In this issue of Blood, Peterson et al demonstrate that inhibition of both Usp9x and Usp24 results in efficient degradation of Mcl-1, induction of apoptosis, and inhibition of tumor growth in B-cell malignancies.1
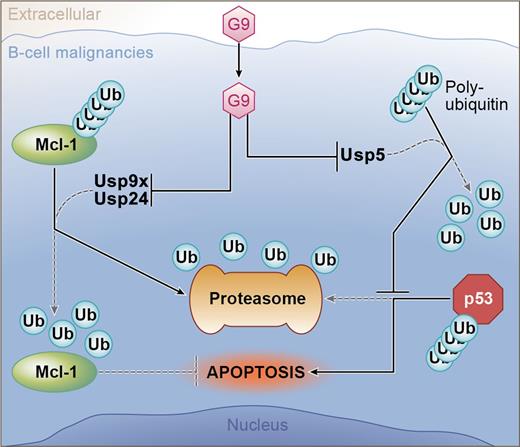
The novel agent G9 can induce apoptosis in B-cell tumors through inhibition of 3 DUBs (Usp9x, Usp24, and Usp5). Usp9x and Usp24 are DUBs for Mcl-1; therefore, G9 treatment allows ubiquitinated Mcl-1 to be degraded by the proteasome. Usp5 targets unanchored polyubiquitin.10 Accumulation of this complex competes for proteasome binding with ubiquitinated p53; therefore, G9 treatment results in the accumulation of proapoptotic p53. Professional illustration by Ken Probst, XavierStudio.
The novel agent G9 can induce apoptosis in B-cell tumors through inhibition of 3 DUBs (Usp9x, Usp24, and Usp5). Usp9x and Usp24 are DUBs for Mcl-1; therefore, G9 treatment allows ubiquitinated Mcl-1 to be degraded by the proteasome. Usp5 targets unanchored polyubiquitin.10 Accumulation of this complex competes for proteasome binding with ubiquitinated p53; therefore, G9 treatment results in the accumulation of proapoptotic p53. Professional illustration by Ken Probst, XavierStudio.
Inhibition of apoptosis is required for tumorigenesis and is a potential barrier to therapeutic activity in cancer. Therefore, directly targeting antiapoptotic proteins is a promising approach for the treatment of B-cell malignancies. Indeed, early reports have suggested preclinical and clinical activity of the Bcl-2/Bcl-xL/Bcl-w inhibitor navitoclax and the Bcl-2–selective inhibitor venetoclax in diseases like B-cell chronic lymphocytic leukemia (B-CLL).2 However, Mcl-1 is not targeted by these agents; therefore, expression of this Bcl-2 family member results in drug resistance. MCL1 is found on a region of chromosome 1 that is amplified in 10% of all cancers, making it likely to be a significant contributor to drug resistance beyond just Bcl-2 inhibitors.3 Additionally, in multiple myeloma, navitoclax and venetoclax are not likely to be effective in most patients, as the survival of most myelomas is primarily dependent on Mcl-1.4 Therefore, strategies to target Mcl-1 could be effective to overcome drug resistance in Bcl-2–dependent diseases like B-CLL and to treat Mcl-1–dependent diseases like myeloma. Currently there are few publications on Mcl-1–selective agents, and thus far they either are early in preclinical studies or have not been particularly potent.5
Although targeting Mcl-1 function holds great promise, targeting its expression could be equally effective and there have been several different approaches tested that are based on the finding that Mcl-1 protein has a short half-life. Inhibition of transcription with CDK9 inhibitors like flavopiridol appears to work in part because Mcl-1 protein is rapidly depleted when transcription is inhibited.6 Filanesib activity has also been attributed to Mcl-1 degradation in cells that are arrested in M-phase.7 However, these approaches are likely to have numerous effects on cells, making it hard to pinpoint the activity to Mcl-1 degradation. The demonstration that the deubiquitinase (DUB) Usp9x regulates Mcl-1 protein stability opened the possibility for more selective targeting.8
Using the DUB inhibitor WP1130, Peterson et al demonstrate inhibition of Usp9x activity, a decrease in Mcl-1 protein, and cleavage of poly(ADP-ribose) polymerase, indicating induction of apoptosis in myeloma, lymphoma, and B-CLL cell lines and/or patient samples. Importantly, the decrease in Mcl-1 is at least partially reversed by addition of proteasome inhibitors, consistent with degradation of Mcl-1 protein. Because WP1130 is known to inhibit multiple DUBs, the authors attempt to confirm their findings by silencing Usp9x. Surprisingly, they find that although silencing Usp9x can induce cell death, it does not appear to affect Mcl-1 protein levels or DUB activity and is significantly weaker than silencing Mcl-1. Together, these findings suggest that Usp9x may not be the only Mcl-1 DUB that is inhibited by WP1130. Usp24 is a structurally similar DUB that has been shown to have overlapping activities with Usp9x. Interestingly, Peterson et al find that it is upregulated in 2 cell lines when Usp9x is silenced and is expressed in patient samples. Unlike Usp9x, silencing of Usp24 resulted in similar death to Mcl-1 silencing and although a decrease in Mcl-1 was observed it was not as great as that seen following Mcl-1 silencing. Several conclusions can be drawn from these findings. First, Usp24 could be the primary DUB for Mcl-1 in myeloma despite higher Usp9x expression levels. Second, the regulation of these 2 DUBs may be distinct, as silencing of Usp24 does not result in upregulation of Usp9x allowing for loss of Mcl-1. This was the case in 1 cell line, and investigation in others is needed. Third, because Usp24 silencing could kill cells as well as Mcl-1 silencing but did not regulate Mcl-1 as efficiently, inhibition of Usp24 likely affects other pathways that influence myeloma cell survival. Finally, targeting multiple DUBs may overcome compensatory changes in expression, thus explaining why WP1130 is more effective than silencing.
Because both Usp9x and Usp24 can play a role in the survival of malignant B cells, one would conclude that a drug like WP1130 would be a promising candidate to move forward toward the clinic. Unfortunately, this compound has solubility issues that limit its effectiveness in vivo. Previously, a structure-activity relationship was performed to identify related compounds that would be more active against Usp9x and Usp24 as well as have better drug-like properties.9 In the current report, the activity of one these compounds, G9, is characterized. G9 is a more potent inhibitor of Usp9x and has better solubility that WP1130. It induces apoptosis in myeloma and lymphoma cell lines in vitro and prevents xenograft tumor growth with little overt toxicity. These are promising findings; however, its ability to reduce tumor burden was not directly tested in these studies. One of the more interesting aspects of G9 activity is that, in addition to inhibiting Usp9x and Usp24, it inhibits Usp5. Inhibition of Usp5 results in an upregulation of p53 and because p53 is not commonly mutated in B-cell malignancies, this may also contribute to the therapeutic potential of this or related agents. Thus, indirectly targeting Mcl-1 via inhibition of DUBs may prove to be more efficacious than direct Mcl-1 inhibition (see figure10 ). However, targeting Mcl-1 degradation in this manner may come at a cost. The data demonstrate that proteasome inhibition reverses the effect of DUB inhibition on Mcl-1 expression. If Mcl-1 that is decorated with ubiquitin remains functional, then combinations with proteasome inhibitors, which are backbone drugs in myeloma treatment, may be antagonistic. This would not be the case with a direct Mcl-1 inhibitor. Additional studies using G9 and future analogs with proteasome inhibitors are needed to see how this promising approach will complement current active agents.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal