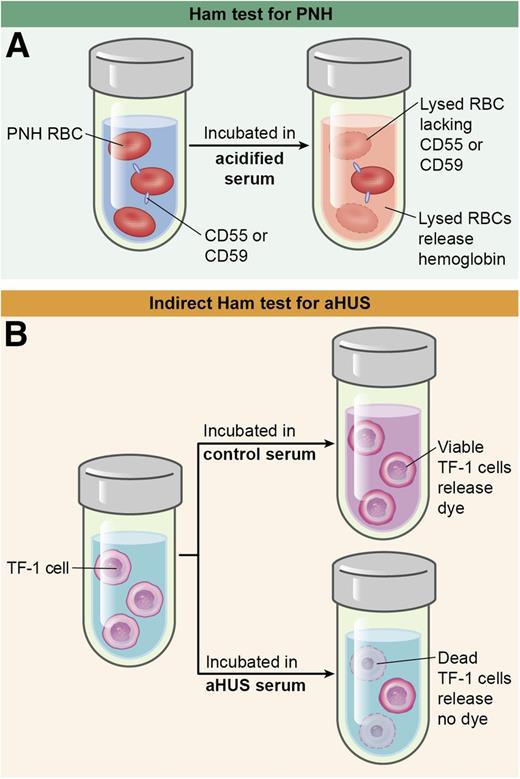
In this issue of Blood, Gavriilaki and colleagues1 describe an assay that could convert atypical hemolytic uremic syndrome (aHUS) from a diagnosis of exclusion into a direct pathophysiologic diagnosis.
(A) The Ham test incubates PNH red cells with acidified serum, which lyses cells that lack glycosylphosphatidylinositol (GPI)-linked complement inhibitors such as CD55 and CD59. (B) The indirect Ham test incubates control or aHUS serum with TF-1 cells that lack these complement regulators. Complement-induced cell death prevents the conversion of WST-1 into formazan, a red dye. RBC, red blood cell. Professional illustration by Ken Probst, XavierStudio.
(A) The Ham test incubates PNH red cells with acidified serum, which lyses cells that lack glycosylphosphatidylinositol (GPI)-linked complement inhibitors such as CD55 and CD59. (B) The indirect Ham test incubates control or aHUS serum with TF-1 cells that lack these complement regulators. Complement-induced cell death prevents the conversion of WST-1 into formazan, a red dye. RBC, red blood cell. Professional illustration by Ken Probst, XavierStudio.
Patients with aHUS usually have microangiopathic hemolytic anemia, thrombocytopenia, and renal failure. However, these features occur in many diseases, including thrombotic thrombocytopenic purpura (TTP), and the more common form of Shiga toxin–expressing Escherichia coli hemolytic uremic syndrome. Making the correct diagnosis is critical because these disorders require different treatment. For example, treatment of aHUS with plasma exchange is associated with up to 8% mortality during the first episode and progression to end-stage renal failure in most survivors. However, treatment with the complement inhibitor eculizumab can halt the thrombotic microangiopathy, prevent or reverse renal failure, and forestall damage to the brain, heart, and other organs,2 which makes sense because aHUS is caused by defects in the regulation of the alternative complement pathway.
Unfortunately, no method has been described that reliably detects the hyperactive alternative complement pathway in aHUS. Relatively few patients have low serum C3 levels, and complement consumption is not specific for aHUS. Complement mutations in aHUS are heterozygous, and the corresponding protein concentrations in blood are not consistently abnormal. At least for now, genetic testing is slow and uninformative in up to 50% of cases. Instead, aHUS is diagnosed by ruling out ADAMTS13 deficiency, Shiga toxin–expressing E coli, and other confounding causes of thrombotic microangiopathy. The patients remaining are said to have aHUS, but for most of them, we have no way to rapidly verify the underlying disease mechanism.
To address this problem, complement activation products have been measured, expecting that impaired complement regulation should increase their levels in blood. On average, levels of anaphylatoxin C5a and the terminal complement complex C5b-9 are higher in aHUS compared with acquired TTP but cannot accurately classify many patients because the ranges overlap.3 These and other biomarkers of complement activation may4 or may not5 be increased for aHUS patients in remission.
A direct assessment of complement dysregulation might be more successful. For example, confocal microscopy showed that more C5b-9 was deposited onto HMEC-1 microvascular endothelial cells by serum from patients with aHUS compared with healthy controls. Interestingly, abnormalities were present during active disease, during remission, and in unaffected carriers of complement mutations.4 This approach appears promising, but plasma from patients with TTP also induces complement deposition on HMEC-1 cells, raising questions about specificity.6
To overcome this roadblock, Gavriilaki et al1 took advantage of their experience with paroxysmal nocturnal hemoglobinuria (PNH) to draw a useful analogy between PNH and aHUS (see figure). Because PNH cells are sensitized to complement damage, PNH-like cells might be sensitive to complement dysregulation in aHUS. This elegant lateral move paid off.
First of all, Gavriilaki et al confirmed that aHUS plasma promotes C5b-9 deposition on Ea.hy926 cells, another human endothelial cell line, and confirmed that the method does not clearly distinguish between aHUS and TTP. However, treating Ea.hy926 cells with a phospholipase to remove cell-surface complement regulatory proteins sensitized the resultant PNH-like cells to the deposition of C5b-9 and improved the discrimination between aHUS and other conditions by confocal immunofluorescence microscopy. Flow cytometry for C5b-9 was also effective and less subjective, but it was challenging to automate. This obstacle was addressed by replacing immunofluorescence detection with a cell-viability end point. Cells were treated with a compound that living cells convert into formazan, a highly colored product. Exposure to aHUS plasma killed the cells and reduced the production of formazan. With this modification, the assay could be performed with a microplate reader.
However, the removal of complement regulators with phospholipase is cumbersome, incomplete, and transient because cells can resynthesize them. This last problem was overcome by using human myeloid TF-1 cells that lack PIGA, which is required to make the phosphatidylinositol anchors of complement inhibitors CD55 and CD59 on cells. With this optimized method, patients with aHUS were easily distinguished from various control groups during active disease and also during remission. Furthermore, with appropriate dilution, defects in complement-dependent cell killing were detected in patients with aHUS even while they were under treatment with eculizumab. This result is unexpected because the therapeutic concentration of eculizumab is high enough to bind and inhibit all plasma complement component C5.
These interesting results suggest that this assay or a descendent of it could be useful to diagnose aHUS and perhaps to identify those at risk for developing aHUS. For instance, TF-1 cells almost certainly express membrane cofactor protein (MCP), a potent complement inhibitor that is not GPI anchored, and elimination or inhibition of MCP might make the cells even more sensitive to aHUS serum. Of course, serum assays cannot detect alternative complement pathway defects caused by mutations in membrane proteins like MCP or thrombomodulin. More importantly, Gavriilaki et al1 studied few subjects, and their results must be validated for many more patients with aHUS, TTP, other relevant diagnoses, and healthy controls. Certain autoimmune disorders characterized by complement-mediated tissue injury may be particularly difficult to distinguish from aHUS.
Nevertheless, if this latter hurdle is overcome, many questions about the pathophysiology of aHUS could be addressed. For example, relatively few persons with complement mutations ever experience aHUS; penetrance in families is estimated to be only 50% by age 45.7 Could a functional assay for complement regulation distinguish between risk groups? Complement factor H (CFH) levels vary from ∼100 µg/mL to 600 µg/mL, and much of this variation is heritable. Are some healthy persons with low CFH at risk for aHUS, and would they exhibit abnormal complement regulation? Many patients with aHUS do not have mutations by current detection methods. Using this assay, might functional complementation tests in vitro localize their defects to specific plasma proteins? More to the point, a robust assay for alternative complement pathway dysregulation could convert aHUS from a diagnosis of exclusion, at least in the acute care setting, into a positive diagnosis based on pathophysiology.
Conflict-of-interest disclosure: The author declares no competing financial interests.