In this issue of Blood, Wang et al report that ex vivo–derived human megakaryocytes infused into mice are trapped in the pulmonary vasculature and release functional platelets into the circulation. Because of the difficulty in scalable generation of ex vivo–derived functional platelets, this strategy may be a substitute for platelet transfusion therapy.1
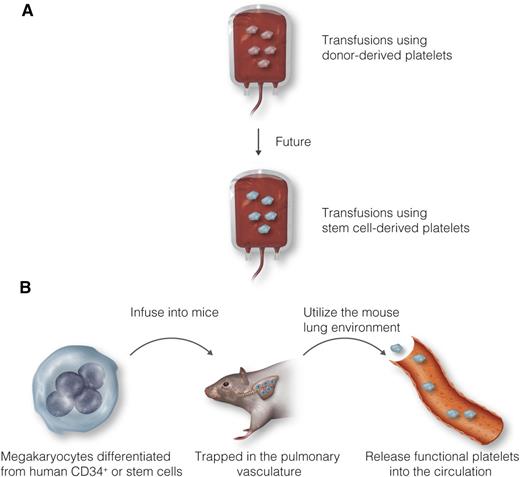
Future of platelet transfusion therapy. (A) Because of numerous concerns about donor-derived platelets, stem cell–derived platelets may be a potential source for transfusion. (B) Instead of generating platelets from human embryonic or induced pluripotent stem cells ex vivo, Wang et al demonstrated that ex vivo–derived human megakaryocytes infused into mice can release functional platelets into the circulation. Professional illustration by Luk Cox, Somersault18:24.
Future of platelet transfusion therapy. (A) Because of numerous concerns about donor-derived platelets, stem cell–derived platelets may be a potential source for transfusion. (B) Instead of generating platelets from human embryonic or induced pluripotent stem cells ex vivo, Wang et al demonstrated that ex vivo–derived human megakaryocytes infused into mice can release functional platelets into the circulation. Professional illustration by Luk Cox, Somersault18:24.
Anucleated blood platelets play a crucial role in hemostasis and thrombosis at the site of vascular injury. Since they have a short life span (7-10 days), platelets are constantly generated from megakaryocytes to maintain normal levels in the blood (150-400 × 103 platelets per microliter). One mature megakaryocyte in the bone marrow produces 2000 to 10 000 platelets in response to thrombopoietin and other cytokines.2 Previous studies also showed that a recombinant protein containing the receptor-binding N-terminal domain of thrombopoietin infused into mice stimulates platelet production from megakaryocytes in the lungs, suggesting that platelet production occurs outside the bone marrow.3
Despite efficient platelet production in the bone marrow and lungs, patients who undergo radiation treatment, chemotherapy, or organ transplant surgery often suffer from bleeding as a result of life-threatening thrombocytopenia (<10-20 × 103 platelets per microliter). Although transfusions using donor-derived platelets remain the most effective way to treat thrombocytopenic patients, numerous concerns have been raised, including the limited shelf life and storage-related deterioration of donor-derived platelets, development of alloantibodies in recipients, and transmission of infectious disease. Thus, many efforts have been put forward to generate functional platelets using human embryonic stem cells and induced pluripotent stem cells (iPSCs) as a potential source for platelet transfusion (see figure panel A). However, the low yield and poor functionality of stem cell (SC)–derived platelets remain a considerable challenge in this strategy.
A previous study showed that infused ex vivo–derived mouse megakaryocytes are trapped in the pulmonary vasculature, where they shed platelets with a normal size that have appropriate surface markers and a 1-day half-life.4 In this study, Wang and colleagues further tested human megakaryocytes in mice (see figure panel B).1 After infusion of ex vivo–derived human megakaryocytes into macrophage-depleted NOD/SCID/interferon-γ–deficient mice, two different pools of human platelets were generated independent of the starting source of megakaryocytes (CD34+ cells derived from human bone marrow, hematopoietic mononuclear cells derived from fetal liver, and iPSCs): in vivo–generated platelets shed from megakaryocytes trapped in the pulmonary vasculature and ex vivo–derived platelets (or platelet-like particles) released from megakaryocytes during culture and/or processing. Intriguingly, in vivo–generated platelets showed characteristics similar to those of human blood platelets in terms of their size, surface markers (CD42a and CD42b), and in vitro and in vivo functionality. However, the authors did not test whether in vivo–generated platelets were able to restore hemostatic function in thrombocytopenic mice or whether CD42a and CD42b make a functional complex. They found that compared with donor platelets, ex vivo–derived platelets exhibited numerous functional defects, including a wide range of sizes and a shorter half-life. In addition, CD42b was shed from ex vivo–derived platelets, and annexin V was expressed on the platelet surface, probably as a result of the enhanced basal activation state. Consistent with previous studies describing functional deficiencies of platelets generated from SC-derived megakaryocytes,5-7 these results suggest that ex vivo–generated platelets may not act like blood platelets.
Although these findings provide evidence that human megakaryocytes infused into mice release functional platelets into the circulation, there might be several hurdles to clear before this strategy can be used in a clinical setting. The critical issue is whether cultured megakaryocytes can be directly infused into humans because large-size megakaryocytes and their aggregates may occlude small vessels after transfusion. Although the concern about SC-derived teratoma formation may be eliminated by transfusing ex vivo–derived megakaryocytes after irradiation, it remains to be determined whether irradiation of the megakaryocytes affects platelet production and function in vivo. Thus, future studies are required to assess the safety of infused ex vivo–derived megakaryocytes into animals. Second, because of the difficulty in obtaining bone marrow–derived megakaryocytes, this strategy still requires megakaryocytes obtained from the differentiation of SCs or megakaryocyte progenitor cells derived from the bone marrow or fetal liver. Because in vitro or ex vivo culture systems do not necessarily mimic the bone marrow environment, ex vivo–derived megakaryocytes are unlikely to act like bone marrow-derived megakaryocytes. Indeed, this speculation is also supported by the results of Wang et al1 showing a low ploidy of ex vivo–derived megakaryocytes and a limited number of in vivo–generated platelets in mice. Third, although the Wang et al study showed the incorporation of in vivo–generated human platelets into the growing mouse platelet thrombus, the number of platelets produced from infused megakaryocytes in a different stage of maturation still needs to be investigated, along with whether in vivo–generated platelets have normal hemostatic function during bleeding.
Because the need for platelet transfusions has been increasing during the past decade, scalable generation of ex vivo–derived platelets is a promising strategy. Recent reports indicated that a large number of platelets can be generated ex vivo by using a bioreactor or a 3-dimensional tissue model that mimics the bone marrow niche.8-10 The long-standing question is whether we can find the optimal conditions for generating a sufficient number of functional platelets that have characteristics similar to those of blood platelets. Because in vivo–generated platelets, unlike ex vivo–derived platelets, are similar to donor platelets in several aspects, this strategy may be a breakthrough in platelet transfusion therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal