To the editor:
Fanconi anemia (FA) is a rare, phenotypically heterogeneous inherited disorder clinically characterized by congenital abnormalities, progressive bone marrow failure (BMF), and a predisposition to develop malignancies.1 Hematopoietic stem cell transplantation (HSCT) is the only curative option for FA patients.2,3 However, finding the best conditioning regimen is still challenging for clinicians. To reduce toxicities, we used progressively lower doses of cyclophosphamide (CY) for conditioning through non-irradiation-based regimens.4-9 A reduced conditioning regimen based on CY (60 mg/kg) alone was proposed by Bomfim et al.10 Survival rates were excellent, but some patients experienced primary (n = 1) or late (n = 4) graft failure (11% of the patients). Moreover, mucositis was a major problem, in that 25 patients (60%) presented grade 3/4 mucositis. The cumulative incidence of chronic graft-versus-host disease (GVHD) was 29%. We hypothesized that tapering the dose of CY to 40 mg/kg and adding fludarabine (FLU) at 90 mg/m2 might improve engraftment, decrease GVHD rates, and eventually improve overall toxicity in FA patients transplanted with human leukocyte antigen (HLA)-matched siblings.
In 2004, the French reference center for aplastic anemia and the French Society of Bone Marrow Transplantation and Cell Therapies (SFGM-TC) recommended using FLU at 90 mg/m2 (30 mg/m2 on days −4, −3, and −2) and CY at 40 mg/kg (10 mg/kg on days −5, −4, −3, and −2) for FA patients transplanted from a matched family donor. Indication for transplantation was based on hematologic complications (transfusions and/or infections). FA patients with morphologic signs of clonal evolution (myelodysplastic syndrome or acute myeloid leukemia) were excluded from the study. All patients in France who received a first allogenic HSCT for FA from a matched related donor between October 2004 and January 2013 using this approach were analyzed (n = 20). Clinical data were prospectively collected using Project Manager Internet Server, an Internet-based data registry system shared by all SFGM-TC centers. Cyclosporin A and mycophenolate mofetil were used as GVHD prophylaxis. Six patients received an in vivo T-cell depletion using antithymocyte globulin because of local policy at their center. The guidelines were approved by the Saint-Louis Hospital Ethics Committee.
The median age at HSCT was 9 years (range 6-19). Stem cell source was bone marrow in 16 cases and matched related cord blood in the remaining transplants. None of the patients received peripheral blood stem cells. All patients had severe or moderate BMF (median hemoglobin: 8.9 g/dL; median platelets: 31 × 103/μL; median neutrophils: 0.88 × 103/μL) at the time of HSCT. Two patients had chromosomal abnormalities (47,XX,i(1)(q10)[10]/48,idem,+8[3] and 47,XX,+der(1;3)(q10;q10)[14]/46,XX[6]); however, neither developed overt myelodysplasia/leukemia before transplant. Patients belonged to complementation groups FANC-A (n = 17) and FANC-G (n = 2). Transplants were performed within a median of 30 months (range 7-143) from FA diagnosis. A median of 3.8. × 108 nucleated marrow cells were infused (range 0.65-8.97).
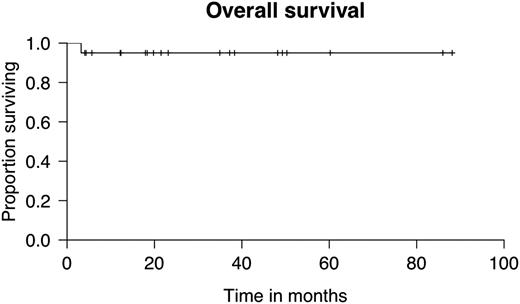
Within a median follow up of 2 years (range 0.2-7.4), overall survival was 95% (Figure 1). Only one patient with an atypical form of FA associated with severe immunodeficiency prior to transplant died subsequently due to uncontrolled cerebral toxoplasmosis. The engraftment rate was 100%, with a median time to neutrophil and platelet recovery of 16.5 days (range 11-28) and 15 days (range 4-29), respectively. No grade 3/4 regimen-related toxicity was observed, and only 1 patient experienced mucositis (grade 2) using this conditioning regimen. Total acute GVHD grade 3/4 was observed in only 3 patients (15%), and chronic GVHD was extensive in 2 patients (10%) and limited in 3 patients (15%). Median alive-patient score on the Karnofsky scale was 100% (range 90% to 100%). No secondary malignancy has been observed in our cohort thus far.
Overall survival after HLA-matched related-donor HSCT conditioned with CY and FLU in FA patients (n = 20).
Overall survival after HLA-matched related-donor HSCT conditioned with CY and FLU in FA patients (n = 20).
The combination of low-dose CY (40 mg/kg) plus FLU (90 mg/m2) in HLA-matched related-donor HSCT in patients with FA resulted in an excellent engraftment rate (100%) with no secondary graft failure, low rates of acute and chronic GVHD, and low rates of regimen-related toxicity, resulting eventually in an excellent overall survival (95% at 2 years). A longer follow-up in this cohort is needed to confirm such excellent results long-term, notably the sustained absence of secondary cancer.
Authorship
Contribution: L.B. wrote the paper and analyzed and interpreted the data; C.S. analyzed the data; J.-H.D., C.J., C.G., and J.S. collected the patients’ data; G.S. designed the study; and R.P.d.L. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lina Benajiba, Service d’Hématologie Greffe, Hôpital Saint Louis, 1, Ave Claude Vellefaux, 75010 Paris, France; e-mail: linabenajiba@yahoo.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal