Abstract
Assessment of minimal residual disease (MRD) is becoming standard diagnostic care for potentially curable neoplasms such as acute lymphoblastic leukemia. In multiple myeloma (MM), the majority of patients will inevitably relapse despite achievement of progressively higher complete remission (CR) rates. Novel treatment protocols with inclusion of antibodies and small molecules might well be able to further increase remission rates and potentially also cure rates. Therefore, MRD diagnostics becomes essential to assess treatment effectiveness. This review summarizes reports from the past 2 decades, which demonstrate that persistent MRD by multiparameter flow cytometry, polymerase chain reaction, next-generation sequencing, and positron emission tomography/computed tomography, predicts significantly inferior survival among CR patients. We describe the specific features of currently available techniques for MRD monitoring and outline the arguments favoring new criteria for response assessment that incorporate MRD levels. Extensive data indicate that MRD information can potentially be used as biomarker to evaluate the efficacy of different treatment strategies, help on treatment decisions, and act as surrogate for overall survival. The time has come to address within clinical trials the exact role of baseline risk factors and MRD monitoring for tailored therapy in MM, which implies systematic usage of highly sensitive, cost-effective, readily available, and standardized MRD techniques.
Introduction
The development of new and effective therapies usually comes along with the need for more sensitive approaches to compare the efficacy of different treatment strategies, and implementation of individualized therapy monitoring strategies to prevent both under- and overtreatment. In the past decade, the landscape of drugs approved for the treatment of multiple myeloma (MM) has rapidly grown, and several agents with novel mechanisms of action are currently in the pipeline.1 This, together with the availability of drugs with well-balanced efficacy/toxicity profiles has recently led to the design of more complex and prolonged treatment strategies.2-7 However, the definition of clinical response criteria and clinical end points has largely remained the same over the past 15 years.8-10 Nevertheless, concepts such as “depth of response,” “minimal residual disease (MRD),” and “surrogate survival markers” have become the subject of extensive research and debate within the MM scientific community (Figure 1) and even the subject of a recent workshop with regulatory agencies.11-15 In this review, we address these concepts and define what remains to be accomplished for optimization of response criteria and full implementation of MRD monitoring in MM into routine clinical practice.
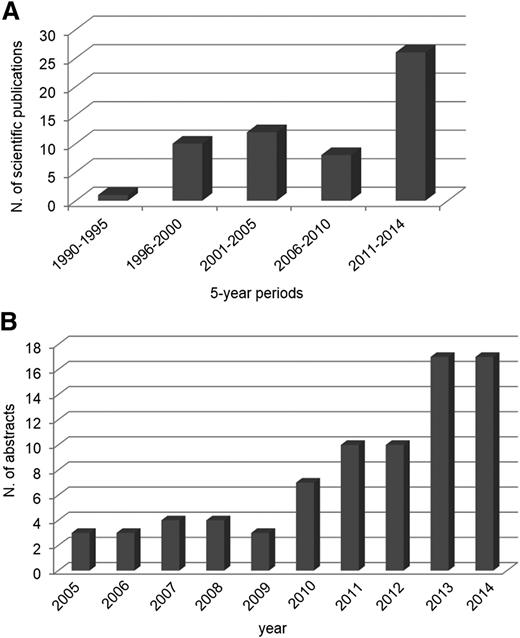
Graphical representation of the increasing number of publications in PUBMED and abstracts reported in the Annual Congress of the American Society of Hematology (ASH) on MM MRD during the past decades. (A) Publications per 5-year periods on MRD studies in MM (PUBMED). (B) Abstracts reported per year at the ASH meetings on MRD studies in MM.
Graphical representation of the increasing number of publications in PUBMED and abstracts reported in the Annual Congress of the American Society of Hematology (ASH) on MM MRD during the past decades. (A) Publications per 5-year periods on MRD studies in MM (PUBMED). (B) Abstracts reported per year at the ASH meetings on MRD studies in MM.
Is depth of response clinically relevant in MM?
For virtually all hematologic malignancies, a direct correlation exists between depth of response and prolonged survival. MM is no exception to such paradigm, and meta-analyses among transplant-eligible and nontransplant candidates have clearly established the link between deep responses such as complete remission (CR) and prolonged survival.16-18 Thus, high-dose therapy (HDT) followed by the incorporation of novel agents into autologous stem cell transplantation (ASCT) trials have significantly improved outcome by achieving higher CR rates.17,19-22 Recent trials with novel agent combinations alone have also resulted in high CR rates (comparable to those previously reported only with HDT/ASCT),23,24 even among patients older than 65,3,25 high-risk patients,26,27 and relapse/refractory MM.28,29 Despite all accumulated evidence, there are still some caveats that should be highlighted. First, achieving the deepest level of remission (ie, CR) is considered to be a prerequisite, not only to prolong survival but also to ultimately achieve cure. Indeed a recent update on Total Therapy trials provides evidence of curability in MM,7 and other long-term analyses have shown that 1 out of 3 patients in CR could potentially be cured (relapse free after 10-years of follow-up).30 Remarkably, also 10% of cases that reach suboptimal response after therapy, such as near CR or (very good) partial response (PR), are relapse free at 10 years.30 This has raised a second question about whether CR is actually needed to achieve long-term survival. Indeed, biologically well-defined patient subgroups with monoclonal gammopathy of undetermined significance (MGUS)-like baseline profiles or specific molecular subtypes can present long-term survival without achieving CR (Figure 2).31-34 However, these patients only represent 10% of total MM patients. Thus, for the vast majority of patients, higher CR rates are indeed needed to increase survival rates and approve (new) treatment regimens.19,21,22,35-38
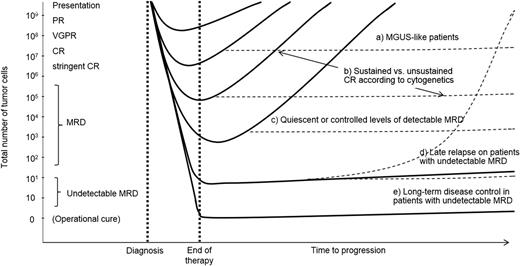
Schematic representation to illustrate the paradigm of the deeper the response, the longer the (progression-free) survival (filled lines). However, distinct biological subgroups exist, and their clinical course may differ from the paradigm (dotted lines): a, those patients with a baseline MGUS-like signature and prolonged survival irrespectively of CR; b, those patients with unsustained CR (high-risk cytogenetics and persistent MRD); c, MRD-positive patients who may also experience extended outcomes if small residual clones are quiescent (MGUS-like) or under control (eg, by immune cells); d, an MRD-negative result does not preclude the risk of relapse, and optimization of MRD monitoring together with follow-up MRD studies are likely crucial to predict relapses early on; e, long-term disease control (ie, functional cure) could potentially be achieved if therapy eradicates (detectable) MRD levels. This is a hypothetical model, which does not translate to the real behavior of individual patients.
Schematic representation to illustrate the paradigm of the deeper the response, the longer the (progression-free) survival (filled lines). However, distinct biological subgroups exist, and their clinical course may differ from the paradigm (dotted lines): a, those patients with a baseline MGUS-like signature and prolonged survival irrespectively of CR; b, those patients with unsustained CR (high-risk cytogenetics and persistent MRD); c, MRD-positive patients who may also experience extended outcomes if small residual clones are quiescent (MGUS-like) or under control (eg, by immune cells); d, an MRD-negative result does not preclude the risk of relapse, and optimization of MRD monitoring together with follow-up MRD studies are likely crucial to predict relapses early on; e, long-term disease control (ie, functional cure) could potentially be achieved if therapy eradicates (detectable) MRD levels. This is a hypothetical model, which does not translate to the real behavior of individual patients.
Do we need a better definition of CR?
A retrospective analysis of 3 randomized European trials for transplant-ineligible patients indicated that ∼40% of CR patients will relapse, and that 20% will die within 4 years after initial therapy.16 Similar results have also been reported for transplant-candidate patients.17,39 In addition, a small fraction of CR patients show early (<1 year) relapse with a very poor survival (≤2 years),40 and similar CR rates after different treatment regimens fail to predict for an overall distinct outcome.4 These data reveal that the quality of CR may largely vary between different regimens. The data also emphasize that current CR criteria, such as negative immunofixation in serum and urine, disappearance of any soft tissue plasmacytomas, and <5% plasma cells (PCs) in bone marrow (BM),8 fail to detect such differences, even among patients who will relapse soon (ie, those who display unsustained CR).
In 2006, the International Myeloma Working Group had already highlighted the need for a new definition of CR and introduced normalization of serum free light chains (sFLCs) and absence of clonal PCs in BM biopsies by immunohistochemistry and/or immunofluorescence as additional requirements to define more stringent CR criteria.8 Since then, only 1 large study has been able to show the superiority of the stringent over conventional CR criteria to define patients’ outcomes,39 whereas other groups failed to demonstrate the utility of the sFLC assay among immunofixation-negative patients,41-43 most likely because the latter groups did not include simultaneous assessment of PC clonality in BM biopsies. Importantly, the vast majority of CR patients after therapy show recovery of normal PCs that exceeds the percentage of clonal PCs,44 implying that solid clonality markers are needed, such as the clonotypic immunoglobulin (Ig) gene sequences. In addition, it has been suggested that the sFLC might be replaced by the heavy-light format45 and become merely a surrogate for recovery of the immune system rather than an MRD monitoring tool.46
Overall, it becomes clear that the definition of CR would benefit from an improvement that matches the dramatic evolution observed in MM treatment. Such improvement can only be achieved by highly sensitive technologies able to detect MRD at very low levels. Recent data by Rawstron et al47 point out that quantitative assessment of tumor load with a cutoff of 10−4 (using multiparameter flow cytometry [MFC]) would be more informative than a positive vs negative categorization, suggesting that a lower cutoff provided by more sensitive assays (eg, next-generation sequencing [NGS] or high-sensitive MFC) will likely improve outcome prediction further. This has already been confirmed by Martinez-Lopez et al using NGS,48 who identified 3 groups of patients with different time to progression (TTP): patients with high (<10−3), intermediate (10−3 to 10−5), and low (>10−5) MRD levels showed significantly different TTP (27, 48, and 80 months, respectively). Accordingly, 10−5 should currently be considered as the target cutoff level for definition of MRD negativity.
Is MRD monitoring ready for prime time in MM?
Over the past decade, MFC and Ig allele-specific oligonucleotide–based quantitative polymerase chain reaction (ASO-PCR) have emerged as the most attractive, well-suited, and sensitive approaches to detect MRD in the BM of MM patients during and after therapy. More recently, preliminary studies have also shown that NGS of Ig genes might be applicable for MRD detection in BM of MM patients. However, because of the frequency of extramedullary relapses, sensitive imaging techniques have also become relevant in assessing low levels of disease outside BM.
MFC
MFC is particularly well-suited to study biological samples containing PCs because this worldwide-available technique allows (1) simultaneous identification and characterization of single PCs based on multiple parameters, (2) evaluation of high cell numbers in a few hours, (3) quantitative assessment of different cell populations and their corresponding antigen expression levels, and (4) combined detection of cell surface and intracellular antigens.49
In recent years, the sensitivity of MFC has increased because of simultaneous assessment of ≥8 markers and evaluation of greater numbers of cells than what was previously feasible with 4-color instruments.50 Single parameters cannot reliably distinguish clonal vs normal PCs, but multiparameter cytometry with evaluation of at least 8 markers in a single tube can readily identify aberrant PC phenotypes at MRD levels if sufficient cell numbers (eg, ≥5 × 106) are evaluated.49 Consensus exists that PC identification markers (CD38 plus CD138) plus discriminatory markers such as CD19, CD27, CD45, CD56, CD81, and CD117 should be simultaneously evaluated for accurate identification of BM PCs and unequivocal distinction between clonal and normal PCs.49-51 It should be noted that normal PCs have a considerably heterogeneous immunophenotype according to the PC maturation process,52,53 but this maturation pathway is highly conserved in all conditions from normal to regenerating and reactive BM samples.50 The understanding of normal PC maturation facilitates the universal MFC-based identification of aberrant PC phenotypes in MM patients (Table 1). This can be further confirmed via the clonal nature of the phenotypically aberrant PCs through cytoplasmic Ig light-chain restriction.50 Because the aberrant phenotypes of clonal PCs are readily distinguishable from normal PCs, flow MRD is applicable in virtually every MM patient without requiring patient-specific diagnostic phenotypic profiles (Table 1). Additionally, discrimination between normal and myeloma PCs is still feasible in the (rare) event of phenotypic shifts from diagnostic to posttreatment MRD samples.54 Most importantly, flow-MRD assays also provide an intra-assay quality check of the whole cell sample via simultaneous detection of B-cell precursors, erythroblasts, myeloid precursors, and/or mast cells. This information is critical to ensure sample quality and to identify hemodiluted BM aspirates that may lead to false-negative results.
Individual features of currently available techniques to monitor MRD in MM
| . | MFC (≥8-color) . | ASO-PCR . | NGS . | PET/CT . |
|---|---|---|---|---|
| Applicability | ∼100% | 60% to 70% | ∼90% | ∼100%* |
| Reproducibility among centers | High | High | Not reported | Moderate at MRD |
| Availability in individual laboratories around the world | High | Intermediate | Limited | Intermediate |
| Diagnostic sample | Important but not mandatory | Mandatory | Mandatory | Important but not mandatory |
| Time | 2-3 h | ≥5 d (follow-up), 3-4 wk (target identification) | ≥7 d | 2 h |
| Cost per sample† | ∼350 USD | ∼500 USD (follow-up), ∼1500 USD at diagnosis (target identification) | ∼700 USD | ∼2000 USD |
| Sensitivity‡ | 10−5 to 10−6 | 10−5 to 10−6 | 10−6 | High (4 mm) |
| Quantitative | Yes (directly; high accuracy) | Yes | Yes | Yes |
| Fresh sample | Needed (<36 h) | Not needed | Not needed | NA |
| Patchy sample | Impacts | Impacts | Impacts | No impact |
| Global cell characterization | Yes | No | No | No |
| Standardization | Ongoing (EuroFlow/IMF) | Yes, since 15 y (EuroMRD) | Not reported | No |
| . | MFC (≥8-color) . | ASO-PCR . | NGS . | PET/CT . |
|---|---|---|---|---|
| Applicability | ∼100% | 60% to 70% | ∼90% | ∼100%* |
| Reproducibility among centers | High | High | Not reported | Moderate at MRD |
| Availability in individual laboratories around the world | High | Intermediate | Limited | Intermediate |
| Diagnostic sample | Important but not mandatory | Mandatory | Mandatory | Important but not mandatory |
| Time | 2-3 h | ≥5 d (follow-up), 3-4 wk (target identification) | ≥7 d | 2 h |
| Cost per sample† | ∼350 USD | ∼500 USD (follow-up), ∼1500 USD at diagnosis (target identification) | ∼700 USD | ∼2000 USD |
| Sensitivity‡ | 10−5 to 10−6 | 10−5 to 10−6 | 10−6 | High (4 mm) |
| Quantitative | Yes (directly; high accuracy) | Yes | Yes | Yes |
| Fresh sample | Needed (<36 h) | Not needed | Not needed | NA |
| Patchy sample | Impacts | Impacts | Impacts | No impact |
| Global cell characterization | Yes | No | No | No |
| Standardization | Ongoing (EuroFlow/IMF) | Yes, since 15 y (EuroMRD) | Not reported | No |
EuroFlow, see www.EuroFlow.org; EuroMRD, see www.EuroMRD.org; IMF, International Myeloma Foundation; PET/CT, positron emission tomography/computed tomography; USD, US dollars. NA, not appropriate.
Specifically for extramedullary disease.
Costs calculated based on both reagent and personnel costs for a medium-size laboratory receiving ∼150 to 200 MRD samples per year.
Defined as minimal percentage of cells detectable within or out of the quantitative range of the method or in size for imaging techniques.
A potential limitation of MFC is that current strategies are designed to characterize the PC compartment and could therefore miss potential MM cancer stem cells with more immature phenotypes, such as postgerminal center memory B cells (Table 1).55 Nevertheless, recent investigations conducted with sensitive ASO-PCR assessment of clonal Ig heavy (IGH) myeloma sequences among fluorescence-activated cell sorter–sorted peripheral blood (PB) B-cell subsets, revealed that such clonotypic cells are either absent or present below highly sensitive limits of detection.56
Highly sensitive MFC-based MRD monitoring (down to 10−5) requires the availability of ≥8-color digital flow cytometers coupled to novel sample preparation procedures that allow fast and cost-effective, routine evaluation of >5 million nucleated cells (for detailed protocols, see www.EuroFlow.org; Table 1). This contrasts with previous MFC studies that defined MRD as the presence of a discrete population of clonal PCs at the 0.01% (ie, 10−4) limit of detection.
The need for extensive expertise to analyze flow cytometric data, together with the lack of well-standardized flow-MRD methods, has been pointed out as the main drawback of MFC immunophenotyping (Table 1).13 Furthermore, conventional visualization of flow cytometric data in bivariate (two-dimensional) dot plots becomes increasingly complex with increasing numbers of parameters.57,58 In recent years, new multivariate computational tools and visualization plots (eg, principal component analysis and canonical analysis) have been developed and integrated into innovative software packages for improved multidimensional identification and classification of different clusters of cells coexisting in a sample. These tools together with the use of normal and malignant reference databases further pave the way for automated detection and tracking of aberrant cell populations that deviate from the normal/reactive phenotypic profiles.50,58 Such innovative flow-MRD strategies are currently being developed by the EuroFlow Consortium under the Black Swan Research Initiative promoted by the International Myeloma Foundation, and it is likely to become the method of choice for accurate, high-sensitive, and automated flow-MRD monitoring in MM.
ASO-PCR
Rearrangements of germ-line V, (D), and J gene segments in the Ig gene complexes (IGH, IGK, and IGL) provide each B cell with specific V(D)J combinations, which together code for the many different variable domains of Ig molecules. The random insertion and deletion of nucleotides at the V(D)J junction sites create highly diverse junctional regions, which represent unique “fingerprint-like” sequences that are most probably different in each B-cell and thus also in each B-cell malignancy. Since the 1990s, these junctional regions (to be identified in each individual patient at diagnosis) have therefore been used as individual tumor-specific targets using Ig ASOs as primers, initially for nested PCR approaches and later for real-time quantitative PCR-based MRD analysis (ASO-PCR). Such Ig targets can be identified and sequenced with standardized technologies in >95% of lymphoid malignancies and used for the design of junctional region–specific oligonucleotides to be applied for sensitive PCR-based detection of low frequencies of malignant cells, down to 1 malignant cell in 104 to 105 normal cells (10−4 to 10−5) (Table 1).59 This time-consuming but sensitive approach has been highly successful for MRD diagnostics in immature B-lineage malignancies, such as acute lymphoblastic leukemia,57,59 and has also been applied in mature B-cell malignancies, such as MM,60,61 reaching good sensitivities and demonstrating predictive value post–stem cell transplantation (SCT)62,63 and in myeloma patients with PR to induction therapy.48,64
However, B-cells can further mature their Ig molecules via somatic hypermutation (SHM) of the functional V(D)J exons, resulting in high-affinity antibodies that are typically produced by postgerminal center memory B cells and PCs. The SHM process also occurs in and around the junctional regions, and therefore, the mutations can change the DNA sequence at the positions of the PCR primers that are used in the MRD studies. This explains why standard PCR primer sets cannot detect each individual IGH, IGK, and IGL gene rearrangement in mature postgerminal center B-cell malignancies.65,66 This is particularly valid for MM, which represents the most mature B-cell stage with heavily mutated Ig genes. To reduce the problem of false-negative results, multiple Ig genes have been targeted in parallel (eg, IGH and IGK), and unmutated Ig rearrangements have also been used as targets (eg, incomplete D-J and deletion of the IGK gene [IGK-Kde] rearrangements).66,67 Nevertheless, it remains difficult to apply the ASO-PCR approach in all MM cases (Table 1), unless other (nonclassical) methods will be used for Ig target detection and sequencing. For example, it might be possible to use Ig leader primers in combination with Constant gene primers at the RNA level to avoid the SHM-mutated sequences; such an approach has not been tested so far.
NGS of Ig genes
Several years ago, high-throughput sequencing (HTS) was introduced for studying the diversity of antigen receptor genes.68 For this purpose, multiplex primer sets65 are used to detect all potential rearrangements in a sample (up to 105 or more). As a logical consequence, HTS has been applied for detection of clonal Ig gene rearrangements, including detection of MRD,69-71 assuming that each rearrangement can be detected and that such detection of rearranged Ig genes is proportional (ie, reflecting their frequency in the original sample). However, comparable to ASO-PCR, HTS is also based on an initial PCR step, using primers that have to anneal to the Ig gene sequences. Logically, some primers are more efficient than others, and SHM in Ig genes further hampers primer annealing in mature B-cell malignancies.65,66 This explains why HTS was not able to detect a reliable Ig PCR target in all MM patients, even when multiple Ig loci (IGH Vh-Jh, Dh-Jh, and IGK) were evaluated.48,70 Another problem in HTS is the quantitation of MRD because the clonal rearrangement is detected between polyclonal Ig rearrangements derived from remaining normal B-cells, of which the frequency might be highly variable, depending on the type of treatment. Finally, whereas the classical ASO-PCR has been standardized and is subjected to frequent (every 6 months) international quality assurance rounds (www.EuroMRD.org), HTS has not (yet) been standardized and lacks quality assurance rounds (Table 1), the MRD HTS data reported so far in MM being restricted to a commercial service-based approach/tool.48,70,72
Recently, NGS has also been evaluated in PB (ie, plasma) from 45 MM patients who received carfilzomid-lenalidomide-dexamethasone (CRD) induction.72 This would represent an attractive minimally invasive approach to overcome the challenge of a patchy BM infiltration. However, preliminary data indicate that clonotypic sequences identified at baseline become undetectable with just a few cycles of chemotherapy, even among electrophoresis-positive patients. Thus, further research is warranted to establish the feasibility of PB (eg, cell- or free DNA–based) MRD monitoring.
MRI and PET/CT
The possibility of patchy BM infiltration or extramedullary involvement with an MRD-negative BM is an additional challenge for both MFC- and PCR-based MRD detection in single BM aspirates. This highlights the value of sensitive imaging techniques to redefine CR among MRD-negative cases by MFC, ASO-PCR, and NGS, both at the intramedullary and extramedullary levels, whenever the subjective nature of the assessments and the concerns regarding reproducibility are overcome. Magnetic resonance imaging (MRI) is the most sensitive noninvasive imaging technique for detection of bone involvement in the spine. It also provides relevant information on the extent and nature of soft tissue disease and the pattern of marrow infiltration (normal, focal, heterogeneous, or diffuse). However, it should be noted that focal lesions may remain hyperintense for several months after therapy, in both responding and nonresponding patients, because of treatment-induced necrosis and inflammation. This can explain some inconsistencies found between serological CR and MRI-based CR.73,74 Consequently, an interval of 3 months has been recommended before MRI monitoring.75
The use of PET/CT combines the imaging of a particular molecular process (eg, fluorodeoxyglucose uptake) with the morphologic images provided by CT data. However, it is important to emphasize that for MRD monitoring (which will pay particular attention to fluorodeoxyglucose uptake rather than lytic bone lesions), both false-negative and false-positive results (in the case of other coexisting infectious or inflammatory processes) may be seen.76
In contrast to traditional imaging techniques, a specific advantage of PET/CT relies on its ability to detect extramedullary disease, which is a sign of spread of the disease outside the BM with an adverse prognostic impact.77 A recent comparison between PET/CT and whole body MRI in transplant-candidate patients showed that, against conventional response criteria, PET/CT had the same sensitivity but higher specificity than whole body MRI. Although the utility of other MRI-based techniques is still under investigation (eg, dynamic contrast-enhanced MRI),78 the current perception is that PET/CT would represent the most effective imaging tool to monitor MRD in MM. However, standardization of response definitions by PET/CT and comparison with other sensitive BM-based MRD methods, including targeted biopsies, is still needed to implement this imaging technique across different clinical studies (Table 1).79
What is the clinical significance of MRD monitoring in MM?
Transplant-eligible patients
Early studies exploring the role of MRD in MM typically used PCR-based methods in the setting of autologous or allogeneic SCT (allo-SCT) because at that time these were considered the only effective treatment approaches (Figure 3).60,63,80-82 With few exceptions,83 most studies concerned relatively small patient series reflecting the challenging nature of PCR-based MRD methods for routine testing (see “ASO-PCR”), but they demonstrated that PCR-MRD monitoring was of prognostic value.60,80-82 In parallel, 3- and 4-color flow-MRD methods were introduced83,84 and shown to be of prognostic value by San Miguel et al85 and Rawstron et al (Figure 3).86 Subsequent comparisons between PCR- and MFC-based MRD monitoring showed that, except for a few discordant cases, both techniques provided highly concordant results (Figure 3).60,64,87,88 The initial positive experience by the Spanish and United Kingdom groups led to the implementation of their corresponding 4- and 6-color MFC approaches in large clinical trials. In the Programa para el Estudio de la Terapéutica en Hemopatías Malignas/Grupo Español de MM (PETHEMA/GEM) 2000 study, flow MRD was identified as the most relevant prognostic factor in a series of 295 newly diagnosed MM patients receiving uniform treatment including HDT/SCT.89 In this trial, MRD negativity after ASCT translated to significantly improved PFS and OS rates. Similarly, in the intensive pathway of the Medical Research Council, UK (MRC) Myeloma IX study, MRD negativity after HDT/ASCT was predictive of favorable PFS and OS.90 More recently, these observations were reproduced using ASO-PCR64 and NGS tools,48 which again confirmed the prognostic value of MRD assessment in transplant-eligible MM patients. Furthermore, Zamagni et al reported that post-ASCT, PET/CT monitoring was also an independent prognostic marker for PFS and OS.91 Recently, similar results have been reported in the allo-SCT setting where the presence of MRD following allo-SCT has been associated with a significantly adverse PFS and OS (Figure 3).92-94
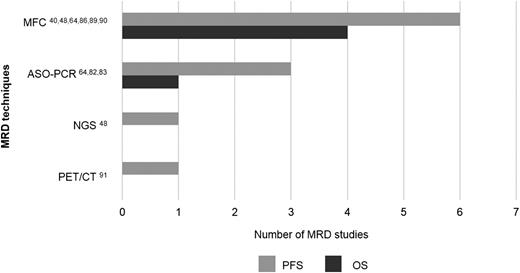
Number of studies published in PUBMED per MRD technique showing prognostic value for progression-free survival (PFS) and overall survival (OS) specifically among patients in CR after therapy. Numbers refer to the literature cited in the present review.
Number of studies published in PUBMED per MRD technique showing prognostic value for progression-free survival (PFS) and overall survival (OS) specifically among patients in CR after therapy. Numbers refer to the literature cited in the present review.
Importantly, all studies showed that PFS of MRD-negative patients at least doubled that of MRD-positive CR patients,40,48,64,88-91 reaching up to a striking 8-year difference in PFS among CR patients by their NGS-MRD status.48 Conversely, both MFC and ASO-PCR showed that CR patients with persistent MRD had significantly inferior OS vs MRD-negative cases.64,89,90 These results support the rationale for implementing MRD assessment to redefine and improve current CR criteria in MM.
Discordant results between MRD (by immunophenotypic, molecular, and imaging techniques) vs conventional response assessment has questioned the sensitivity and specificity of MRD monitoring over traditional paraprotein measurement. However, it has been shown that MRD-negative patients in near CR/PR have a favorable prognosis, which has been hypothesized to be because of the long M-protein half-lives that, in selected patients, could disappear over time43 ; other factors such as the phenomenon of continued response after HDT/ASCT without further therapy, as well as the impact of additional therapy, should also be considered. Irrespectively of all the above-mentioned factors, our most recent observations indicate that approximately two-thirds of MRD-negative cases in near CR/PR achieved CR in a median of 2 months (B.P., L. Rosiñol, M.B. Vidriales, M.A. Montalban, N.C. Gutierrez, M.L. Martín-Ramos, N. Puig, J. Martinez-Lopez, M.V. Mateos, L. Cordón, A. Oriol, M.J. Terol, M.A. Echeveste, J. De la Rubia, J.J. Lahuerta, J. Blade, and J.F. San Miguel, manuscript in preparation). Such observations also highlight the importance of the 2 consecutive protein response assessments before the institution of any new therapy to confirm a response category and also unravel how immunofixation alone may be suboptimal to evaluate the added value of sequential treatment strategies (eg, HDT/ASCT followed by consolidation and/or maintenance).
Elderly nontransplant candidate patients
The prognostic value of MRD assessment was not investigated outside of the SCT setting until recently, when the incorporation of novel agents into the treatment of patients who were not fit for HDT/ASCT showed increased CR rates and prolonged survival.3 Puig et al have recently demonstrated that among patients treated according to the PETHEMA/GEM2005MAS65 protocol, those in molecular CR after induction had a PFS not yet reached, whereas MRD-positive patients had a significantly shorter PFS (median 31 months; P = .03).64 Because MRD levels measured by ASO-PCR- and NGS-based approaches correlate well,70 when Martinez-Lopez studied young and elderly patients separately, the prognostic significance of achieving MRD negativity by deep-sequencing was equally observed.48 Regarding MFC, in the MRC myeloma IX protocol only a few patients achieved flow CR after induction regimens without proteasome inhibitors, and these showed nonsignificantly superior PFS.90 In contrast, in the PETHEMA/GEM2005MAS65 study patients were monitored after 6 induction cycles with bortezomib, melphalan, and prednisone or bortezomib, thalidomide, and prednisone, and, within a subset of 102 cases in CR/(very good) PR, 30% attained MRD negativity with PFS and OS rates at 3 years of 90% and 94%, respectively.41 A recent update of this study3 after a median follow-up >5 years shows median PFS and OS rates not yet reached for patients in flow CR after bortezomib, melphalan, and prednisone (but not bortezomib, thalidomide, and prednisone) induction. These results suggest that MRD monitoring is also clinically relevant in elderly patients. Because MRD-negative cases after 2 different regimens should experience similar outcomes,95 this study also unraveled that the 4-color MFC assay originally performed was underpowered for ultrasensitive detection of MRD.3 This has been recently confirmed by comparing deep-sequencing vs 4-color MFC-based MRD monitoring in younger and elderly MM patients,48 indicating that MRD prognostication is improved when more sensitive (ie, lower) limits of detection (ie, ≤1 tumor cell in 100 000 vs 10 000 normal cells; 10−5 vs 10−4) are reached.
Standard-risk vs high-risk cytogenetic patients
In MM, it has been suggested that attaining deep levels of remission (CR) could be critical only for patients with high-risk disease, whereas those with more indolent biology may not particularly benefit.14,15 However, after the PETHEMA/GEM reported that risk assessment by fluorescence in situ hybridization and flow-MRD monitoring were of independent prognostic value in transplant-eligible patients,89 Rawstron et al have reproduced and confirmed that the presence of MRD is a strong predictor of outcome in patients with both favorable and adverse cytogenetic profiles.90 In fact, the percentage of patients achieving MRD negativity was identical between standard- and high-risk cytogenetic patient subgroups (∼60%).90 Further analyses by the PETHEMA/GEM have shown that combined cytogenetic evaluation of PCs at diagnosis plus MRD assessment after HDT/ASCT (day +100) provided a powerful discriminator of outcomes, which also resulted in a highly effective approach to identify patients with unsustained CR and dismal outcomes (2-years median OS for cases with baseline high-risk cytogenetics plus persistent MRD).39 Collectively, these results confirm the superiority of MRD assessment over conventional response criteria to predict outcome in distinct MM genetic subgroups.
MRD and treatment schema
So far, no clinical trial has randomized MM patients according to their MRD status and, thereby, investigated the role of MRD for individualized therapy. However, many studies have shown the value of MRD diagnostics for evaluation of the efficacy of specific treatment stages and, therefore, potential treatment decisions (Figure 4). For example, both the Spanish and the United Kingdom study groups have shown that MRD kinetics before and after HDT/ASCT allow the identification of chemosensitive (MRD-negative cases at 2 time points), intermediate, and chemoresistant patients (MRD-positive patients at 2 time points).89,90 For the latter, it could be hypothesized that consolidation is needed to improve outcomes that, with maintenance alone, were significantly inferior vs the remaining cases.89,90 When such analysis is restricted to CR patients after induction, those failing to eradicate MRD levels before HDT/ASCT will show significantly superior PFS if MRD negativity is achieved after HDT/ASCT, and their outcome becomes superimposable to that of cases that were already MRD negative before HDT/ASCT (B.P., L. Rosiñol, M.B. Vidriales, M.A. Montalban, N.C. Gutierrez, M.L. Martín-Ramos, N. Puig, J. Martinez-Lopez, M.V. Mateos, L. Cordón, A. Oriol, M.J. Terol, M.A. Echeveste, J. De la Rubia, J.J. Lahuerta, J. Blade, and J.F. San Miguel, manuscript in preparation). These results suggest not only that MRD kinetics is more informative than single time-point assessments, but also that this information may be useful to address specific clinical questions (eg, early vs delayed HDT/ASCT for CR patients after induction).96
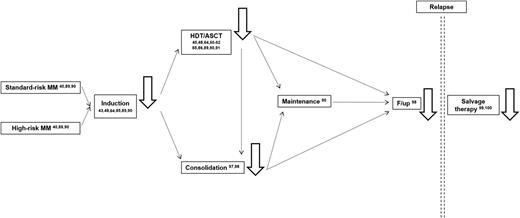
MRD monitoring (ie, black solid line arrows) has been reported (numbers refer to the literature cited in the present review) to be prognostically informative among cytogenetically defined standard- and high-risk MM patients after induction chemotherapy, HDT/ASCT, and consolidation; during follow-up; and after salvage therapy.
MRD monitoring (ie, black solid line arrows) has been reported (numbers refer to the literature cited in the present review) to be prognostically informative among cytogenetically defined standard- and high-risk MM patients after induction chemotherapy, HDT/ASCT, and consolidation; during follow-up; and after salvage therapy.
Maintenance therapy represents another illustrating example. In the Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) VEL-03-096 study, Ladetto et al reported PFS rates at median follow-up of 100% vs 57% for patients in molecular-CR vs MRD-positive cases, respectively.97 In a recent update, these authors confirmed the significantly superior PFS and OS observed for patients attaining molecular CR.98 Because no maintenance therapy was given in this study, one might hypothesize that for those cases that failed to achieve MRD negativity despite being in CR/near CR after consolidation, maintenance could have potentially been an effective approach to eradicate MRD levels and improve outcome. In line with this hypothesis, Rawstron et al have shown that 1 out of 4 MRD-positive patients randomized to the maintenance arm of the intensive treatment pathway of the MRC-myeloma IX study turned into MRD negative and experienced significantly prolonged PFS vs the abstention arm.90
Concluding remarks and future directions
Overall, the experience of several cooperative groups using different MRD techniques indicates that persistence of MRD is always an adverse prognostic feature, even among CR patients. Consequently, it would be safer to make clinical decisions based on MRD positivity rather than on MRD negativity because the patchy pattern of BM infiltration typically observed in MM leads to a degree of uncertainty regarding MRD-negative results (ie, are clonal PCs truly absent, or is it because of nonrepresentative BM sampling?). Some of these limitations could be potentially overcome in flow- and/or molecular-MRD-negative cases by parallel usage of sensitive imaging techniques, although these approaches may also give false-negative results.76,79,91 Thus, it may be envisioned that if treatment decisions are made according to patients’ MRD status, follow-up MRD studies would also become useful to detect MRD reappearance preceding clinical relapse.98
Recently, Barlogie et al have shown that the vast majority of CR patients (94%) achieving long-term survival (10 years relapse free), were also MRD negative.7 By contrast, at least one-third of MM patients achieving CR after initial therapy will not experience a survival benefit because of persistent MRD. However, attaining deep remissions is not a prerequisite for some patients to achieve long-term disease control,7,30 and more accurate identification of such patients should also become a research priority (Figure 2).
MRD clearance is achievable in the era of novel and more effective treatment strategies and it is predictive of superior outcomes. Thus, MRD could potentially be used as a biomarker to evaluate the efficacy of treatment at different stages (induction, transplantation, consolidation, and/or maintenance; Figure 4) and as a surrogate for OS.
Because of their poor prognosis, 2 specific patient subgroups could be ideal to investigate the role of MRD monitoring as a clinical end point for novel treatment modalities and a surrogate biomarker for OS: patients with baseline high-risk cytogenetics and those with relapsed disease. Both patient subgroups reflect the unmet need for novel agents; at the same time, achieving MRD negativity has also resulted in superior outcome in both groups.40,89,90,99,100 The choice of MRD technology for monitoring will depend on how individual centers’ priorities adjust to the specific advantages that each tool has to offer, highly sensitive and automated flow MRD being particularly attractive in assessing BM response (Table 1). In turn, extensive research is still warranted to determine how to best integrate medullary and extramedullary MRD monitoring.
In other hematologic malignancies, baseline risk factors and MRD monitoring have an established and complementary role to individualized treatment. Over the past 2 decades, several groups have consistently confirmed the added value of MRD in MM. Therefore, now also in MM, the time has come to establish the role of baseline risk factors and MRD monitoring for tailored therapy. This requires the introduction of standardized, highly sensitive, cost-effective, and broadly available MRD techniques in all clinical trials.
Acknowledgments
This work was supported by grants from the Cooperative Research Thematic Network (RD12/0036/0058 and RD12/0036/0048 of the Red de Cancer [Cancer Network of Excellence] FEDER); Instituto de Salud Carlos III, Spain, Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS: PI060339, 06/1354, 02/0905, 01/0089/01-02, PS09/01897/01370, G03/136; Sara Borrell: CD13/00340); and Asociación Española Contra el Cáncer (GCB120981SAN), Spain. The study was also supported internationally by the International Myeloma Foundation Black Swan Research Initiative and Junior Grant Proposals and the Multiple Myeloma Research Foundation research fellow award.
Authorship
Contribution: B.P., J.J.M.v.D., and A.O. wrote, reviewed, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Orfao, Cancer Research Center, Paseo de la Universidad de Coimbra s/n, 37007 Salamanca, Spain; e-mail: orfao@usal.es.