Database, National Heart, Lung, and Blood Institute Grand Opportunity Exome Sequencing Project, and 1000 Genomes Project). In contrast, c.589+1G>C was found in the heterozygous state in both parents of the proband and in the homozygous state in her siblings (Figure 1B). A genealogy search revealed that the parents were first cousins once removed (Figure 1A), which provides an explanation for the sharing of this rare mutation.
SLC29A1 encodes the equilibrative nucleoside transporter 1 (ENT1; also called SLC29A1), which enables facilitated diffusion of hydrophilic nucleosides across the plasma membrane.9 Of note, SLC29A1 is highly expressed in early erythroid cells (supplemental Figure 1), and ENT1 is particularly abundant in the red cell membrane.10 From a therapeutic point of view, ENT1 plays an important role in the cellular uptake of nucleoside analog drugs, such as ribavirin, which is widely used in the treatment of hepatitis C.11,12 To determine the effect of the c.589+1G>C mutation, we set up the analysis of ENT1 expression by immunoblotting red cell membrane extracts (supplemental Figure 2). As shown in Figure 1D, we detected no ENT1 protein in the proband and hence concluded that c.589+1G>C defines a null allele of SLC29A1, explaining how the proband could have developed an alloantibody to ENT1.
As homozygotes for a null allele of SLC29A1, the proband and her siblings are the first reported individuals lacking the complete expression of ENT1. They showed no gross developmental abnormities and had a normal lifestyle, at least until age 45 to 50 years, when they were contacted for this study. However, strikingly, all experienced frequent attacks of pseudogout since they were 18 to 20 years old (mainly in the hands but also in the feet, elbow, and knee), which was not the case for their parents and children. Hand x-rays indicated multiple small calcifications around the joints (Figure 1E; supplemental Figure 3). Ectopic calcification/mineralization was also observed in the hips, pubic symphysis, and lumbar discs of the proband at age 47 (supplemental Figures 4 and 5). Serum levels of urea, uric acid, creatinine, and electrolytes were in the normal range (supplemental Table 2). Although it is virtually impossible to prove that ENT1 deficiency is solely responsible for the ectopic mineralization observed in the proband and her siblings, recent data with Slc29a1−/− mice fully support this hypothesis.13,14 The precise role of ENT1 in bone homeostasis remains to be established in mice and humans, but it is most likely linked to its ability to transport adenosine, which has emerged as a key regulator of bone metabolism.15 In fact, other genes of the adenosine pathway have been found to be responsible for calcification disorders.16,17
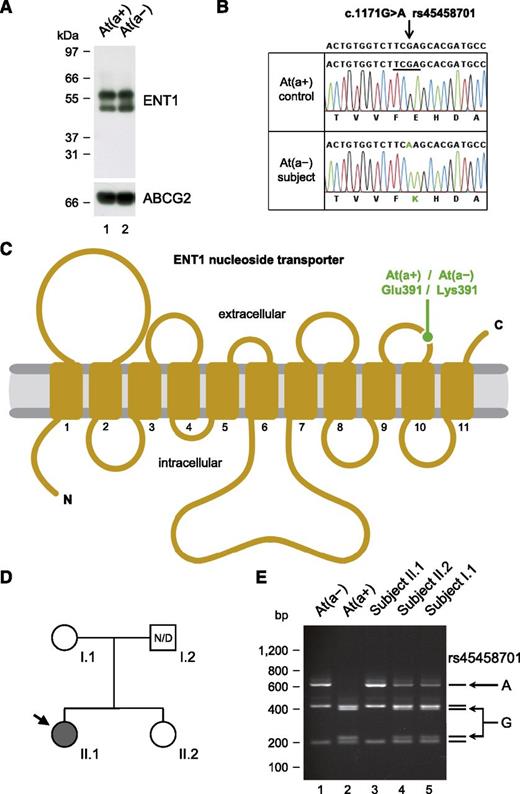
As mentioned above, it was suggested that the proband did not express the protein carrying the Ata antigen. We therefore analyzed ENT1/SLC29A1 in At(a−) people. In line with the reactivity of the proband’s antibody with At(a−) red cells, ENT1 was found to be equally present in At(a+) and At(a−) red cells (Figure 2A). Sequencing of SLC29A1 in 5 At(a−) individuals revealed that all were homozygous for a G-to-A transition in exon 12 (c.1171G>A in NM_004955; Figure 2B) encoding the amino acid change Glu391Lys. Glutamine 391 is located in the fifth extracellular loop of the ENT1 transporter18 (Figure 2C), and the nonconservative substitution p.Glu391Lys likely disrupts the epitope of anti-Ata. Nevertheless, p.Glu391Lys was shown not to alter the activity of the ENT1 transporter19 so that those with At(a−), in contrast to the 3 siblings reported above, have normal ENT1 function.
Homozygosity for the p.Glu391Lys variation in the ENT1 nucleoside transporter is responsible for the At(a−) blood type in people of African ancestry. (A) Western blot analysis of ENT1 (performed as in Figure 1D) in an At(a−) subject and an At(a+) control, showing the presence of ENT1 in individuals with the At(a−) blood type. (B) Detail of SLC29A1 sequencing in an At(a−) subject and an At(a+) control, showing the homozygosity for the A allele of rs45458701 in individuals with the At(a−) blood type. Reference sequence (NC_000006) is indicated at the top, and the Taqα1 restriction site that is absent in the A allele but present in the G allele is underlined. (C) Topology diagram of the human ENT1 nucleoside transporter (adapted from Sundaram et al18 ) highlighting the location of the p.Glu391Lys variation encoded by the A allele of rs45458701; transmembrane domains are numbered 1 to 11. (D) Pedigree of the family of an At(a−) patient (arrow); gray- and white-filled symbols represent individuals with the At(a−) and At(a+) blood types, respectively; N/D, no data. (E) Polymerase chain reaction-restriction fragment length polymorphism analysis of rs45458701 in At(a−) and At(a+) controls (lanes 1 and 2) and in the family depicted in D (lanes 3-5).
Homozygosity for the p.Glu391Lys variation in the ENT1 nucleoside transporter is responsible for the At(a−) blood type in people of African ancestry. (A) Western blot analysis of ENT1 (performed as in Figure 1D) in an At(a−) subject and an At(a+) control, showing the presence of ENT1 in individuals with the At(a−) blood type. (B) Detail of SLC29A1 sequencing in an At(a−) subject and an At(a+) control, showing the homozygosity for the A allele of rs45458701 in individuals with the At(a−) blood type. Reference sequence (NC_000006) is indicated at the top, and the Taqα1 restriction site that is absent in the A allele but present in the G allele is underlined. (C) Topology diagram of the human ENT1 nucleoside transporter (adapted from Sundaram et al18 ) highlighting the location of the p.Glu391Lys variation encoded by the A allele of rs45458701; transmembrane domains are numbered 1 to 11. (D) Pedigree of the family of an At(a−) patient (arrow); gray- and white-filled symbols represent individuals with the At(a−) and At(a+) blood types, respectively; N/D, no data. (E) Polymerase chain reaction-restriction fragment length polymorphism analysis of rs45458701 in At(a−) and At(a+) controls (lanes 1 and 2) and in the family depicted in D (lanes 3-5).
Interestingly, c.1171G>A in SLC29A1 corresponds to the minor allele of rs45458701 that has only been detected in people of African ancestry (supplemental Table 3) like the At(a−) blood type. In the United States, the NHLBI GO Exome Sequencing Project found 49 carriers of the A allele of rs45458701 by sequencing 2203 African Americans and none of 4300 European Americans. As a Taqα1 restriction site is absent in the A allele of rs45458701 (Figure 2B), we developed a polymerase chain reaction-restriction fragment length polymorphism assay to detect it (supplemental Figure 6). We thus analyzed 8 reference anti-Ata sera, including Mrs Augustine’s serum, as well as the family of a British patient who was recently identified as At(a−) (Figure 2D-E). In all these cases (of African ancestry), we also found the At(a−) blood type associated with homozygosity for the A allele of rs45458701.
In conclusion, we provide evidence that allelic variations in SLC29A1 underlie a new blood group system. Typing rs45458701 (c.1171G>A in SLC29A1) could be implemented in blood group genotyping platforms to identify people with the At(a−) blood type. Outside transfusion medicine, we recommend looking for SCL29A1 loss-of-function mutations (or alternatively for the Augustine-null blood type) in patients with periarticular calcifications of unknown cause.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the proband and her siblings for generously contributing time and material to this study. The authors also thank J. Poole, P.-Y. Le Pennec, M. Nagler, A. Brownell, D. Gien, P. Martin, G. Nicolas, M. Bonny, and M. Le Gall for their help and assistance.
Mass spectrometry analysis was funded by the Vermont Genetics Network (National Institutes of Health National Institute of General Medical Sciences/IDeA Networks of Biomedical Research Excellence grant 8P20GM103449) (to B.A.B.).
Authorship
Contribution: G.D., B.A.B., V.H., C.S., S.G., and L.A. performed experiments and analyzed data; G.D., B.A.B., L.M., H.H., E.L., and T.P. contributed essential material; G.D., B.A.B, J.-P.C., and T.P. discussed the results and commented on the manuscript; L.A. designed and supervised the study, made the figures, and wrote the manuscript; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lionel Arnaud, National Institute of Blood Transfusion, 6 rue Alexandre Cabanel, 75015 Paris, France; e-mail: lionel.arnaud.contact@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal