Key Points
Rapamycin and Flt3L are synergistic in Treg induction when coadministered with antigen, resulting in improved tolerance induction.
pDCs are required for efficient Treg induction and selectively expanded with Flt3L/rapamycin because of high mTOR activity.
Abstract
CD4+CD25+FoxP3+ regulatory T cells (Treg) are critical elements for maintaining immune tolerance, for instance to exogenous antigens that are introduced during therapeutic interventions such as cell/organ transplant or gene/protein replacement therapy. Coadministration of antigen with rapamycin simultaneously promotes deletion of conventional CD4+ T cells and induction of Treg. Here, we report that the cytokine FMS-like receptor tyrosine kinase ligand (Flt3L) enhances the in vivo effect of rapamycin. This occurs via selective expansion of plasmacytoid dendritic cells (pDCs), which further augments the number of Treg. Whereas in conventional DCs, rapamycin effectively blocks mammalian target of rapamycin (mTOR) 1 signaling induced by Flt3L, increased mTOR1 activity renders pDCs more resistant to inhibition by rapamycin. Consequently, Flt3L and rapamycin synergistically promote induction of antigen-specific Treg via selective expansion of pDCs. This concept is supported by the finding that Treg induction is abrogated upon pDC depletion. The combination with pDCs and rapamycin is requisite for Flt3L/antigen-induced Treg induction because Flt3L/antigen by itself fails to induce Treg. As coadministering Flt3L, rapamycin, and antigen blocked CD8+ T-cell and antibody responses in models of gene and protein therapy, we conclude that the differential effect of rapamycin on DC subsets can be exploited for improved tolerance induction.
Introduction
Regulatory T cells (Treg) are critical in central and peripheral tolerance to self-antigens as well as exogenous antigens. Because of their ability to suppress immune responses, ex vivo expanded CD4+CD25+FoxP3+ Treg are used to prevent graft-versus-host disease in bone marrow transplants and are tested in clinical trials for autoimmune diseases. Treg can also be induced in vivo and play important roles in tolerance to cell and organ transplants, oral tolerance, and tolerance to therapeutic proteins in the treatment of genetic diseases.
One method of inducing antigen-specific CD4+CD25+FoxP3+ Treg is to introduce the antigen in the presence of rapamycin. The macrolide immunosuppressant rapamycin (sirolimus) can inhibit intracellular signaling through mammalian target of rapamycin (mTOR; a serine/threonine kinase) complex 1 by binding to the immunophilin FK506 binding protein-12 (FKBP-12).1 Thereby, rapamycin inhibits cycle progression of activated T cells, leading to T-cell anergy or deletion,1 and inhibits the T-cell stimulatory activity of dendritic cells (DCs),2,3 resulting in impaired cytokine-driven cellular activation and selective depletion of T helper (Th) 1, Th2, and Th17 cells.4 This is associated with an increased expansion of CD4+CD25+FoxP3+ Treg in response to reduced mTOR signaling.5-9 Our previous studies have shown that rapamycin, when coadministered with protein or peptide antigen, can suppress inhibitory antibody formation to factor (F) VIII and FIX in treatment of hemophilia A and B.10-12 This approach was further improved by addition of the cytokine interleukin (IL) 10.11,12
Treg homeostasis is controlled by DCs, so that increased numbers of DCs lead to a corresponding accumulation of Treg.13 Hence, expansion of DCs, using the ligand for the FMS-like receptor tyrosine kinase Flt3 (CD135) indirectly leads to expansion of existing peripheral Treg.14,15 These observations prompted us to hypothesize that Treg induction with antigen/rapamycin combined with Treg expansion via Flt3L-induced DC proliferation should be synergistic and may represent an ideal strategy for effective in vivo Treg induction. FLT3 is a transmembrane glycoprotein expressed in stem and early hematopoietic precursor cells in the bone marrow, immature thymocytes, and steady-state DCs.14 Its cognate ligand (Flt3L) is a hematopoietic growth factor with essential functions in early progenitor and DC generation and is involved in the proliferation, differentiation, development, and mobilization of these cells in the bone marrow, peripheral blood, and lymphoid organs.16,17 Flt3/Flt3L signaling is critical to the generation and steady-state expansion of both the conventional (CD11c+, CD8+CD11c+) and plasmacytoid (CD11cmid-loPDCA-1+) subsets of DCs.18,19 Flt3−/− or Flt3L−/− mice show deficient hematopoiesis and reduced DC numbers and, consequently, also reduced Treg numbers.16,20
The molecular signaling pathways underlying Flt3L activity in DC development are only partially defined but include a role for signal transducer and activator of transcription (STAT) 3.21,22 However, a recent report has shown that Flt3L mediates its signaling through the phosphatidylinositol 3-kinase (PI3K)–mTOR pathway and is thus impaired by rapamycin.23 PI3K hyperactivation, through deletion of the negative regulator phosphatase and tensin homolog, causes increased DC proliferation.24 The serine/threonine kinase protein kinase B (PKB, also known as AKT) regulates multiple biological processes by binding various molecules, one of which is the lipid kinase PI3K.24 Importantly, mTOR is a pivotal downstream mediator of the PI3K/AKT pathway.25 Rapamycin-induced inhibition of mTOR signaling in DCs is associated with changes in DC generation, expansion, activation, and maturation.18,26-28 In particular, rapamycin inhibited the expansion of DCs in Flt3L-treated mice by 40% to 50% in 2 prior studies.2,23 These findings would argue against our hypothesis and instead predict that rapamycin should block Flt3L-induced DC and ultimately Treg expansion.
Here, we demonstrate that rapamycin and Flt3L can be used synergistically to substantially improve in vivo Treg induction. In this regimen, rapamycin blocks expansion of conventional DCs (cDCs) but not plasmacytoid DCs (pDCs), resulting in improved Treg induction, which is pDC dependent. Increased mTOR activity in pDCs makes their Flt3L signaling pathway more resistant to rapamycin.
Materials and methods
Mouse strains and experiments
All experimental animals were 6- to 10-week-old male mice and housed under special pathogen-free conditions. BDCA-2 (CLEC4C)-DTR transgenic C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). These mice have a simian diphtheria toxin receptor (DTR) under the transcriptional control of the pDC-sepcific BDCA2 promoter, so that administration of diphtheria toxin (DT) depletes pDCs.29 OT-II (B6.Cg-Tg(TcraTcrb)425Cbn; C57BL/6; Jackson Laboratories) are transgenic for a CD4 T-cell receptor (TCR) specific for chicken ovalbumin (OVA323-339) . Hemophilia A mice with a deletion in exon 16 of the F8 gene (BALB/c F8e16−/−) were as published.30,31 DO11.10-tg x Rag-2−/− BALB/c mice (transgenic for TCR for OVA323-339 presented by MHCII I-Ad) were from Taconic (Hudson, NY). Drug treatments and gene and protein therapy experiments were as published and are explained in further detail in supplemental Methods (see the Blood Web site).11,12,32-34
Flow cytometry
Intracellular staining for forkhead box P3 (FoxP3) was performed with the FoxP3 staining kit (eBioscience, San Diego, CA). Activation markers for CD4+ T cells (L-selectin [CD62L], CD44, and CD69) and Treg (cytolytic T lymphocyte-associated antigen [CTLA] 4, CD62L, Helios, and glucocorticoid-induced tumor necrosis factor receptor [GITR]) were analyzed. Apoptosis of CD4+ T cells were measured with the annexin V (PE)-7-AAD kit (BD Biosciences, San Jose, CA). DC subsets were enumerated using antibodies to CD11c, SiglecH, B220, plasmacytoid dendritic cell antigen 1 (PDCA-1), and Flt3 receptor surface expression. Samples were acquired on the LSR II flow cytometer (BD Biosciences) and analyzed using FCS Express 4 (DeNovo Software, Los Angeles, CA).
Depletion of pDCs
Model 1: In DO11.10-tg x Rag-2−/− mice, pDCs were depleted using purified anti-PDCA-1 antibody (anti-CD317, clone 927; eBioscience).35 Mice received 2 weekly injections of anti-PDCA-1 antibody (500 μg). Model 2: BALB/c mice received 1 × 107 magnetically sorted CD4+CD25− T cells from DO11.10-tg x Rag-2−/− mice. Recipient animals were pDC depleted with 5 IV injections of PDCA-1 antibody (200 μg/mouse), clone 120G8 (Novus Biologicals, Littleton, CO).36 Induced OVA-specific Treg, derived from donor DO11.10 CD4+ T cells, were detected with the KJ1-26 antibody (eBioscience) combined with CD4, FoxP3, and CD25 stains. Model 3: BDCA-2 DTR mice were injected with 1 × 107 cell trace violet (CTV)–labeled CD4+CD25− T cells magnetically sorted from OT-II mice.29,31 Recipient animals were pDC depleted with DT (100 ng/mouse, intraperitoneally [IP], 3 times per week; List Biologicals, Campbell, CA). OVA-specific Treg stain with MR9-4 antibody (eBioscience) and transferred OT-II cells are identified as CTV+. All 3 models: During conditions of pDC depletion, mice were simultaneously treated with Flt3L/OVA323-339/rapamycin (3 times per week) for 3 to 3.5 weeks, followed by analysis of splenic Treg (compared with mice that had not been subjected to pDC depletion).
Western blotting
Splenic DCs were enriched using the mouse pan DC isolation kit (Miltenyi Biotec, Auburn, CA), and pDCs and cDCs purified with the FACS Aria II cell-sorter (BD Biosciences). Cells (5 × 105/mL) were serum starved for 2 hours in RPMI 1640 media, then stimulated with 2 μg/mL Flt3L, 100 nM rapamycin, or Flt3L/rapamycin for 60 minutes. Cell lysates were probed with antibodies against phospho-mTOR (p-mTORSer2448) or β-actin (Cell Signaling Technology, Danvers, MA).
Results
Flt3L enhances activation and apoptosis of OVA323-339-specific CD4+ T cells
Our earlier studies have shown that protein or peptide antigen coadministered with rapamycin was effective in inducing antigen-specific CD4+CD25+FoxP3+ Treg while deleting effector CD4+ T cells.10-12,37 Using OVA-specific TCR-transgenic DO11.10 (DO11.10-tg x Rag-2−/−) mice, which lack Treg, we showed that IL-10 further improved the regimen.11,12 Here, we set out to test whether biological agents other than IL-10 synergize with rapamycin in antigen-specific Treg induction and T effector cell deletion. To this end, DO11.10-tg x Rag-2−/− mice were injected 3 times per week for 4 weeks with rapamycin and the OVA-derived peptide OVA323-339, which is specific for DO11.10. Simultaneously, we coinjected one of the following recombinant proteins: IL-10, IL-2, Flt3L, Fc-glucocorticoid-induced tumor necrosis factor receptor-ligand (Fc-GITR-L), or a combination of Flt3L and Fc-GITR-L (Flt3L/Fc-GITR-L). These molecules were selected for the following reasons: (1) IL-2 is an important growth factor for Treg, which constitutively expresses part of its receptor31 ; (2) Flt3L has been shown to indirectly expand Treg through increasing DC numbers14 ; and (3) the Fc-GITR-L fusion protein dimer preferentially enhances proliferation of Treg, which naturally have highly upregulated expression of GITR.32
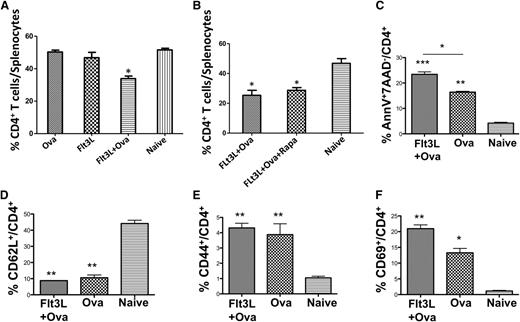
Each of these reagents was able to increase deletion of conventional CD4+ T cells (37% to 75% decrease in OVA-specific CD4+ T cells as compared with untreated animals; Figure 1A) and improve Treg induction (to 7% to 15% of CD4+ T cells; Figure 1B). Compared with IL-10 (the molecule we had originally used), Flt3L and Fc-GITR-L significantly further improved CD4+ T-cell deletion. A combination of Flt3L and Fc-GITR-L did not further increase the effect of either molecule alone. Surprisingly, coadministering antigen, rapamycin, and Flt3L most consistently increased the Treg population, even though Flt3-Flt3L signaling has been suggested to involve the mTOR pathway, which is directly impaired by rapamycin (Figure 1B-C).32 To better understand how Flt3L and rapamycin might work synergistically in Treg induction, we administered Flt3L alone, in combination with OVA323-339, or with both OVA323-339 and rapamycin. We found that Flt3L in combination with the OVA323-339 peptide was sufficient to deplete conventional CD4+ T cells by 35% to 45% (Figure 2A-B), whereas addition of rapamycin had no further effect on the T-cell depletion (Figure 2B and supplemental Figure 1). CD4+ T cells from both mice treated with OVA323-339 or Flt3L/OVA323-339 had an activated phenotype (decreased CD62L+, increased CD69+, CD44+ expression; Figure 2D-F). Addition of Flt3L increased CD69 expression and the proportion of AnnexinV+ CD4+ T cells to >20% (Figure 2C). We conclude that coadministering Flt3L and OVA323-339 caused activation and apoptosis of OVA323-339-specific conventional CD4+ T cells, consistent with activation-induced cell death.
Multiple molecules can be used synergistically with rapamycin to decrease conventional CD4+ T cells and induce Treg. Percentage of CD4+ T cells (A) or CD25+FoxP3+ Treg/CD4+ T cells (B) in spleens of DO11.10-tg x Rag-2−/− BALB/c mice IP injected 3 times per week for 4 weeks with 100 μg of OVA323-339 plus rapamycin (4 mg/kg) and either Flt3L (80 μg/kg), Fc-GITR-L (8 mg/kg), a combination of Flt3L/Fc-GITR-L, IL-2 (50 ng/kg), or IL-10 (50 ng/kg). Untreated naïve animals serve as controls (n = 4-5 per group). Data are average ± standard deviation (SD). Statistically significant differences were determined by 2-way analysis of variance (ANOVA). (C) Examples of Treg induction with Flt3L/OVA323-339/rapamycin compared with OVA323-339/rapamycin and untreated control mouse.
Multiple molecules can be used synergistically with rapamycin to decrease conventional CD4+ T cells and induce Treg. Percentage of CD4+ T cells (A) or CD25+FoxP3+ Treg/CD4+ T cells (B) in spleens of DO11.10-tg x Rag-2−/− BALB/c mice IP injected 3 times per week for 4 weeks with 100 μg of OVA323-339 plus rapamycin (4 mg/kg) and either Flt3L (80 μg/kg), Fc-GITR-L (8 mg/kg), a combination of Flt3L/Fc-GITR-L, IL-2 (50 ng/kg), or IL-10 (50 ng/kg). Untreated naïve animals serve as controls (n = 4-5 per group). Data are average ± standard deviation (SD). Statistically significant differences were determined by 2-way analysis of variance (ANOVA). (C) Examples of Treg induction with Flt3L/OVA323-339/rapamycin compared with OVA323-339/rapamycin and untreated control mouse.
Flt3L enhances activation-induced cell death of CD4+ T cells in response to antigen, an effect that is not further enhanced by rapamycin. (A-B) Depletion of OVA-specific CD4+ T cells in spleens of DO11.10-tg Rag-2−/− BALB/c mice treated with OVA323-339, Flt3L, Flt3L/OVA323-339, or a combination of Flt3L/OVA323-339/rapamycin. Untreated, naïve animals served as controls (n = 5 per experimental group). (C) Percentage of CD4+ T cells showing early apoptosis (Annexin V+7-AAD−) after treatment with OVA323-339 or Flt3L/OVA323-339, compared with untreated control animals. Expression of activation markers CD62L (D), CD44 (E) and CD69 (F) in mice treated with OVA323-339 or Flt3L/OVA323-339, or in naïve, untreated animals. Data are average ± SD. Statistical differences were determined by 2-way ANOVA with Dunnett’s multiple comparison posttest analysis, using data from naïve mice as a control group, against which the other treatment groups were tested.
Flt3L enhances activation-induced cell death of CD4+ T cells in response to antigen, an effect that is not further enhanced by rapamycin. (A-B) Depletion of OVA-specific CD4+ T cells in spleens of DO11.10-tg Rag-2−/− BALB/c mice treated with OVA323-339, Flt3L, Flt3L/OVA323-339, or a combination of Flt3L/OVA323-339/rapamycin. Untreated, naïve animals served as controls (n = 5 per experimental group). (C) Percentage of CD4+ T cells showing early apoptosis (Annexin V+7-AAD−) after treatment with OVA323-339 or Flt3L/OVA323-339, compared with untreated control animals. Expression of activation markers CD62L (D), CD44 (E) and CD69 (F) in mice treated with OVA323-339 or Flt3L/OVA323-339, or in naïve, untreated animals. Data are average ± SD. Statistical differences were determined by 2-way ANOVA with Dunnett’s multiple comparison posttest analysis, using data from naïve mice as a control group, against which the other treatment groups were tested.
Rapamycin is required for Treg induction
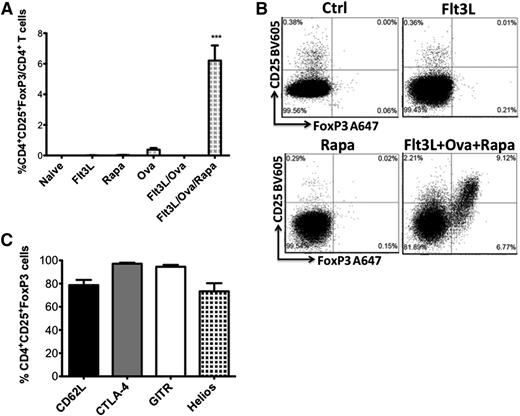
The induction of OVA323-339-specific CD4+CD25+FoxP3+ Treg was dependent on the presence of rapamycin in the treatment cocktail, even though Flt3L without rapamycin was sufficient to delete activated CD4+ T effector cells. Neither Flt3L alone nor Flt3L/OVA323-339 were sufficient in inducing Treg in DO11.10-tg x Rag-2−/− mice (Figure 3A-B and supplemental Figure 2A). Rapamycin by itself also failed to induce Treg. When a combination of Flt3L/OVA323-339/rapamycin was administered, Treg increased from undetectable to 5% to 9% (mean 6.2 ± 0.99) of CD4+ T cells. These results were confirmed in 2 independent experiments (Figure 3A-B and supplemental Figure 2A). Induced Treg expressed CD62L, CD154/CTLA-4, GITR, and Helios (Figure 3C and supplemental Figure 2B).
Rapamycin is required for Treg induction. Flt3L alone or with antigen fails to induce Treg. (A) Induction of OVA-specific CD4+CD25+FoxP3+ Treg cells in DO11.10-tg Rag-2−/− BALB/c mice treated with Flt3L/OVA323-339/rapamycin combination. Mice treated with OVA323-339, Flt3L, Flt3L/OVA323-339, or rapamycin only failed to generate Treg (n = 3-5 per group). Statistical differences were determined by 1-way ANOVA with Bonferonni’s multiple comparison posttest analysis. All groups tested were significantly different from the Flt3L/OVA323-339/Rapa treatment group (P < .0001). (B) Representative examples of stains for CD25 and FoxP3 (gated off CD4+ cells) for naïve controls, Flt3L-treated, rapamycin-treated, and Flt3L/OVA323-339/rapamycin-treated mice. (C) Expression of CTLA-4, Helios, CD62L, and GITR molecules in the induced Treg. Data are average ± SD (n = 5 per group).
Rapamycin is required for Treg induction. Flt3L alone or with antigen fails to induce Treg. (A) Induction of OVA-specific CD4+CD25+FoxP3+ Treg cells in DO11.10-tg Rag-2−/− BALB/c mice treated with Flt3L/OVA323-339/rapamycin combination. Mice treated with OVA323-339, Flt3L, Flt3L/OVA323-339, or rapamycin only failed to generate Treg (n = 3-5 per group). Statistical differences were determined by 1-way ANOVA with Bonferonni’s multiple comparison posttest analysis. All groups tested were significantly different from the Flt3L/OVA323-339/Rapa treatment group (P < .0001). (B) Representative examples of stains for CD25 and FoxP3 (gated off CD4+ cells) for naïve controls, Flt3L-treated, rapamycin-treated, and Flt3L/OVA323-339/rapamycin-treated mice. (C) Expression of CTLA-4, Helios, CD62L, and GITR molecules in the induced Treg. Data are average ± SD (n = 5 per group).
Flt3L selectively expands pDCs in the presence of rapamycin
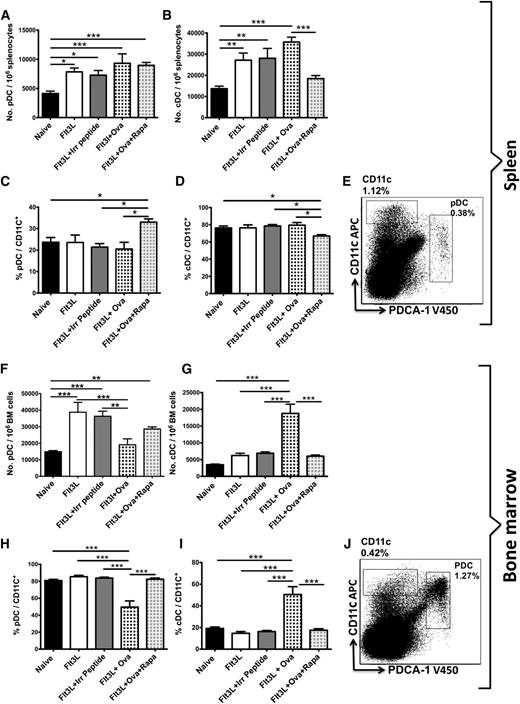
Upon administering Flt3L (80 μg/kg, 3 per week for 3 weeks) to DO11.10-tg x Rag-2−/− mice,14,38 we found a significant expansion of pDCs (CD11c+PDCA-1+) in the spleen and bone marrow (Figure 4A,F). Whereas the number of cDCs (CD11chi) in the spleen had increased, Flt3L caused only a minor expansion of these cells in the bone marrow (Figure 4B,G). Coinjecting the OVA323-339 antigen together with Flt3L further expanded the number of cDCs in the spleen and bone marrow (Figure 4B,G), while limiting expansion of pDCs in bone marrow (Figure 4F). Surprisingly, treatment of DO11.10-tg x Rag-2−/− mice with Flt3L/OVA323-339/rapamycin abrogated cDC expansion (Figure 4B,G) while allowing expansion of pDCs to occur to a level similar to that in mice receiving Flt3L alone (Figure 4A,F). Thus, in response to Flt3L/OVA323-339/rapamycin, the DC population became enriched for pDCs with a corresponding proportional reduction of cDCs (Figure 4C-D,H-I). The sum of results obtained thus far suggest that selective pDC expansion augments Treg induction.
Flt3L expands pDCs and cDCs in the spleen and bone marrow. Rapamycin blocks cDC expansion (but not pDC expansion). Total numbers of pDC (CD11c+PDCA-1+) (A) and cDC (CD11chi) (B) DC subsets in DO11.10-tg Rag-2−/− BALB/c mice per 106 splenocytes. Mice (n = 6-10) were treated 3 times per week for 3 weeks IP with Flt3L, Flt3L/OVA323-339, Flt3L/OVA323-339/ rapamycin, or Flt3L/irrelevant peptide (FIX peptide). Enumeration of pDCs (C) and cDCs (D) as a percentage of total DCs (CD11c+). (E) Representative dot plot of naïve DO11.10-tg Rag-2−/− BALB/c mice splenocytes showing gating scheme for pDCs and cDCs. Total numbers of pDC (CD11c+PDCA-1+) (F) and cDC (CD11chi) (G) DC subsets in DO11.10-tg Rag-2−/− BALB/c mice per 106 bone marrow cells. Mice (n = 6-10) were treated 3 times per week for 3 weeks IP with Flt3L, Flt3L/ OVA323-39, Flt3L/OVA323-339/ rapamycin, or Flt3L with an irrelevant peptide (FIX peptide). Enumeration of pDCs (H) and cDCs (I) as a percentage of total DCs (CD11c+). (J) Representative dot plot of naïve DO11.10-tg Rag-2−/− BALB/c mice bone marrow cells showing gating scheme for pDCs and cDCs. Plots are representative of data from 6 animals per experimental group. Statistical differences were determined by 2-way ANOVA with Bonferonni’s posttest comparisons.
Flt3L expands pDCs and cDCs in the spleen and bone marrow. Rapamycin blocks cDC expansion (but not pDC expansion). Total numbers of pDC (CD11c+PDCA-1+) (A) and cDC (CD11chi) (B) DC subsets in DO11.10-tg Rag-2−/− BALB/c mice per 106 splenocytes. Mice (n = 6-10) were treated 3 times per week for 3 weeks IP with Flt3L, Flt3L/OVA323-339, Flt3L/OVA323-339/ rapamycin, or Flt3L/irrelevant peptide (FIX peptide). Enumeration of pDCs (C) and cDCs (D) as a percentage of total DCs (CD11c+). (E) Representative dot plot of naïve DO11.10-tg Rag-2−/− BALB/c mice splenocytes showing gating scheme for pDCs and cDCs. Total numbers of pDC (CD11c+PDCA-1+) (F) and cDC (CD11chi) (G) DC subsets in DO11.10-tg Rag-2−/− BALB/c mice per 106 bone marrow cells. Mice (n = 6-10) were treated 3 times per week for 3 weeks IP with Flt3L, Flt3L/ OVA323-39, Flt3L/OVA323-339/ rapamycin, or Flt3L with an irrelevant peptide (FIX peptide). Enumeration of pDCs (H) and cDCs (I) as a percentage of total DCs (CD11c+). (J) Representative dot plot of naïve DO11.10-tg Rag-2−/− BALB/c mice bone marrow cells showing gating scheme for pDCs and cDCs. Plots are representative of data from 6 animals per experimental group. Statistical differences were determined by 2-way ANOVA with Bonferonni’s posttest comparisons.
Treg induction by Flt3L/antigen/rapamycin is pDC dependent
In order to determine directly whether Treg induction was indeed dependent on the number of pDCs, we depleted pDCs by intravenously injecting animals with a monoclonal antibody directed against PDCA-1 (clone 927; 2 500 μg doses separated by 2 weeks). Mice continued to receive Flt3L/OVA323-339/rapamycin during the course of anti-PDCA-1 injections for a complete treatment schedule of 3.5 weeks (Figure 5A). In mice that had received the PDCA-1 antibody, splenic pDCs were depleted by ∼40% (supplemental Figure 3A), as compared with mice that had received only the Flt3L/OVA323-339/rapamycin combination. Strikingly, the spleens of pDC-depleted mice contained sevenfold fewer Treg (Figure 5B), demonstrating that Treg induction with this protocol was indeed largely pDC dependent. We found the same effect in BALB/c mice in which pDCs had been depleted with a second antibody, namely the anti-PDCA-1 clone 120G8. Into these mice, 1 × 107 CD4+CD25− cells purified from DO11.10-tg x Rag2−/− mice had been adoptively transferred (Figure 5C), and during the course of the experiment, Flt3L/OVA323-339/rapamycin was administered (Figure 5C and supplemental Figure 4). When transplanted cells were identified with the clonotypic KJ1-26 antibody, the induction of OVA323-339-specific Treg (KJ1-26+CD25+FoxP3+) was significantly reduced in pDC-depleted recipient mice (∼76% depletion; supplemental Figure 3B), when compared with control recipients (Figure 5D).
Treg induction by Flt3L/antigen/rapamycin cocktail is pDC dependent. (A) Experimental timeline of DO11.10-tg Rag-2−/− BALB/c mice that received 2 weekly injections of PDCA-1 antibody (clone 927) to deplete pDCs. Mice were injected 3 times per week for 3.5 weeks with Flt3L/OVA323-339/rapamycin during the course of PDCA-1 antibody administration. Flt3L/OVA323-339/rapamycin was continued for 2 more weeks after PDCA-1 antibody treatment. (B) Treg induction was substantially lower in pDC-depleted mice after Flt3L/OVA323-339/rapamycin treatment. Data are average ± SD (n = 6 per group). Statistical differences were determined by the Student t test. (C) Experimental timeline of BALB/c mice that received 5 IV injections of PDCA-1 antibody (clone 120G8) over 3 weeks. Mice were infused with 1 × 107 CD4+CD25− effector T cells from DO11.10-tg x Rag2−/− mice 1 day after the first PDCA-1 antibody injection. Mice continued to receive Flt3L/OVA323-339/rapamycin combination during the course of PDCA-1 antibody administration. Control mice only received Flt3L/OVA323-339/rapamycin treatment. (D) Induction of OVA specific (KJ1-26+) Treg from transplanted donor (DO11.10) cells was significantly lower in pDC-depleted mice after Flt3L/OVA323-339/rapamycin treatment. Data are average ± SD (n = 8 per group). Statistical differences were determined by the Student t test. (E) Experimental timeline of BDCA-2-DTR mice that received IV injections of DT (3 times per week for 3 weeks) to deplete pDCs. Mice continued to receive Flt3L/OVA323-339/rapamycin combination during the course of pDC depletion. Control mice only received Flt3L/OVA323-339/rapamycin treatment. (F) Increased OVA specific (MR9-4+) Treg in Flt3L/OVA323-339/rapamycin treated control mice as compared with naïve animals, which is abrogated by pDC depletion. Data are average ± SD (n = 4 per group). Statistical differences were determined by 1-way ANOVA with Bonferonni’s multiple comparison posttest analysis.
Treg induction by Flt3L/antigen/rapamycin cocktail is pDC dependent. (A) Experimental timeline of DO11.10-tg Rag-2−/− BALB/c mice that received 2 weekly injections of PDCA-1 antibody (clone 927) to deplete pDCs. Mice were injected 3 times per week for 3.5 weeks with Flt3L/OVA323-339/rapamycin during the course of PDCA-1 antibody administration. Flt3L/OVA323-339/rapamycin was continued for 2 more weeks after PDCA-1 antibody treatment. (B) Treg induction was substantially lower in pDC-depleted mice after Flt3L/OVA323-339/rapamycin treatment. Data are average ± SD (n = 6 per group). Statistical differences were determined by the Student t test. (C) Experimental timeline of BALB/c mice that received 5 IV injections of PDCA-1 antibody (clone 120G8) over 3 weeks. Mice were infused with 1 × 107 CD4+CD25− effector T cells from DO11.10-tg x Rag2−/− mice 1 day after the first PDCA-1 antibody injection. Mice continued to receive Flt3L/OVA323-339/rapamycin combination during the course of PDCA-1 antibody administration. Control mice only received Flt3L/OVA323-339/rapamycin treatment. (D) Induction of OVA specific (KJ1-26+) Treg from transplanted donor (DO11.10) cells was significantly lower in pDC-depleted mice after Flt3L/OVA323-339/rapamycin treatment. Data are average ± SD (n = 8 per group). Statistical differences were determined by the Student t test. (E) Experimental timeline of BDCA-2-DTR mice that received IV injections of DT (3 times per week for 3 weeks) to deplete pDCs. Mice continued to receive Flt3L/OVA323-339/rapamycin combination during the course of pDC depletion. Control mice only received Flt3L/OVA323-339/rapamycin treatment. (F) Increased OVA specific (MR9-4+) Treg in Flt3L/OVA323-339/rapamycin treated control mice as compared with naïve animals, which is abrogated by pDC depletion. Data are average ± SD (n = 4 per group). Statistical differences were determined by 1-way ANOVA with Bonferonni’s multiple comparison posttest analysis.
In a third set of experiments, pDCs were depleted by injecting DT into BDCA-2-DTR mice, which caused up to 97% depletion of these cells (supplemental Figure 3C). In control mice, Flt3L/OVA323-339/rapamycin induced an OVA-specific Treg response as judged by analysis with MR9-4 antibody (which is specific for V beta 5.1/5.2, which is part of the I-Ab/OVA323-339-specific CD4+ TCR; Figure 5E-F and supplemental Figure 5B). Importantly, induction of MR9-4+CD25+FoxP3+ cells was abrogated upon pDC depletion (Figure 5F). By contrast, we did not find an effect of pDC depletion on Treg induction among CTV-labeled OT-II CD4+CD25− T cells adoptively transferred into these BDCA-2-DTR mice (supplemental Figure 5A,C). However, far fewer transferred OT-II cells were found at the end of the experiment when compared with DO11.10-tg CD4+ T cells transferred into BALB/c mice (supplemental Figure 3B-C), suggesting that the drug treatment more effectively deleted OT-II cells, which may have limited conversion to Treg.
Finally, in 2 of these experimental systems, pDC depletion further reduced OVA323-339-specific CD4+ T cells (supplemental Figure 3A,C), suggesting that pDCs to some extent may also limit deletion of effector T cells.
Rapamycin only partially blocks mTOR phosphorylation in pDCs
Recent reports have shown that rapamycin inhibits Flt3-Flt3L signaling in DCs by interfering with the PI3K-mTOR pathway. However, because substantially higher doses of rapamycin are required to block Flt3L-induced expansion of pDCs than of cDCs (Figure 6A), we examined potential underlying mechanisms causing this differential effect. First, higher expression of Flt3, the receptor for Flt3L, on the surface of pDCs compared with cDCs might be a contributing factor (supplemental Figure 6). Second, when purified pDCs and cDCs (5 × 105 cells per mL) were cultured ex vivo with Flt3L, p-mTORSer2448 expression was differentially upregulated (ie, to a more limited extent in cDCs than in pDCs; Figure 6B-C and supplemental Figure 7). Third, whereas rapamycin downregulated p-mTORSer2448 in pDCs or cDCs, rapamycin only partially blocked Flt3L-induced p-mTORSer2448 signaling in pDCs, whereas complete blockage was observed in cDCs.
Flt3 receptor expression and mTOR signaling in pDC and cDC subsets. (A) Effect of increasing rapamycin dose on pDC and cDC numbers. DO11.10-tg x Rag-2−/− BALB/c mice (n = 4 per group) were treated three times per week for 3 weeks IP with Flt3L/OVA323-339/increasing doses of rapamycin: 4 mg/kg (1× rapa), 8 mg/kg (2× rapa) and 16 mg/kg (3× rapa). Total numbers of pDCs and cDCs were enumerated and statistical differences compared by 1-way ANOVA with Dunnett’s multiple comparison posttest using naïve animals as the control group, against which all other treatment groups were tested. (B) Histogram overlays showing differential p-mTORSer2448 expression in splenic pDCs and cDCs of naïve DO11.10-tg x Rag-2−/− BALB/c mice on in vitro incubation for 60 minutes with Flt3L (red histogram), rapamycin (blue histogram), or a combination of Flt3L/rapamycin (purple histogram). Shown is 1 representative of 2 independent experiments. (C) Graphical representation of p-mTORSer2448 expression in pDC and cDC populations from panel B. Data show showing percent positive cells for each treatment, with histogram subtraction applied against the control group, which represents unstimulated cells. Histogram subtraction was applied using FCS express 4.0 software. The graphs represent data from 2 independent experiments. One-way ANOVA with Tukey’s multiple comparison test was used to calculate significance. (D) Representative western blot images of splenic pDCs and cDCs probed for p-mTORSer2448 (upper) and actin (lower). Flow-sorted pDCs and cDCs from spleens of naïve, non-pretreated DO11.10-tg x Rag-2−/− mice (top) or mice that received repeated Flt3L injections for 10 days (bottom), were serum starved for 2 hours and treated for 60 minutes with 2 μg/mL Flt3l, 100 nM rapamycin, or a combination of Flt3L/rapamycin. Images for pmTORSer2448 and β-actin were analyzed by the ImageJ densitometric software. Normalized relative density of pmTOR to β-actin is represented for both sets of western blot images.
Flt3 receptor expression and mTOR signaling in pDC and cDC subsets. (A) Effect of increasing rapamycin dose on pDC and cDC numbers. DO11.10-tg x Rag-2−/− BALB/c mice (n = 4 per group) were treated three times per week for 3 weeks IP with Flt3L/OVA323-339/increasing doses of rapamycin: 4 mg/kg (1× rapa), 8 mg/kg (2× rapa) and 16 mg/kg (3× rapa). Total numbers of pDCs and cDCs were enumerated and statistical differences compared by 1-way ANOVA with Dunnett’s multiple comparison posttest using naïve animals as the control group, against which all other treatment groups were tested. (B) Histogram overlays showing differential p-mTORSer2448 expression in splenic pDCs and cDCs of naïve DO11.10-tg x Rag-2−/− BALB/c mice on in vitro incubation for 60 minutes with Flt3L (red histogram), rapamycin (blue histogram), or a combination of Flt3L/rapamycin (purple histogram). Shown is 1 representative of 2 independent experiments. (C) Graphical representation of p-mTORSer2448 expression in pDC and cDC populations from panel B. Data show showing percent positive cells for each treatment, with histogram subtraction applied against the control group, which represents unstimulated cells. Histogram subtraction was applied using FCS express 4.0 software. The graphs represent data from 2 independent experiments. One-way ANOVA with Tukey’s multiple comparison test was used to calculate significance. (D) Representative western blot images of splenic pDCs and cDCs probed for p-mTORSer2448 (upper) and actin (lower). Flow-sorted pDCs and cDCs from spleens of naïve, non-pretreated DO11.10-tg x Rag-2−/− mice (top) or mice that received repeated Flt3L injections for 10 days (bottom), were serum starved for 2 hours and treated for 60 minutes with 2 μg/mL Flt3l, 100 nM rapamycin, or a combination of Flt3L/rapamycin. Images for pmTORSer2448 and β-actin were analyzed by the ImageJ densitometric software. Normalized relative density of pmTOR to β-actin is represented for both sets of western blot images.
These cytofluorometry-based conclusions on p-mTORSer2448 expression were confirmed by western blotting (Figure 6D). Naïve mice had low basal expression of p-mTORSer2448 in pDCs, which was substantially increased upon Flt3L treatment ex vivo (Figure 6D, top) and comparatively resistant to inhibition by rapamycin. In contrast, p-mTORSer2448 induction in cDCs was considerably lower upon Flt3L treatment and completely inhibited by rapamycin or Flt3L/rapamycin treatment. Densitometric analysis of mTORSer2448 expression relative to β-actin expression was used to quantitate western blot data (Figure 6D). In DCs isolated from mice that had been injected daily for 10 days with 80 μg Flt3L/kg, a higher basal level of p-mTORSer2448 was observed in both pDCs and cDCs (Figure 6D, bottom). However, a similar pattern of differential p-mTORSer2448 inhibition was observed. Taken together, the data show that rapamycin less effectively inhibited the mTOR signaling pathway in pDCs as compared with cDCs.
Flt3L combined with rapamycin prevents immune responses in gene and protein therapy
To evaluate whether empowering Treg cells in vivo would block CD8+ T-cell responses, C57BL/6 mice were pretreated with combinations of Flt3L/OVA323-339, OVA323-339/rapamycin, or Flt3L/OVA323-339/rapamycin, followed by intramuscular injection of our scAAV1-CMV-OVA vector.34 When OVA-specific CD8+ T-cell responses were quantified by tetramer stain (Figure 7A), control mice (vector only treated) and Flt3L/OVA323-339-treated mice showed a substantial response at both the 2-week (11.2 ± 8% and 10.7 ± 8.2%) and 4-week time points (4.4 ± 2.6% and 8.4 ± 2.9%; Figure 7B). In contrast, tetramer positive CD8+ T-cell responses were low to undetectable in OVA323-339/rapamycin- and Flt3L/OVA323-339/rapamycin-treated mice at both the 2-week (0.1 ± 0.1%) and 4-week time points (0.9 ± 1.4% and 1.3 ± 2.1%, undetectable in 2/3 mice in each group; Figure 7B).
Flt3L combined with rapamycin prevents antigen-specific immune response in gene and protein therapy models. (A) Experimental timeline of C57BL/6 mice that were treated with combinations of Flt3L/OVA323-339, OVA323-339/rapamycin, or Flt3L/OVA323-339/rapamycin (3 times per week for 3 weeks). Treatment was followed by challenge with scAAV1-CMV-OVA, delivered by intramuscular injection. (B) OVA323-339-specific CD8+ responses from blood were enumerated at 2 and 4 weeks postvector injection. Representative dot plots of OVA323-339-specific tetramer labeling are shown for 2 treatment conditions: Flt3L/OVA323-339 and Flt3L/OVA323-339/rapamycin. (C) Experimental timeline. Male F8e16−/−BALB/c mice were treated 3 times per week IV with combinations of Flt3L/rapamycin/low-dose FVIII (0.3 IU/mL), or rapamycin/FVIII, or Flt3L/FVIII for 4 weeks (using 0.3 IU FVIII per dose per mouse). Mice were subsequently challenged with FVIII replacement therapy (1 IU IV, 1 time per week for 4 more weeks). Control mice received the FVIII challenge only (ie, without prior immune tolerance regimen). Blood was collected on weeks 7 and 9. (D) Antibody titers against FVIII were determined by Bethesda assay and by FVIII-specific IgG1 enzyme-linked immunosorbent assay. (E) Timeline for male F8e16−/−BALB/c mice that were treated three times per week IV with Flt3L/low-dose FVIII (0.3 IU/mL) for 4 weeks. Mice were subsequently challenged with FVIII replacement therapy (1 IU IV, 1 time per week for 4 more weeks). (F) Mice pretreated with the Flt3L/FVIII combination developed high-titer inhibitor inhibitors (“Pre’), which further increased after FVIII challenge (“Post”). IgG1 responses were similarly elevated.
Flt3L combined with rapamycin prevents antigen-specific immune response in gene and protein therapy models. (A) Experimental timeline of C57BL/6 mice that were treated with combinations of Flt3L/OVA323-339, OVA323-339/rapamycin, or Flt3L/OVA323-339/rapamycin (3 times per week for 3 weeks). Treatment was followed by challenge with scAAV1-CMV-OVA, delivered by intramuscular injection. (B) OVA323-339-specific CD8+ responses from blood were enumerated at 2 and 4 weeks postvector injection. Representative dot plots of OVA323-339-specific tetramer labeling are shown for 2 treatment conditions: Flt3L/OVA323-339 and Flt3L/OVA323-339/rapamycin. (C) Experimental timeline. Male F8e16−/−BALB/c mice were treated 3 times per week IV with combinations of Flt3L/rapamycin/low-dose FVIII (0.3 IU/mL), or rapamycin/FVIII, or Flt3L/FVIII for 4 weeks (using 0.3 IU FVIII per dose per mouse). Mice were subsequently challenged with FVIII replacement therapy (1 IU IV, 1 time per week for 4 more weeks). Control mice received the FVIII challenge only (ie, without prior immune tolerance regimen). Blood was collected on weeks 7 and 9. (D) Antibody titers against FVIII were determined by Bethesda assay and by FVIII-specific IgG1 enzyme-linked immunosorbent assay. (E) Timeline for male F8e16−/−BALB/c mice that were treated three times per week IV with Flt3L/low-dose FVIII (0.3 IU/mL) for 4 weeks. Mice were subsequently challenged with FVIII replacement therapy (1 IU IV, 1 time per week for 4 more weeks). (F) Mice pretreated with the Flt3L/FVIII combination developed high-titer inhibitor inhibitors (“Pre’), which further increased after FVIII challenge (“Post”). IgG1 responses were similarly elevated.
Next, we evaluated the protocol for suppression of inhibitor formation in FVIII protein replacement therapy. Hemophilia A mice (BALB/c F8e16−/−, n = 6) were IV injected with a cocktail of Flt3L/low-dose FVIII/rapamycin for 4 weeks. Four groups of control animals received Flt3L/FVIII, rapamycin/FVIII, Flt3L/rapamycin, or nothing. Subsequently, mice were challenged with weekly IV injections of FVIII (1 IU) for 1 month, followed by measurement of inhibitor formation against FVIII (Figure 7C). Mice that had received FVIII/rapamycin treatment developed significantly lower inhibitor titers (∼40 BU/mL) than control animals (70 BU/mL) (Figure 7D), which were further reduced (to ∼10 BU/mL) when Flt3L was included in the treatment cocktail.
Inhibitor titers were unaffected in mice that were pretreated with Flt3L/rapamycin without FVIII, followed by challenge with FVIII, indicating that the observed effects reflected tolerance induction to FVIII rather than nonspecific suppression that may have persisted after the regimen was stopped. Consistent with this conclusion was the observation that the regimen caused only transient changes in various immune cell frequencies in immune competent mice, which subsequently responded normally to an unrelated antigen (supplemental Figures 8 and 9). Anti-FVIII immunoglobulin (Ig) G1 titers displayed a similar pattern as inhibitor titers (Figure 7D). Interestingly, mice that were pretreated with Flt3L/FVIII (Figure 7E) developed very high-titer antibodies to FVIII (Figure 7F), indicating that in the absence of rapamycin, Flt3L/antigen treatment immunized rather than tolerized the mice to FVIII.
Discussion
Immune tolerance can be induced by tipping the balance from an effector to a Treg response, a strategy that can be applied to cell and organ transplantation, treatment of autoimmune diseases, replacement therapies for genetic diseases, and treatment of allergies.6,7,10,11,39 Rapamycin is particularly useful in this regard. The drug blocks the PI3K-mTOR signaling cascade via mTOR complex 1 and downstream activation of STAT4, STAT6, or STAT3 in conventional CD4+ T cells.5,40 Consequently, the activated T cell undergoes programmed cell death. At the same time, rapamycin increases transforming growth factor β levels in vivo and upregulates FoxP3 expression in CD4+ cells, thereby facilitating Treg induction.41,42 Because Treg have a downregulated mTOR pathway and preferentially use STAT5 and other signaling molecules, intracellular signaling can proceed in the presence of rapamycin.5,8,43-45
In this study, we found that the tolerogenic effects of a rapamycin/antigen cocktail can be further enhanced by addition of several alternative third components, including cytokines or a ligand for the costimulatory molecule GITR, which is constitutively expressed by CD4+CD25+FoxP3+ Treg. Fc-GITR-L directly acts on Treg, stimulating their expansion in vitro and in vivo while maintaining their suppressive phenotype.32 The cytokine Flt3L is an attractive molecule that has already shown safety in clinical trials and is in clinical development for improved hematopoietic stem cell transplantation (registered at www.clinicaltrials.gov/ as #NCT01465139). Our new study demonstrates that Flt3L can be used synergistically with rapamycin for improved Treg induction, thereby enhancing immune tolerance. Flt3L and rapamycin had a synergistic rather than additive effect on Treg induction because in the absence of rapamycin, no Treg were induced. Flt3L on its own promotes proliferation of already existing Treg indirectly by expanding DCs but does not promote de novo induction of Treg.14,23 The combination of Flt3L and antigen may enhance effector T-cell deletion but fails to induce tolerance, suggesting that Treg induction (through the addition of rapamycin) and/or limited cDC expansion is required.
Flt3L-induced signaling in pDCs is more resistant to the mTOR inhibitor rapamycin
Rapamycin blocked Flt3L-induced cDC expansion in bone marrow and spleen, resulting in selective expansion of pDCs, which suggested to us that an increase in pDCs was responsible for the augmented Treg induction. This DC subset is able to produce large amounts of type I interferon and thus has important innate immune functions, such as in antiviral responses. However, pDCs can also be manipulated into assuming a nonactivated state (“tolerogenic pDCs”), characterized by incapacity to induce an effector T-cell response while promoting Treg expansion.46,47 For example, pDCs have been implicated in regulating oral tolerance and self-tolerance.48-50 The question arises why rapamycin blocked cDC but not pDC expansion. Given the previous evidence that intracellular signaling downstream of Flt3L-Flt3 occurs through the mTOR pathway, we compared the effects of Flt3L and rapamycin on purified cDCs and pDCs and found that pDCs had a more robust basal p-mTORSer2448 signal, thus rendering this cell type more resistant to inhibition by rapamycin. This conclusion is further supported by a recent study by Agudo et al, who found that miR-126, a microRNA known to regulate angiogenesis in vascular endothelial cells, is within the immune system uniquely expressed in pDCs.37 Within pDCs, miR-126 functions to suppress the translation of Tsc1, a negative regulator of mTOR, thereby upregulating mTOR activity.37 Increased mTOR activity is critical for survival of pDCs (in part through interaction with vascular endothelial growth factor receptor 2 signaling) and also for their function in innate immunity by upregulating expression of several innate response genes. A remaining unresolved issue is that pDC development from bone marrow precursors was found to be particularly susceptible to inhibition by rapamycin. However, miR-126 expression gradually increases during pDC development, and it is thus possible that the expansion that we observed in the presence of rapamycin primarily resulted from proliferation of differentiated pDCs. In fact, we found that pDCs also had greater expression of the Flt3 receptor and may thus also be more receptive to Flt3L.
Robust Treg induction is pDC dependent
In 3 of 4 experimental models that we tested, depletion of pDCs substantially reduced Treg induction by the Flt3L/rapamycin/antigen regimen. In a breast cancer model, pDC depletion resulted in decreased Treg numbers (and thus increased effector T-cell responses), further supporting a critical role for pDCs in Treg induction.36 Interestingly, we recently found that expansion of Treg using Flt3L is less effective in mice lacking GITR-L, a molecule that has been described in the literature to be expressed by pDCs.51,52 It is therefore possible that pDCs aid in Treg induction via the GITR-L/GITR costimulatory pathway. One also needs to consider that rapamycin not only modulates T-cell but also DC function. For example, adoptively transferred rapamycin-conditioned DCs inhibit organ allograft rejection and graft-versus-host disease following hematopoietic cell transplantation.1 In searching for a mechanism to explain the tolerogenic function in pDCs treated with our drug cocktail, we interrogated expression of several molecules that have been associated with a regulatory phenotype in DCs.46,53-55 The chemokine receptor CCR9 is selectively expressed on pDCs of immature phenotype in vivo and has been implicated with inducing Treg in culture.56 In our study, we observed a high surface expression of CCR9 on naïve splenic pDCs (∼85%) as compared with cDCs (∼8%) (data not shown). Importantly, this high expression of CCR9 was retained in all treatment conditions, suggesting that pDCs maintain an immunoregulatory phenotype upon Flt3L/antigen/rapamycin treatment rather than maturing into immune response–promoting cells. However, we did not find evidence for the upregulation of the metabolic enzyme indoleamine-pyrrole 2,3-dioxygenase or of the costimulatory molecule inducible T cell costimulator (ICOS) ligand on DCs (or of ICOS on Treg) upon Flt3L/antigen/rapamycin administration (data not shown). However, plasma samples showed increased transforming growth factor β, which is required for peripheral Treg induction (supplemental Figure 10).
Implications for immune tolerance therapy
The therapeutic relevance of our findings is illustrated by the results in gene and protein therapies. Because pretreatment of hemophilia A mice with Flt3L/rapamycin alone (ie, without presence of FVIII antigen) failed to suppress inhibitor formation, the effect was not because of general immune suppression. Rather, FVIII-specific tolerance was established. The protocol led to only minor and highly transient decreases in lymphocyte populations, and cell numbers recovered within a month after stopping the regimen. Interestingly, others found that rapamycin and Flt3L could be combined to improve engraftment of cardiac allografts in mice.38 Consistent with our findings, these authors also noticed an increase in pDC and Treg frequencies (without however further addressing the underlying mechanism).38 These data illustrate the wide range of potential applicability of the Flt3L/rapamycin combination. Conversely, these findings have implications for immunotherapy, as discussed previously for breast cancer.36 DC expansion via Flt3L has been proposed to enhance immune responses against tumors, and rapamycin and Flt3L can be combined for immunotherapy as shown in a glioma model.57 However, our data demonstrate the critical importance of drug dosing in this fine balance between immunity and tolerance and suggest that rapamycin doses need to be limited in cancer therapy to avoid efficient Treg induction, which is further enhanced by selective pDC expansion when combined with Flt3L. Conversely, further increased doses of rapamycin limit pDC expansion and may thus negatively impact tolerance induction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Celldex Therapeutics for the kind gift of recombinant human Flt3L. The authors also thank Drs Brian Brown and Judith Aguda for advice, and the flow cytometry core of the University of Florida Health Cancer Center for technical assistance.
This work was supported by grants from the National Institutes of Health (P01 HL078810 from the National Heart, Lung and Blood Institute [R.W.H. and C.T.], R01 DK052510 from the National Institute of Diabetes and Digestive and Kidney Diseases [C.T.], and R01 AI51390 [R.W.H.] and T32 AI007110 [G.L.R.] from the National Institute of Allergy and Infectious Diseases).
National Institutes of Health
Authorship
Contribution: M.B., D.S., S.N., S.R.P.K., G.L.R., and D.M.M. performed experiments; all authors participated in data analysis and interpretation; M.B., D.S., C.T., and R.W.H. designed experiments; G.L. and C.T. contributed critical reagents; M.B., G.L., C.T., and R.W.H. wrote the manuscript; and R.W.H. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland W. Herzog, University of Florida, Cancer and Genetics Research Complex, 2033 Mowry Rd, Gainesville, FL 32610; e-mail:rherzog@ufl.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal