Key Points
Increased IL-15 expression in leukemic lymphoblasts is associated with activation of NK cells.
The CNS may be an immunologic sanctuary protecting lymphoblasts from NK-cell activity.
Abstract
Central nervous system acute lymphoblastic leukemia (CNS-ALL) is a major clinical problem. Prophylactic therapy is neurotoxic, and a third of the relapses involve the CNS. Increased expression of interleukin 15 (IL-15) in leukemic blasts is associated with increased risk for CNS-ALL. Using in vivo models for CNS leukemia caused by mouse T-ALL and human xenografts of ALL cells, we demonstrate that expression of IL-15 in leukemic cells is associated with the activation of natural killer (NK) cells. This activation limits the outgrowth of leukemic cells in the periphery, but less in the CNS because NK cells are excluded from the CNS. Depletion of NK cells in NOD/SCID mice enabled combined systemic and CNS leukemia of human pre-B-ALL. The killing of human leukemia lymphoblasts by NK cells depended on the expression of the NKG2D receptor. Analysis of bone marrow (BM) diagnostic samples derived from children with subsequent CNS-ALL revealed a significantly high expression of the NKG2D and NKp44 receptors. We suggest that the CNS may be an immunologic sanctuary protected from NK-cell activity. CNS prophylactic therapy may thus be needed with emerging NK cell-based therapies against hematopoietic malignancies.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy.1 Involvement of the central nervous system (CNS) by ALL is a major clinical problem. CNS prophylaxis consisting of intrathecal chemotherapy and/or cranial irradiation and high-dose systemic chemotherapy significantly reduced CNS recurrence.2-5 However, these therapies are associated with substantial acute and long-term neurotoxicity.6-9 Moreover, CNS relapse remains a major therapeutic obstacle in ALL, accounting for 30% of the relapses.10-15
Despite its clinical importance, little is known about the mechanisms that cause CNS leukemia.16 We previously reported the association between increased expression of interleukin-15 (IL-15) mRNA in ALL blasts and increased risk for CNS involvement.17 Several studies suggest an oncogenic role of IL-15 in hematologic malignancies.18-21 Williams et al22 recently proposed that IL-15 enhances growth of leukemic cells in the growth factor-poor environment of the cerebrospinal fluid (CSF). Overall, however, the biological role of IL-15 in CNS-ALL remains unclear.
IL-15 displays pleiotropic functions by acting on various immune cells including natural killer (NK) cells. NK cells are a subset of lymphocytes of the innate immune system that play an important role in cancer immune surveillance through their capacity to recognize and kill transformed cells without prior sensitization. NK cell-mediated cytotoxicity is regulated by the balance of signals transmitted by activating and inhibitory receptors on conjunction with a target cell.23,24 The development, survival, and activation of NK cells are predominantly regulated by IL-15.25-30 Moreover, IL-15 was shown to augment NK cell cytotoxicity against tumor cells by upregulating the expression of NKG2D and NKp44 receptors on NK cells, as well as the expression of cytotoxic effector molecules.31-33
Here, we describe experiments that demonstrate that NK-cell activation by leukemic cells expressing IL-15 can lead to control of residual disease in the periphery but to a lesser extent in the CNS because of a lack of NK-cell penetration into the brain. This could explain the association between IL-15 expression and CNS relapses of ALL and, importantly, suggests the need for CNS-directed prophylaxis in emerging protocols of antileukemia therapies with NK cells.
Materials and methods
Cells
Constructs and generation of stable cell lines
The murine IL-15 construct, in which the signal peptide of IL-2 is fused to the mouse IL-15 cDNA, was obtained from Hugh Brady and subcloned into the pCEFL expression vector. 018Z cells stably expressing luciferase and cherry fluorescent proteins were generated by transduction with the Cherry 2A luciferase _pLNT/Sffv-MCS/ccdB provided by Dr Vaskar Saha.
In vivo models
Experiments were performed in specific pathogen-free units and were approved by the institutional animal experiments committees. See “Results” for a detailed description of the experiments. For imaging, mice were anesthetized with ketamine-xylazine or isoflurane and were intraperitoneally injected with 150 mg/kg d-luciferin (Promega). Bioluminescent imaging (IVIS; Caliper Life Sciences) of anesthetized mice was performed 10 minutes thereafter. A bioluminescent image was acquired for 1 minute with medium binning, analyzed with Living Image software version 4.2 (Caliper Life Science), and quantified using a region of interest set to total flux (photons per second) or average radiance (photons per second per centimeter squared). All images were set to the same scale based on the negative controls (mice with phosphate-buffered saline [PBS] injection).36
Histopathology and hematoxylin and eosin staining of formalin-fixed brain and eyes were performed by routine methods.
NK-cell depletion
NK cells were depleted by intraperitoneal injections of 50 µL rabbit anti-Asialo GM1 (Cedarlane Laboratories) 1 day before leukemia transplantation and then once every 5 days. To ensure NK depletion, mice were monitored weekly for peripheral blood NK cells by flow cytometry using NKp46 antibody.
Antibodies and fusion proteins
The antibodies used are as follows: Alexa647-conjugated anti-mouse CD335 (NKp46), eFluor450-conjugated anti-mouse CD69 (eBioscience), allophycocyanin (APC)/phycoerythrin (PE)-conjugated anti-human CD19, PE-conjugated anti-human CD14, fluorescein isothiocyanate (FITC)-conjugated anti-CD3, PE-conjugated anti-human CD45 (IQ Products), APC/PE/Pacific Blue -conjugated anti-human CD56, Pacific Blue-conjugated anti-human CD16, APC-conjugated anti-human CD314 (NKG2D), biotin-conjugated anti-mouse NKG2D, strepavidin-conjugated PE/APC, APC/PE-conjugated anti-mouse CD49b (DX5), APC/PE-conjugated anti-human CD336 (NKp44), BV421 mouse anti-human CD107a, PE anti-human HLA A/B/C (W6/32) 7-amino-actinomycin D (7-AAD) (BioLegend), purified mouse anti-human DNAM-1 (DX11), mouse anti-human NKG2D (#149810), mouse anti-human IL-15 monoclonal antibody (mAb; R&D Systems), isotype control mouse immunoglobulin (Ig)G1, mouse anti-human MICA, mouse anti-human MICB, mouse anti-human ULBP2 mAb, APC-conjugated F(ab9)2 goat anti-human IgG, FITC-conjugated F(ab9)2 goat anti-human IgG, and APC-conjugated F(ab9)2 goat anti-mouse IgG (Jackson). Antibodies used for human cell surface labeling are as follows: PE-CyE5-conjugated CD10 mAb and phycoerythrin-Texas red–conjugated CD19 mAb (Beckman Coulter). The production of human Fc-fusion proteins NKp44, human NKp46, murine NKp46(Ly94), and human and murine NKG2D was previously described.37-39
Flow cytometry
018Z cells were incubated on ice with various human Fc-fusion proteins (40 mg/mL) or labeled with anti-MICA, anti-MICB, or anti-ULBP2, washed, and stained with APC- or FITC-conjugated anti-human-IgG or with APC- or FITC-conjugated anti-mouse IgG. For analysis of cells prepared from hematopoietic organs, red blood cell lysis was performed using red blood cell lysis buffer (BioLegend). Brains were homogenized in PBS followed by separation on a 40% Percoll (GE Healthcare) gradient to eliminate residual fat tissue before staining.
To reduce FcgII/IIIR-mediated antibody binding, 1 µL of anti-mouse CD32/CD16 (mouse BD Fc block) was added to all samples before staining. Dead cells were excluded using 7-AAD staining. Cells were analyzed on a Gallios flow cytometer (Beckman Coulter) or FACS Canto II (BD Biosciences), and data were analyzed using either Kaluza or FlowJo (Tree Star) software.
Isolation and culture of primary NK cells
Primary human NK cells were isolated from peripheral blood from healthy donors using a human NK-cell isolation kit, which is based on the negative selection approach (RosetteSep; STEMCELL Technologies). Purity (>85-90%) was confirmed by flow cytometry, and cells were cultured with IL-2 (300 U/mL) in CellGro stem cell serum-free growth medium (CellGenix) supplemented with 10% heat inactivated human serum, 1 mM sodium pyruvate, 2 mM l-glutamine, minimum essential medium nonessential amino acids, 1% penicillin/streptomycin, and 10 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]. Following in vitro culture with IL-2, total non-NK-cell contamination level was <5% with no CD3+ cells.
KIR and HLA genotyping
KIR genotyping was performed with the KIR Genotyping SSP Kit (Invitrogen). HLA typing was performed using LUMINEX-TM technology and Immucor Transplant Diagnostic (Stamford, CT) kits to obtain HLA A*, B*, and C* loci typing at low/intermediate resolution.
Cytotoxicity assays
Cytotoxic activity of primary human NK cells against ALL cells was tested in a 5-hour killing assay as previously described.40 For NKG2D blocking experiments, NK cells were preincubated for 1 hour at 4°C with 10 μg/mL anti-human NKG2D mAb (Clone 149810; R&D Systems, Minneapolis, MN) or with isotype control. 7-AAD staining was applied to exclude dead cells. The percentage of specific cytotoxicity was calculated as follows: (experimental killing – spontaneous killing)/(maximum killing – spontaneous killing) × 100.
Quantification of NKG2D (KLRK1) and NKp44 (NCR2) receptor and ligand mRNA in ALL BM samples was done in the same set of patients originally used in the IL-15 report,17 except for 2 patients lacking additional material. Informed consent was obtained, and the protocol was approved by local and central ethical committees. All patients on ALL–Berlin Frankfurt Munster 2000 underwent diagnostic lumbar puncture and CSF examination at admission to the participating hospital, cranial magnetic resonance imaging, and inspection of the retina. Only CNS 1 patients (no blasts in CSF cytospins) were included as CNS-negative controls. In all but 1 sample (70% blasts), the specimens contained >90% blasts. The expression level of the succinate dehydrogenase complex subunit A (SDHA) gene was used to normalize for differences in input cDNA. QuantiTect primer assays were used to measure mRNA abundance of the NKG2D (KLRK1), NKp44, and SDHA genes (Qiagen). Melting curve analyses were performed to verify the amplification specificity. Each sample was tested in duplicate. The expression ratio was calculated as 2n, where n was the CT value difference normalized by the CT difference of a calibrator sample.
Statistical analysis
Results of in vivo or in vitro experiments are shown by mean ± standard error (SE) or standard deviation, respectively. Data were analyzed using GraphPad Prism 5 software, and comparisons between groups were performed by paired or unpaired Student t test as appropriate. P < .05 was considered statistically significant. Comparison of NKG2D (KLRK1) and NKp44 expression between patient groups was performed applying the Mann-Whitney U test.
Results
Expression of IL-15 in mouse T-25 T-cell lymphoblasts increases mice survival associated with clinical symptoms for CNS involvement and activation of NK cells
To test the hypothesis that increased expression of IL-15 will be associated with CNS leukemia, we used the T-cell leukemia/lymphoma model described by Hochman et al.41,42 T-25 cells, not expressing endogenous IL-15, were transfected with an expression vector encoding murine IL-15 (designated T-25-IL15; supplemental Figure 1, available on the Blood Web site) and transplanted intraperitoneally into BALB/c neonates. Constitutive expression of IL-15 significantly enhanced the survival of mice (Figure 1A). Remarkably, mice injected with T-25-IL15 cells had pronounced clinical CNS symptoms (eg, ataxia, side walk, spin when held by the tail) not observed in control mice (Figure 1B). The most dramatic manifestation was ocular involvement that was observed only in mice injected with T-25-IL15 (Figure 1C). Ocular involvement has been previously shown in this model and in humans with advanced CNS leukemia.42-44 Lymphoblasts isolated from the eye continue to secrete the IL-15 protein (Figure 1D).
Constitutive expression of IL-15 in T-25 lymphoma cells increases survival of mice and is associated with clinical CNS symptoms. (A) Kaplan-Meier survival curve of 7-day-old BALB/C mice injected with either T-25-IL15 (n = 27) or T-25 cells (n = 28) (P < .0001, log-rank test). (B) Kaplan-Meier curve depicting the accumulated rate of CNS symptoms. Mice were monitored every day for clinical symptoms for CNS involvement including spin when held by tail, ataxia, and side-walk. These symptoms were present only in the T-25-IL15 group. (C) Representative picture of a T-25-IL15-injected mouse with ocular involvement. Note the opacity of the left eye (arrow) caused by accumulation of lymphoma cells in the left anterior chamber. (D) IL-15 secretion was measured by enzyme-linked immunosorbent assay in cells derived from the eyes of T-25-IL15-injected mice (n = 2). The T-25-injected mouse served as a control. (E-F) Hematoxylin and eosin staining of brain sections derived from T-25- (upper) and T-25-IL15-injected mice (lower), showing infiltration of lymphoma cells in the subarachnoid space. Histopathology analysis revealed that there was no difference in the number of mice with CNS involvement between both groups. (G) T-25-IL15-injected mice exhibited a significant increase in peripheral blood NK cells. Values presented as means ± SE (P = .01, t test).
Constitutive expression of IL-15 in T-25 lymphoma cells increases survival of mice and is associated with clinical CNS symptoms. (A) Kaplan-Meier survival curve of 7-day-old BALB/C mice injected with either T-25-IL15 (n = 27) or T-25 cells (n = 28) (P < .0001, log-rank test). (B) Kaplan-Meier curve depicting the accumulated rate of CNS symptoms. Mice were monitored every day for clinical symptoms for CNS involvement including spin when held by tail, ataxia, and side-walk. These symptoms were present only in the T-25-IL15 group. (C) Representative picture of a T-25-IL15-injected mouse with ocular involvement. Note the opacity of the left eye (arrow) caused by accumulation of lymphoma cells in the left anterior chamber. (D) IL-15 secretion was measured by enzyme-linked immunosorbent assay in cells derived from the eyes of T-25-IL15-injected mice (n = 2). The T-25-injected mouse served as a control. (E-F) Hematoxylin and eosin staining of brain sections derived from T-25- (upper) and T-25-IL15-injected mice (lower), showing infiltration of lymphoma cells in the subarachnoid space. Histopathology analysis revealed that there was no difference in the number of mice with CNS involvement between both groups. (G) T-25-IL15-injected mice exhibited a significant increase in peripheral blood NK cells. Values presented as means ± SE (P = .01, t test).
Surprisingly, histopathology analysis of brains isolated from 25 mice (12 mice injected with T-25-IL15 cells and 13 control mice) close to their time of death revealed that both groups exhibited lymphoblastic infiltration of the subarachnoid space, indicating that IL-15 is not necessary for blast entry into the CNS (Figure 1E-F). We therefore hypothesized that the IL-15-expressing cells activated NK cells that reduced the aggressiveness of the systemic leukemia, allowing sufficient time for development of symptomatic CNS and ocular disease. Consistent with this hypothesis, the percentage of peripheral activated blood NK cells (NKp46, CD69 positive) was increased significantly in mice transplanted with T-25-IL15 (Figure 1G; supplemental Figure 2)
Enhanced control of peripheral ALL but not CNS leukemia in NK-cell–proficient NOD/SCID mice compared with NK-cell–deficient NOD/SCID/IL2Rγ-null mice
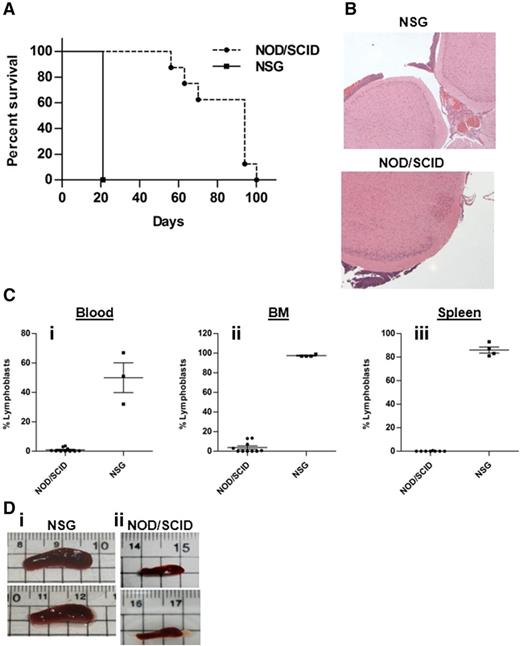
To study another model for human CNS leukemia with physiologic IL-15 levels, we obtained 018Z ALL cells derived from a B-cell precursor ALL of a child with suspected CNS involvement that died during induction chemotherapy. These cells causes isolated CNS leukemia in NOD/SCID mice.45 To examine whether the 018Z-induced CNS phenotype is associated with NK cell activation, we used 2 strains of immune-deficient mice: NOD/SCID mice containing some functional NK cells46 and NOD/SCID/IL2Rγ null (NSG), in which there are no NK cells.47 NOD/SCID and NSG mice were intravenously injected with 107 and 5 × 106 018Z cells, respectively, and killed on the appearance of clinical CNS symptoms or the development of severe clinical disease. The median survival of the NOD/SCID mice was 80 days after transplantation, and 5 of 10 developed hind limbs paralysis or ataxia. In contrast, all NSG mice developed hind limb paralysis 3 weeks after transplantation (Figure 2A). Histopathologic examination of brain sections showed subarachnoid CNS leukemia infiltration in both NOD/SCID and NSG mice (Figure 2B). Flow cytometry of human CD10+CD19+ leukemic cells revealed significant involvement of BM, spleen, and blood in the NSG mice but not in the NOD/SCID mice (Figure 2C). Similarly, enlarged spleens were present in NSG but not in NOD/SCID mice (Figure 2D). Thus, 018Z cells cause isolated CNS leukemia in NOD/SCID mice but a more aggressive combined CNS and peripheral leukemia in the NK-deficient NSG mice.
018Z human pre-B-ALL cells induce isolated CNS leukemia in NOD/SCID mice and combined CNS/peripheral leukemia in NSG mice. (A) A Kaplan-Meier survival curve of NOD/SCID (n = 10) and NSG (n = 4) mice injected intravenously with 018Z cells (P = .001; log-rank test). (B) Representative hematoxylin and eosin staining of brain sections from NOD/SCID (lower; n = 10) and NSG (upper; n = 3) mice presenting leukemia infiltration in the subarachnoid space. (C) Flow cytometry analysis of peripheral leukemia percentage in the (i) blood, (ii) BM, and (iii) spleen using antibodies for CD10 and CD19. Values are means ± SE (P < .0001; unpaired t test). (D) Representative pictures of spleens taken from (i) NSG or (ii) NOD/SCID mice.
018Z human pre-B-ALL cells induce isolated CNS leukemia in NOD/SCID mice and combined CNS/peripheral leukemia in NSG mice. (A) A Kaplan-Meier survival curve of NOD/SCID (n = 10) and NSG (n = 4) mice injected intravenously with 018Z cells (P = .001; log-rank test). (B) Representative hematoxylin and eosin staining of brain sections from NOD/SCID (lower; n = 10) and NSG (upper; n = 3) mice presenting leukemia infiltration in the subarachnoid space. (C) Flow cytometry analysis of peripheral leukemia percentage in the (i) blood, (ii) BM, and (iii) spleen using antibodies for CD10 and CD19. Values are means ± SE (P < .0001; unpaired t test). (D) Representative pictures of spleens taken from (i) NSG or (ii) NOD/SCID mice.
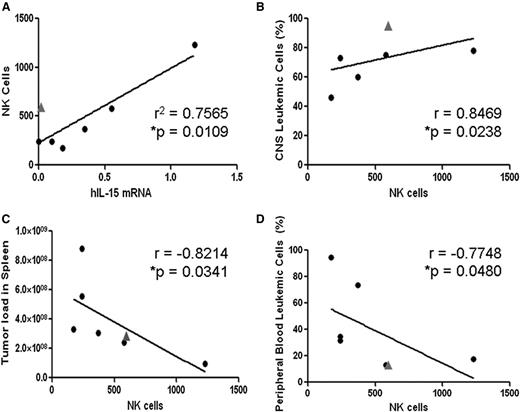
To examine the general relevance of these observations, we analyzed the leukemic phenotypes of 2 primary ALL cell xenografts in the 2 mouse strains (supplemental Figure 3). For both samples, the peripheral leukemic load was significantly lower in NOD/SCID mice, whereas in both strains, there was substantial CNS infiltration. To study the correlations with NK cells, we analyzed an additional 7 xenografts of primary ALLs in NOD/SCID mice,48 depicting combined CNS/peripheral leukemia. As shown in Figure 3A, there was a positive correlation between the levels of IL-15 mRNA in the ALL blasts and the level of NK cells with 1 outlier (marked with a triangle in Figure 3A), in which an increase in NK cells was observed despite the absence of IL-15 expression. Interestingly, these ALL cells carried the t(1;19)(q23 p13) chromosomal translocation that is known to be associated with increased risk for CNS involvement.49,50 Increased NK cells (351 NK cells/105 splenic cells) were also observed in another t(1;19)(q23 p13) NOD/SCID xenograft with CNS leukemia (patient A in supplemental Figure 3). Thus, t(1;19)(q23 p13) may stimulate NK cells in an IL-15-independent manner. Importantly, increased NK-cell counts were associated with decreased peripheral leukemia and increased CNS infiltration of xenografted leukemic cells in the NOD/SCID mice (Figure 3B-D).
Associations of NK-cell infiltration in spleens of human ALL-bearing NOD/SCID with levels of IL-15 mRNA expression and with leukemic infiltrates of CNS and peripheral organs. (A) Linear regression analysis of splenic mouse NK cells (DX5- and NKG2D-positive cells per 105 splenic cells) as a function of IL-15 mRNA expression in infiltrating human ALL cells (determined by qRT-PCR, values normalized to β actin mRNA levels72,73 ). The outlier (gray triangle) is a xenograft from a patient with t(1;19)-positive ALL demonstrating significant NK-cell accumulation despite no IL-15 expression. (B-D) Increasing splenic NK-cell recruitment is significantly associated with (B) increased infiltration of human ALL cells in the recipient’s CNS and (C-D) lower systemic leukemia burden in spleen and peripheral blood (Spearman correlation).
Associations of NK-cell infiltration in spleens of human ALL-bearing NOD/SCID with levels of IL-15 mRNA expression and with leukemic infiltrates of CNS and peripheral organs. (A) Linear regression analysis of splenic mouse NK cells (DX5- and NKG2D-positive cells per 105 splenic cells) as a function of IL-15 mRNA expression in infiltrating human ALL cells (determined by qRT-PCR, values normalized to β actin mRNA levels72,73 ). The outlier (gray triangle) is a xenograft from a patient with t(1;19)-positive ALL demonstrating significant NK-cell accumulation despite no IL-15 expression. (B-D) Increasing splenic NK-cell recruitment is significantly associated with (B) increased infiltration of human ALL cells in the recipient’s CNS and (C-D) lower systemic leukemia burden in spleen and peripheral blood (Spearman correlation).
018Z cells induced aggressive peripheral and CNS leukemia in NK-cell–depleted NOD/SCID mice
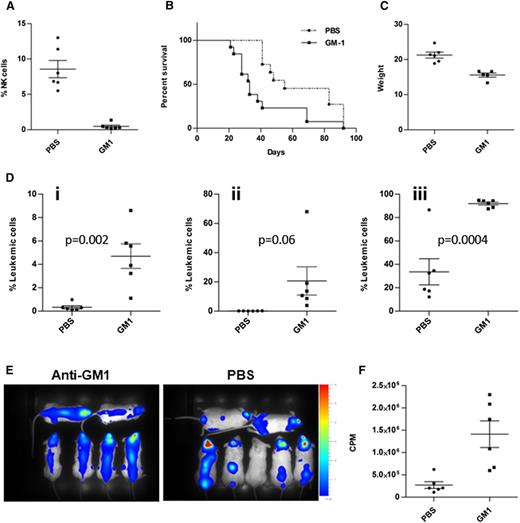
To study directly the hypothesis that the leukemia was confined to the CNS in NOD/SCID mice because of the activation of NK cells in the periphery, we injected 018Z cells expressing both mCherry and luciferase reporter genes into NK cell-depleted and nondepleted NOD/SCID mice. NK-cell depletion was achieved by intraperitoneal injection of anti-Asialo GM1 twice a week. As depicted in Figure 4A, the percentage of NK cells after treatment with anti-Asialo GM1 was 0.5 ± 0.1 compared with 8.5 ± 1.2 in PBS-injected control mice. NK cells remained depleted throughout the experiment.
Depletion of NK cells reduced survival of mice and enhanced peripheral and CNS leukemia. NOD/SCID mice were intraperitoneally injected with either anti-Asialo GM1 (n = 13) or sterile PBS (n = 11) and then transplanted with 107 cells intravenously. (A) Confirmation of NK depletion by flow cytometry analysis of peripheral NK cells in blood samples from mice injected with anti-Asialo GM1 compared with PBS controls (P < .0001). (B) Kaplan-Meier survival curve depicting reduced survival of the NK-cell–depleted mice (P = .003, log-rank test). (C) Reduced weight of NK-cell–depleted mice on the day of euthanasia (P = .0005, t test). (D) Flow cytometry analysis of 018Z-cherry-positive cells in the (i) blood, (ii) BM, and (iii) brain. Data presented are from 3 independent experiments. Values are means ± SE. Statistical analysis was performed by t test. (E) Bioluminescent imaging of mice 27 days after transplantation with 018Z cells expressing the luciferase reporter: NK-cell–depleted mice (left) and PBS-injected mice (right). (F) Quantification of the images in E by Living Image Software. The leukemic load is represented as counts per minute (CPM; P = .004, t test).
Depletion of NK cells reduced survival of mice and enhanced peripheral and CNS leukemia. NOD/SCID mice were intraperitoneally injected with either anti-Asialo GM1 (n = 13) or sterile PBS (n = 11) and then transplanted with 107 cells intravenously. (A) Confirmation of NK depletion by flow cytometry analysis of peripheral NK cells in blood samples from mice injected with anti-Asialo GM1 compared with PBS controls (P < .0001). (B) Kaplan-Meier survival curve depicting reduced survival of the NK-cell–depleted mice (P = .003, log-rank test). (C) Reduced weight of NK-cell–depleted mice on the day of euthanasia (P = .0005, t test). (D) Flow cytometry analysis of 018Z-cherry-positive cells in the (i) blood, (ii) BM, and (iii) brain. Data presented are from 3 independent experiments. Values are means ± SE. Statistical analysis was performed by t test. (E) Bioluminescent imaging of mice 27 days after transplantation with 018Z cells expressing the luciferase reporter: NK-cell–depleted mice (left) and PBS-injected mice (right). (F) Quantification of the images in E by Living Image Software. The leukemic load is represented as counts per minute (CPM; P = .004, t test).
Similar to the phenotype observed in NSG mice, NK cell-depleted NOD/SCID mice developed aggressive systemic disease characterized by reduced survival (Figure 4B; P = .003, log-rank test), substantial weight loss (Figure 4C), and significant increase of leukemic blasts in the blood, BM, and brain (Figure 4Di-Diii). Bioluminescent imaging of mice 27 days after transplantation supported these findings (Figure 4E-F). Thus, the increased burden of leukemia in the absence of NK cells was associated with an aggressive combined BM and CNS phenotype.
Poor penetration of NK cells to the CNS in mice with CNS leukemia
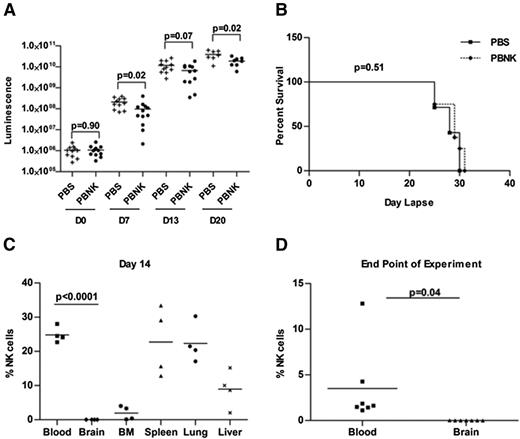
Our hypothesis that NK cells regulate development of isolated CNS leukemia assumes that NK cells do not penetrate the CNS, even in the presence of CNS leukemia that may be associated with compromise of the blood-brain-CSF barrier. We therefore injected NSG mice intravenously with 2 × 105 018Z McCherry/luciferase-labeled leukemic cells. Six days later, mice received either PBS or 107 human peripheral blood NK cells (PBNK) intravenously. Mice were followed by bioluminescent imaging at days 0, 7, 13, and 20 after NK treatment and euthanized at the time when the debilitating symptoms developed (supplemental Figure 4). Although mice that received this single dose of NK-cell treatment did have slower tumor progression, as quantified by bioluminescence (Figure 5A), this did not translate to increased survival (Figure 5B). At the end of experiment, there was extensive infiltration of peripheral hematopoietic organs and of the CNS in both groups. Analysis of NK-cell trafficking to specific tissues shows that human NK cells can be seen in the blood and peripheral tissues at day 14 after NK-cell treatment (Figure 5C); however, no NK cells are seen in the CNS at day 14 or at the end of experiment when the mice became moribund (Figure 5C-D).
In vivo antitumor responses by expanded human NK cells in the 018Z-engrafted NSG mouse model. Mice were intravenously injected with 2 × 105 018Z tumor cells and imaged by bioluminescence after 6 days to confirm tumor establishment. Then mice received either PBS (n = 11 mice) or 1 × 107 human PBNK cells (n = 12 mice) at day 0 with IL-2 and IL-15 daily for the first week and IL-2 every other day for 3 more weeks. (A) Bioluminescent analyses indicate significant delay in tumor growth in mice treated with PBNK cells at days 7 and 20. (B) Kaplan-Meier survival curve of NK cell-treated and control mice. These survival data use 7 mice from the PBS-treated group and 8 mice from the PBNK cell-treated group. (C) NK-cell engraftment was evaluated by flow cytometry. Mice blood, brain, BM, spleen, lung, and liver tissues were collected from 4 mice at day 14 after PBNK treatment. NK-cell survival/engraftment was evaluated by flow cytometry for CD45+CD56+ cells in the live cell population. (D) Analysis of NK cells (CD45+CD56+ cells) in the blood and CNS at the end of the experiment when the remaining animals became moribund (n = 7 in each group). These studies demonstrate lack of penetration of NK cells into the CNS with significantly decreased NK cells in the CNS compared with peripheral blood and tissues. All statistical analyses were performed by the Mann-Whitney test in Prism 4.0.
In vivo antitumor responses by expanded human NK cells in the 018Z-engrafted NSG mouse model. Mice were intravenously injected with 2 × 105 018Z tumor cells and imaged by bioluminescence after 6 days to confirm tumor establishment. Then mice received either PBS (n = 11 mice) or 1 × 107 human PBNK cells (n = 12 mice) at day 0 with IL-2 and IL-15 daily for the first week and IL-2 every other day for 3 more weeks. (A) Bioluminescent analyses indicate significant delay in tumor growth in mice treated with PBNK cells at days 7 and 20. (B) Kaplan-Meier survival curve of NK cell-treated and control mice. These survival data use 7 mice from the PBS-treated group and 8 mice from the PBNK cell-treated group. (C) NK-cell engraftment was evaluated by flow cytometry. Mice blood, brain, BM, spleen, lung, and liver tissues were collected from 4 mice at day 14 after PBNK treatment. NK-cell survival/engraftment was evaluated by flow cytometry for CD45+CD56+ cells in the live cell population. (D) Analysis of NK cells (CD45+CD56+ cells) in the blood and CNS at the end of the experiment when the remaining animals became moribund (n = 7 in each group). These studies demonstrate lack of penetration of NK cells into the CNS with significantly decreased NK cells in the CNS compared with peripheral blood and tissues. All statistical analyses were performed by the Mann-Whitney test in Prism 4.0.
Taken together, the NK depletion experiment in NOD/SCID mice (Figure 4) and the analysis of human NK-cell distribution in NSG mice (Figure 5C) show that, although NK cells appear to mediate anti-ALL activity in the periphery, the lack of NK cells in the CNS demonstrates that this region remains a sanctuary against NK cell-mediated activity.
B-cell precursor ALL cell line 018Z secretes endogenous IL-15 and is efficiently killed by primary NK cells via NK2GD
We next investigated the mechanisms of NK-cell activity on 018Z human leukemic cells. Consistent with the previously reported association between IL-15 production by ALL cells and CNS involvement,17,22 018Z secreted IL-15 (Figure 6A). As our previous in vivo results indicated that 018Z leukemia growth was regulated by murine NK cells, we performed a standard killing assay in which 018Z cells were prelabeled with carboxyfluorescein diacetate succinimidyl ester and incubated with IL-2-activated primary human NK cells. Consistent with the mouse experiments, 018Z cells were efficiently killed by human NK cells relatively to the resistance of another B-cell precursor leukemia cell line (REH) that does not secrete IL-15 (Figure 6B). Thus, 018Z cells express IL-15 and are sensitive to NK cytotoxicity.
018Z cells are efficiently killed by primary NK cells through interaction with NKG2D receptors. (A) IL-15 levels (enzyme-linked immunosorbent assay) in the medium of various ALL cell lines after overnight incubation at 37°C. (B) 018Z and REH cells were incubated for 5 hours with human primary NK cells at various effector to target (E:T) ratios. Specific killing of 018Z cells was significantly higher than that of REH cells for all E:T ratios. *P < .001; **P < .0001 (t test). (C) HLA-A/B/C expression on REH (black line) and 018Z cells (dashed line). (D) Binding of Fc-fusion proteins of NK receptors to 018Z cells. 018Z cells were incubated with various human Fc-fusion proteins of NK receptors followed by labeling with goat anti-hIgG (black line): (i) hNKG2D-Ig, (ii) mNKG2D-Ig, (iii) NKp44-Ig, (iv) hNKp46-Ig, (v) mNKp46-Ig, and (vi) NKp30-Ig. Nonspecific binding was detected by staining with human Fc-protein (filled gray). (vii) Expression of the hNKG2D ligands on 018Z cells. Purified mouse anti MICA (black line), MICB (dotted), and ULBP2 (dashed), followed by labeled goat anti-mIgG (filled gray). Results are representative of 3 independent experiments. (E-F) Killing assay of 018Z cells by IL-2-activated human primary NK cells with or without blocking antibodies for NKG2D and DNAM-1, respectively. Pretreatment of NK cells with blocking antibodies for NKG2D significantly reduced the killing of 018Z cells. Values are shown as means ± standard deviation of triplicates. *P < .00001 (t test, no mAb or isotype control compared with anti-hNKG2D).
018Z cells are efficiently killed by primary NK cells through interaction with NKG2D receptors. (A) IL-15 levels (enzyme-linked immunosorbent assay) in the medium of various ALL cell lines after overnight incubation at 37°C. (B) 018Z and REH cells were incubated for 5 hours with human primary NK cells at various effector to target (E:T) ratios. Specific killing of 018Z cells was significantly higher than that of REH cells for all E:T ratios. *P < .001; **P < .0001 (t test). (C) HLA-A/B/C expression on REH (black line) and 018Z cells (dashed line). (D) Binding of Fc-fusion proteins of NK receptors to 018Z cells. 018Z cells were incubated with various human Fc-fusion proteins of NK receptors followed by labeling with goat anti-hIgG (black line): (i) hNKG2D-Ig, (ii) mNKG2D-Ig, (iii) NKp44-Ig, (iv) hNKp46-Ig, (v) mNKp46-Ig, and (vi) NKp30-Ig. Nonspecific binding was detected by staining with human Fc-protein (filled gray). (vii) Expression of the hNKG2D ligands on 018Z cells. Purified mouse anti MICA (black line), MICB (dotted), and ULBP2 (dashed), followed by labeled goat anti-mIgG (filled gray). Results are representative of 3 independent experiments. (E-F) Killing assay of 018Z cells by IL-2-activated human primary NK cells with or without blocking antibodies for NKG2D and DNAM-1, respectively. Pretreatment of NK cells with blocking antibodies for NKG2D significantly reduced the killing of 018Z cells. Values are shown as means ± standard deviation of triplicates. *P < .00001 (t test, no mAb or isotype control compared with anti-hNKG2D).
This sensitivity could not be explained by reduction in HLA expression, as 018Z and REH expressed similar levels of HLA class I (Figure 6C). As killer-cell immunoglobulin-like receptor (KIR)/KIR-ligand disparity influences NK-cell lysis of leukemic cells,51 we genotyped the NK-cell donor and target cells. KIR2DL1, KIR2DL2, and KIR2DL3 inhibitory receptors were expressed on the NK cells, whereas both 018Z and REH cells expressed ligands for KIR2DL1 (Cw*04 and Cw*06), and in addition, 018Z cells expressed HLA-Cw*07, a KIR2DL2/3 ligand (supplemental Figure 5). Thus, despite an increased expression of inhibitory ligands, 018Z cells were susceptible to killing by NK cells. These results indicate that KIR/HLA mismatch is not a major factor contributing to the increased susceptibility of 018Z cells to cytotoxicity.
Therefore, we further investigated the expression of ligands for NK-activating receptors.52-55 Because some cellular ligands for NK receptors (NKRs) are yet unknown, we characterized their expression by 018Z cells using Fc-fusion proteins of NKRs.40 Flow cytometry was used to examine the binding of Fc-fusion proteins of human and mouse NKG2D, human NKp30, NKp44, and NKp46, and mouse LY94 (NKp46 homolog) to putative ligands on the surface of 018Z cells. We found that 018Z cells express ligands recognized by both human and mouse NKG2D, as well as ligands for the human NKp44 receptor (Figure 6Di-Dvi). We further examined the expression of known specific human NKG2D ligands: MHC class І-related proteins A and B (MICA/MICB) and the UL16 binding protein 2 (ULBP2) on 018Z cells. As shown in Figure 6Dvii, 018Z cells express all 3 ligands, with the highest expression for ULBP2. Interestingly, ULBP2 was reported to also be effectively recognized by mouse NKG2D,33 which could explain the in vivo sensitivity of 018Z to murine NK (Figure 4). As NKp44 is not expressed by murine NK, expression of ligands to NKp44 by 018Z did not contribute to its NK-mediated growth retardation in mice. For the human NK scenario, because the human NK-resistant REH cell line (Figure 6B) expressed cell surface ligands to NKp44 but a very low level of ligands to NKG2D (data not shown), we hypothesized that the higher sensitivity of 018Z to human NK cells was mediated by NKG2D. Indeed, using blocking antibodies to NKG2D, we show that the killing of 018Z is primarily mediated by NKG2D (Figure 6E). The NEC2/DNAM-1 interaction was reported to be involved in the pathway of acute lymphoblastic leukemia cell recognition by NK cells.56 However, blocking the DNAM-1/PVR-nectin-2 recognition did not affect the lysis of 018Z by NK (Figure 6F). Together, our experiments suggest that the increased sensitivity to lysis by NK of the IL-15-secreting 018Z is mediated by the NKG2D/NKG2D ligand interaction.
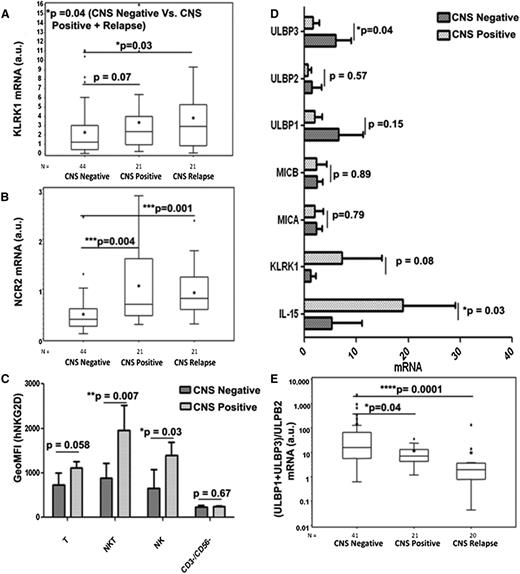
Expression of NK-cell receptors and ligands in primary pediatric ALL diagnostic BM samples and association with CNS leukemia
To further establish the role of NK cells, its relevance to childhood ALL, and its association with CNS leukemia, we first examined the mRNA expression of NKG2D (KLRK1) and NKp44 (NCR2) receptors in ALL BM samples originally used in the IL-15 report.17 Clinical and biological characteristics of the samples analyzed are shown in Table 1. Expression of both NKp44 and NKG2D was analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of cDNAs derived from BM samples of patients who were (1) CNS negative at initial diagnosis with no CNS relapse (n = 44); (2) CNS positive at initial diagnosis (n = 21); and (3) CNS negative at initial diagnosis with subsequent CNS relapse (n = 21). As depicted in Figure 7A, expression levels of NKG2D (KLRK1) were associated with CNS leukemia (when comparing group CNS positive vs negative and CNS negative vs CNS negative with subsequent CNS relapse; P = .07 and P = .03, respectively). Similarly, expression levels of NKp44 (NCR2) in BM were significantly higher in CNS involving leukemia (P = .004 and P = .001, respectively; Figure 7B).
Characteristics at initial diagnosis of 44 CNS-negative patients with acute lymphoblastic leukemia, 21 CNS-positive patients, and 21 patients subsequently relapsing with CNS involvement
| . | CNS negative (n = 44) (%) . | CNS positive (n = 21) (%) . | CNS relapse (n = 21) (%) . | P* . |
|---|---|---|---|---|
| Age (years) | ||||
| 1 to <10 | 28 (63.6) | 14 (66.7) | 14 (66.7) | |
| ≥10 | 16 (36.4) | 7 (36.3) | 7 (36.3) | 1.000 |
| Sex | ||||
| Male | 26 (59.1) | 12 (57.1) | 16 (76.2) | |
| Female | 18 (40.9) | 9 (42.9) | 5 (23.8) | .390 |
| Presenting white blood cell count/µL | ||||
| <10 000 | 7 (15.9) | 3 (14.3) | 6 (28.6) | |
| 10 000 to <50 000 | 20 (45.4) | 8 (38.1) | 5 (23.8) | |
| 50 000 to <100 000 | 9 (20.5) | 4 (19.0) | 4 (19.0) | |
| ≥100 000 | 8 (18.2) | 6 (28.6) | 6 (28.6) | .636 |
| Immunophenotype | ||||
| B-precursor | 44 (100.0) | 21 (100.0) | 18 (85.7) | |
| T-ALL | 0 | 0 | 3 (14.3) | .808 |
| BCR/ABL | ||||
| Negative | 44 (100.0) | 18 (85.6) | 19 (90.4) | .104 |
| Positive | 0 | 1 (4.8) | 2 (9.6) | |
| Not known | 0 | 2 (9.6) | 0 | |
| ETV6/RUNX1 | ||||
| Negative | 44 (100.0) | 16 (76.2) | 18 (85.7) | .01 |
| Positive | 0 | 4 (19.1) | 2 (9.5) | |
| Not known | 0 | 1 (4.7) | 1 (4.8) | |
| MLL/AF4 | ||||
| Positive | 0 | 0 | 0 | |
| Treatment group | ||||
| Standard risk | 18 (41.0) | 8 (38.1) | 2 (9.5) | |
| Intermediate risk | 9 (20.4) | 7 (33.3) | 14 (66.7) | |
| High risk | 17 (38.6) | 6 (28.6) | 5 (23.8) | .007 |
| . | CNS negative (n = 44) (%) . | CNS positive (n = 21) (%) . | CNS relapse (n = 21) (%) . | P* . |
|---|---|---|---|---|
| Age (years) | ||||
| 1 to <10 | 28 (63.6) | 14 (66.7) | 14 (66.7) | |
| ≥10 | 16 (36.4) | 7 (36.3) | 7 (36.3) | 1.000 |
| Sex | ||||
| Male | 26 (59.1) | 12 (57.1) | 16 (76.2) | |
| Female | 18 (40.9) | 9 (42.9) | 5 (23.8) | .390 |
| Presenting white blood cell count/µL | ||||
| <10 000 | 7 (15.9) | 3 (14.3) | 6 (28.6) | |
| 10 000 to <50 000 | 20 (45.4) | 8 (38.1) | 5 (23.8) | |
| 50 000 to <100 000 | 9 (20.5) | 4 (19.0) | 4 (19.0) | |
| ≥100 000 | 8 (18.2) | 6 (28.6) | 6 (28.6) | .636 |
| Immunophenotype | ||||
| B-precursor | 44 (100.0) | 21 (100.0) | 18 (85.7) | |
| T-ALL | 0 | 0 | 3 (14.3) | .808 |
| BCR/ABL | ||||
| Negative | 44 (100.0) | 18 (85.6) | 19 (90.4) | .104 |
| Positive | 0 | 1 (4.8) | 2 (9.6) | |
| Not known | 0 | 2 (9.6) | 0 | |
| ETV6/RUNX1 | ||||
| Negative | 44 (100.0) | 16 (76.2) | 18 (85.7) | .01 |
| Positive | 0 | 4 (19.1) | 2 (9.5) | |
| Not known | 0 | 1 (4.7) | 1 (4.8) | |
| MLL/AF4 | ||||
| Positive | 0 | 0 | 0 | |
| Treatment group | ||||
| Standard risk | 18 (41.0) | 8 (38.1) | 2 (9.5) | |
| Intermediate risk | 9 (20.4) | 7 (33.3) | 14 (66.7) | |
| High risk | 17 (38.6) | 6 (28.6) | 5 (23.8) | .007 |
χ2 or Fisher’s exact test.
ETV6, ets variant 6; RUNX1, runt-related transcription factor 1.
Expression of NKG2D and NKp44 receptors and ligands on NK cells and the association with CNS leukemia. Box plots demonstrating the positive association between the expressions of (A) NKG2D/KLRK1 and (B) NKp44/NCR2, respectively, and CNS involvement in leukemia in the entire set of 86 primary BM samples. (C) Geometric mean fluorescence intensity of human NKG2D protein expression on T cells (CD45+/CD3+/CD56−/CD19−), natural killer T (NKT) cells (CD45+/CD3+/CD56+/CD19−), NK cells (CD45+/CD3−/CD56+/CD19−), and CD45+/CD3−/CD56−/CD19+/− cells in a subset of 6 CNS-negative and 3 CNS-positive primary BM samples. (D) qRT-PCR of IL-15, NKG2D/KLRK1, MICA, MICB, ULBP1, ULBP2, and ULBP3 in the same set as C. mRNA values are normalized to SDHA expression. (E) Association of (ULBP1 + ULBP3/ULBP2) mRNA ratio and CNS involvement in leukemia in the entire set of BM samples. Data shown were derived through qRT-PCR after reverse transcription using RNA of BM mononuclear cells at initial diagnosis. The line within the box plots of A, B, and E corresponds to the median value, the star is the mean value, the box length is the interquartile range, and the lines emanating from the box (whiskers) extend to the smallest and largest observations; outliers are indicated. a.u., arbitrary units. Statistical analyses were done by applying the 2-tailed Student t-test.
Expression of NKG2D and NKp44 receptors and ligands on NK cells and the association with CNS leukemia. Box plots demonstrating the positive association between the expressions of (A) NKG2D/KLRK1 and (B) NKp44/NCR2, respectively, and CNS involvement in leukemia in the entire set of 86 primary BM samples. (C) Geometric mean fluorescence intensity of human NKG2D protein expression on T cells (CD45+/CD3+/CD56−/CD19−), natural killer T (NKT) cells (CD45+/CD3+/CD56+/CD19−), NK cells (CD45+/CD3−/CD56+/CD19−), and CD45+/CD3−/CD56−/CD19+/− cells in a subset of 6 CNS-negative and 3 CNS-positive primary BM samples. (D) qRT-PCR of IL-15, NKG2D/KLRK1, MICA, MICB, ULBP1, ULBP2, and ULBP3 in the same set as C. mRNA values are normalized to SDHA expression. (E) Association of (ULBP1 + ULBP3/ULBP2) mRNA ratio and CNS involvement in leukemia in the entire set of BM samples. Data shown were derived through qRT-PCR after reverse transcription using RNA of BM mononuclear cells at initial diagnosis. The line within the box plots of A, B, and E corresponds to the median value, the star is the mean value, the box length is the interquartile range, and the lines emanating from the box (whiskers) extend to the smallest and largest observations; outliers are indicated. a.u., arbitrary units. Statistical analyses were done by applying the 2-tailed Student t-test.
We then extended these findings to the protein level and tested the cell surface expression of NKG2D and NCR2 proteins in the immune cells within those primary ALL BM samples with additional material available (6 CNS-negative and 3 CNS-positive samples). Although the NCR2 mRNA level significantly differed in primary ALL BM samples, we could not detect significant cell surface expression of the NCR2 protein in the subset analyzed. In contrast, significant enhancement of cell surface expression of the NKG2D protein was observed in T, NKT, and NK cells in BM diagnostic samples from CNS-positive patients (Figure 7C). In accordance, the qPCR analysis of the mRNA of these specific samples manifested enhancement of KLRK1 mRNA and of IL-15 mRNA (Figure 7D).
Enhanced expression of NKG2D ligands by pediatric B-ALL cells was previously reported, particularly for ULBP1 and ULBP3.56 We thus assessed mRNA expression levels of the specific NKG2D ligands in these ALL BM samples. Interestingly, ULBP1 and ULBP3 mRNA expression levels were enhanced in the CNS-negative compared with the CNS-positive samples (Figure 7D). ULBP3, and to a lesser extent ULBP1, was shown to be secreted in exosomes and thus could facilitate inhibition of NKG2D-mediated NK function.57,58 We therefore analyzed ULBP1, ULBP2, and ULBP3 in the entire set of 68 diagnostic BM samples and calculated the (ULBP1 + ULBP3/ULBP2) mRNA expression ratio for each ALL BM sample. Figure 7E clearly shows that the ratios in the CNS-negative group were significantly higher than the ratios in the CNS-positive and CNS relapse groups (note that the y-axis scale is logarithmic). Thus, the high expression of the NKG2D receptor in infiltrating NK cells coupled with lower expression of inhibitory NKG2D ligands in the BM at the time of diagnosis of ALL correlated with CNS leukemia.
Discussion
The occurrence of CNS leukemia is largely explained by neurotropism of lymphoblasts and the poor penetration of antileukemic drugs into the CNS. Here we demonstrate that the CNS is also an immunologic sanctuary for ALL cells.
We sought a mechanistic explanation to our previously reported association between IL-15 expression in ALL blasts and the increased risk for CNS relapse of ALL.17 Consistent with the known regulation of NK cells by IL-15,25-30 we observed that the expression of IL-15 in cell lines and in primary ALL cells was associated with activation of NK cells. Our data suggest that NK cells do not directly regulate leukemic blast penetrance and survival in the CNS. Rather, neurotropism is caused by endogenous properties of ALL cells. For example, William et al22 suggested that IL-15 may be directly involved in the survival and proliferation of cells in the CNS. Similarly, Krause et al49 showed that MERTK kinase may regulate the CNS tropism of t(1;19) ALL. Our studies of mouse and human cell lines and primary ALL cells demonstrate that NK cells activated by IL-15 or other mechanisms56,59 inhibit systemic peripheral leukemia but fail to enter the brain and control the CNS leukemia.
Recently, there has been significant progress in NK cell-based therapies of acute leukemias. In addition to exploiting mismatch of NK inhibitory receptors during stem cell transplantation,60-65 direct treatment of ALL with allogeneic or autologous NK cells has been recently proposed.56,66-70 Our findings may be important for emerging NK-based therapies of acute leukemias, suggesting the need for additional prophylactic CNS-directed therapy.
A role for autologous NK cells in ALL surveillance has been suggested by genetic studies indicating that possessing more NK-activating KIR genes reduces the risk of developing leukemia.71 Interestingly, increased NK sensitivity associated with increased NK ligand expression has been recently demonstrated in BCR-ABL1 ALLs, a leukemia that is highly associated with CNS involvement.56 Our mechanistic studies imply a major role for NKG2D in NK surveillance of endogenous ALL cells. We observed increased mRNA and protein expression of NKG2D on NK cells from diagnostic BM of ALL patients with CNS involvement. This increase may be caused by IL-15 or alternatively by reduction in the secretion of “decoy” ULBP ligands.57,58
This is the first report suggesting that NK-cell activity against ALL is excluded from the CNS, and it raises multiple questions. For example, should CNS prophylaxis be added to leukemia therapeutic protocols with NK cells? Can increased NKG2D and a lower ratio of ULBP1 + ULBP3/ULBP2 expression in diagnostic BM be translated into biomarkers for CNS relapse and for sensitivity to autologous NK cells? These and other questions emerging from our discoveries need to be tested in prospective clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Profs Vaskar Saha and Hugh Brady for providing reagents; Birthe Fedders, Inna Muler, and Rivka Kalt for technical assistance; and Prof Ofer Mandelboim for advice.
This study was supported in part by the Israel Science Foundation 757/07 Bikura grant (to S.I. and J.H.) and I-CORE Center grant 41/11 (to S.I.); the WLBH Foundation (S.I. and D.K.); European Research Area NET grant Translational Research in Childhood Acute Lymphoblastic Leukemia (S.I. and M. Stanulla); United States–Israel Binational Science Foundation grant 2011123 (to K.S.C. and A.P.); Israel Science Foundation grant 304/12 (to A.P.); the International Graduate School in Molecular Medicine Ulm (V.M.); German Research Foundation grant SFB1074 (to K.-M.D. and L.H.M.); and the Max-Eder Junior Research Program by the Deutsche Krebshilfe (D.M.S.). This study was done as part of the requirement for PhD thesis of Tel Aviv University and Ben Gurion University for L.F.-L. and A.S., respectively.
Authorship
Contribution: L.F.-L. S.I., G.C., and M. Stanulla initiated the project; L.F.-L., A.S., A.B.-S., Z.N., L.H.M., K.-M.D., D.K., J.H., D.M.S., A.P., and S.I. planned experiments; L.F.-L., A.S., A.B.-S., C.M., Z.N., V.M., S.F., H.B., C.V., K.S.C., R.L., D.M.S., L.H.M., and G.C. performed and analyzed experiments; L.H.M., K.-M.D., D.M.S., G.C., D.K., A.P., and S.I. further analyzed experiments; C.E., M. Stanulla, M. Schrappe, and G.C. provided and analyzed patient data; L.F.-L., A.S., L.H.M., D.M.S., D.K., A.P., and S.I. wrote the manuscript; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shai Izraeli, Sheba Medical Center, Tel Hashomer, Ramat Gan 52621, Israel; e-mail: sizraeli@sheba.health.gov.il; and Angel Porgador, The Shraga Segal Department of Microbiology, Immunology and Genetics, Faculty of Health Sciences and the National Institute of Biotechnology in the Negev, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; e-mail: angel@bgu.ac.il.
References
Author notes
L.F.-L. and A.S. contributed equally to this work.