Key Points
β2GPI complexed with HLA class II molecules was found to be a target for autoantibodies in APS.
More than 80% of patients with APS possess autoantibodies against β2GPI/HLA class II complexes.
Abstract
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by thrombosis and/or pregnancy complications. β2-glycoprotein I (β2GPI) complexed with phospholipid is recognized as a major target for autoantibodies in APS; however, less than half the patients with clinical manifestations of APS possess autoantibodies against the complexes. Therefore, the range of autoantigens involved in APS remains unclear. Recently, we found that human leukocyte antigen (HLA) class II molecules transport misfolded cellular proteins to the cell surface via association with their peptide-binding grooves. Furthermore, immunoglobulin G heavy chain/HLA class II complexes were specific targets for autoantibodies in rheumatoid arthritis. Here, we demonstrate that intact β2GPI, not peptide, forms a complex with HLA class II molecules. Strikingly, 100 (83.3%) of the 120 APS patients analyzed, including those whose antiphospholipid antibody titers were within normal range, possessed autoantibodies that recognize β2GPI/HLA class II complexes in the absence of phospholipids. In situ association between β2GPI and HLA class II was observed in placental tissues of APS patients but not in healthy controls. Furthermore, autoantibodies against β2GPI/HLA class II complexes mediated complement-dependent cytotoxicity against cells expressing the complexes. These data suggest that β2GPI/HLA class II complexes are a target in APS that might be involved in the pathogenesis.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by arterial or venous thrombosis and pregnancy complications, including recurrent spontaneous abortion.1,2 APS is associated with antiphospholipid (aPL) antibodies that bind to anionic phospholipid and serum protein complexes.3-5 Interactions between aPL antibodies and vascular endothelial cells are thought to be involved in the pathogenesis of APS.6-9 β2-glycoprotein I (β2GPI) is the main phospholipid-binding molecule recognized by aPL antibodies5,10,11 and is produced predominantly by hepatocytes, although some endothelial cells of blood vessels and placental villous tissue also express it.12,13 Plasma β2GPI circulates in a circular conformation with the aPL antibody epitopes being cryptic.14 When β2GPI associates with anionic phospholipids such as cardiolipin (CL), the circular structure of plasma β2GPI is converted to a linear form, leading to exposure of the major epitope for aPL antibodies.14-19 Therefore, β2GPI bound to negatively charged phospholipids or negatively charged plates is used clinically to detect antibodies.20 However, autoantibodies against the β2GPI associated with phospholipids are detected in less than half the patients with clinical manifestations of APS,21-23 suggesting the existence of additional targets of the autoantibodies. In addition, β2GPI is a secreted protein and is generally not present on the cell surface; therefore, how aPL antibodies bind vascular endothelial cells and induce thrombosis or pregnancy complications has remained unclear.
Specific human leukocyte antigen (HLA) class II alleles are associated with susceptibility to APS, as in other autoimmune diseases.24-27 Because peptide repertoires presented on different HLA class II alleles differ,28,29 it has been proposed that specific peptide-HLA class II combinations affect T-cell development and/or tolerance, which may confer susceptibility or resistance to autoimmune diseases.30 Nonetheless, the mechanisms by which HLA class II gene polymorphisms regulate susceptibility to autoimmune diseases are unknown.
Misfolded cellular proteins are generally eliminated by the process of endoplasmic reticulum-associated degradation31 and would not be exposed to the immune system. Recently, however, we found that misfolded proteins are rescued from degradation and transported to the cell surface without processing to peptides when they associate with the peptide-binding groove of HLA class II molecules in the endoplasmic reticulum (ER).32,33 Structural analyses of major histocompatibility complex (MHC) class II molecules have revealed that both ends of the MHC class II peptide-binding groove are open. Therefore, it is possible that MHC class II molecules might bind linear epitopes exposed on misfolded proteins. Indeed, several studies have suggested that MHC class II molecules have the capacity to associate with denatured proteins at the cell surface.34-36 Furthermore, immunoglobulin G (IgG) heavy chains thus transported to the cell surface by HLA class II alleles associated with rheumatoid arthritis (RA) susceptibility were specifically recognized by autoantibodies from RA patients.33 Because HLA class II expression on nonlymphoid cells, including endothelial cells, is frequently observed in various autoimmune diseased tissues,37-41 we hypothesized that misfolded proteins rescued from protein degradation by HLA class II molecules might be targets for autoantibodies in autoimmune diseases. Here, we addressed whether structurally altered β2GPI is transported to the cell surface by HLA class II molecules and is recognized by autoantibodies in APS patients. Strikingly, 100 (83.3%) of the 120 APS patients, including those whose aPL antibody titers were within normal range, possessed autoantibodies against β2GPI/HLA class II complexes. Furthermore, autoantibodies from APS patients mediated complement-dependent cytotoxicity against cells expressing both β2GPI and HLA class II molecules. Our findings provide new insights into the pathogenesis of APS and also into an unexpected function of HLA class II molecules in autoimmune diseases.
Materials and methods
Sera and placental tissue samples
The collection and use of human sera and placental tissues was approved by the institutional review boards of Hokkaido University, Kobe University, Kyoto University, Dohgo Spa Hospital, and Osaka University. Written informed consent was obtained from all participants according to the relevant guidelines of the institutional review boards. The diagnosis of APS was based on the preliminary classification criteria for definite APS.1,2 Sera from 63 of the 120 APS patients were derived from patients with secondary APS complicated by systemic lupus erythematosus. Sera from 50 healthy controls were purchased from George King Bio-Medical, Inc.
Measurement of anticardiolipin antibody, anti-β2GPI antibody, and lupus anticoagulant
Anticardiolipin (aCL) antibody was detected by using CL complexed with serum phospholipid-binding protein, and anti-β2GPI antibody was detected by using β2GPI bound to negatively charged plates as previously reported.20,42 Normal ranges of aCL antibody-IgG (<18.5 IgG phospholipid) and anti-β2GPI antibody-IgG (<2.2 U) were established previously by using 132 healthy controls with 99th percentile cutoff values.22 Lupus anticoagulant was measured by 3 clotting tests as previously reported.43
Plasmids
Complementary DNAs (cDNAs) prepared from pooled human peripheral blood mononuclear cells (3H Biomedical) were cloned into the pME18S or pCAGGS expression vectors. cDNA sequences for HLA class II were based on information contained in the Immunogenetics/HLA Database (http://www.ebi.ac.uk/imgt/hla/index.html). HLA-DRB1*04:04 containing a covalently attached HLA-Cw4 peptide (GSHSMRYFSTSVSWPGR) was generated as previously described.44 Domain I-deleted β2GPI cDNA (acid residues 80-345) were cloned into the pCAGGS expression vector containing a human signaling lymphocytic activation molecule signal sequence. 293T cells were transiently transfected by using Polyethylenimine Max (Polyscience) and were analyzed 2 days after transfection.
Antibodies
HL40 (EXBIO), L243 (American Type Culture Collection), FL-254 (Santa Cruz Biotechnology), and TAL.1B5 (Dako) were used to detect HLA-DR by flow cytometry, immunoprecipitation, western blotting, and immunohistochemistry, respectively. Anti-FLAG monoclonal antibody (mAb) (M2, Sigma-Aldrich), anti-His mAb (Wako), and rabbit anti-β2GPI antibody (specific to domains IV and V; HPA001654; Atlas Antibodies) were used for flow cytometry and western blotting. EY2C9, a human aPL mAb derived from an APS patient,45 was purified from EY2C9-producing cells. Stained cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously.46 Briefly, cells were lysed in buffer containing 0.5% NP-40. The immunoprecipitates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and were blotted. Total cell lysates were also analyzed by immunoblotting.
Immunohistochemistry and in situ proximity-ligation assay (PLA)
Paraffin-embedded tissue sections from APS patients (n = 6) and individuals without APS (n = 6) were stained with anti-β2GPI and anti-HLA-DR antibodies, followed by Alexa 647- or Alexa 555-conjugated goat anti-rabbit IgG or mouse IgG antibodies (Molecular Probes). A Duolink was used for PLA according to the manufacturer’s instructions (Olink Bioscience). The assayed tissue sections were analyzed by Axioplan 2 fluorescence microscopy (Zeiss).
Effect of exogenous β2GPI on EY2C9 mAb binding to HLA class II–expressing cells
Primary endothelial cells (human dermal microvascular endothelial cells; Lonza) were stimulated with interferon γ (IFN-γ; 500 U/mL; Miltenyi Biotec) and tumor necrosis factor α (TNF-α; 20 ng/mL; Miltenyi Biotec) for 24 hours. Thereafter, β2GPI (GenWay) was added to the medium at the concentration found in serum (200 μg/mL). HLA-DR7, invariant chain (Ii), and HLA-DM were transfected into 293T cells, and β2GPI (200 μg/mL) was added to the transfectants 24 hours later. Cells cultured in the presence of β2GPI for 48 hours were stained with EY2C9 mAb and anti-HLA-DR mAb (L243).
Competitive inhibition assay
β2GPI was purified from the culture supernatants of 293T cells transfected with FLAG-tagged β2GPI using anti-FLAG M2 affinity gels (Sigma-Aldrich). β2GPI bound to anti-FLAG M2 affinity gels was eluted by DYKDDDDK (FLAG) peptide (150 ng/μL; Wako). Purity of the β2GPI was more than 90% as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis. β2GPI and EY2C9 mAb (1 μg/mL) were incubated for 12 hours at 4°C in the presence or absence of CL (50 μg/mL; Sigma-Aldrich). 293T cells cotransfected with β2GPI, HLA-DR7, and green fluorescent protein (GFP) were stained with EY2C9 mAb preincubated with β2GPI and/or CL. Binding of EY2C9 mAb to GFP-expressing cells was measured by flow cytometry.
Determination of anti-β2GPI/HLA class II complex antibody titer
A serum in which aCL antibody titer was high (47 IgG phospholipid) and anti-β2GPI/HLA-DR7 complex antibody was detectable after 106-fold dilution was used as a standard throughout this study. The aβ2GPI/HLA-DR7 complex antibody titer of the standard serum was defined as 100 U. β2GPI, HLA-DR7, and GFP were transfected into 293T cells. Mean fluorescence intensities (MFIs) of IgG binding to GFP-positive and -negative cells in sequentially diluted standard sera (102- to ∼106-fold dilution) were analyzed by flow cytometry. Specific IgG binding to the β2GPI/HLA-DR7 complexes was calculated by subtracting the MFI of IgG binding to GFP-negative cells from the MFI of IgG binding to GFP-positive cells. A standard curve was generated from the specific IgG binding to β2GPI/HLA-DR7 complexes in sequentially diluted standard sera. The anti-β2GPI/HLA-DR7 complex antibody titer of each serum was calculated from the standard curve. The normal range of anti-β2GPI/HLA-DR7 complex antibody titers (<1.8 U) was established by using 100 healthy controls with 99th percentile cutoff values.
Complement-mediated cytotoxicity of aPL antibody against cells expressing β2GPI and HLA-DR7
β2GPI, HLA-DR (HLA-DRA*01:01 and DRB1*07:01), and GFP were co-transfected into 293T cells, and GFP-expressing cells were purified by using a cell sorter (FACSAria) 2 days after transfection. The purified transfectants were mixed with aPL (EY2C9) or control human IgM mAb (Calbiochem) on ice for 30 minutes followed by incubation with 1:10 diluted rabbit complement (Cedarlane) at 37°C for 30 minutes. Dead cells were stained with propidium iodide dye, and their proportions were determined by flow cytometry.
Statistics
To assess the significance of the correlation, Pearson’s product-moment correlation coefficient was used and the correlation coefficient (r) and P value of the linear regression line were calculated. Student t test and Mann-Whitney U test were used to determine the significance of differences. P values of <.05 were regarded as statistically significant.
Results
β2GPI complexed with HLA class II molecules is recognized by aPL antibody
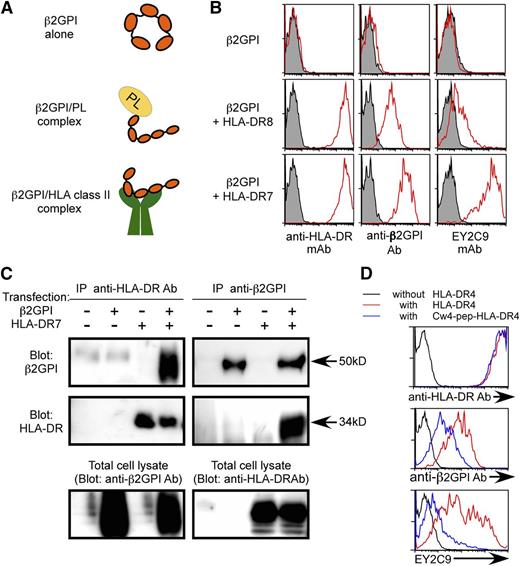
Free β2GPI has a circular conformation whereas phospholipid-bound β2GPI has a linear conformation that is accessible by aPL antibodies (Figure 1A).14-18 Because β2GPI is a secreted serum protein, it is not generally detected on the surface of even the cells that produce it. In order to analyze whether β2GPI is transported to the cell surface by HLA class II molecules in a manner similar to IgG heavy chain,33 β2GPI was cotransfected into 293T cells together with GFP and HLA-DRA*01:01 and DRB1*07:01 (HLA-DR7) or HLA-DRA*01:01 and DRB1*08:01 (HLA-DR8), and cell surface expression of β2GPI on GFP-positive cells was analyzed. β2GPI was not detected on the surface of cells transfected with β2GPI alone (Figure 1B). In contrast, it was found on the surface of cells co-transfected with HLA-DR7, an APS susceptibility allele.24-27 Cells cotransfected with HLA-DR8, an allele not associated with APS susceptibility, expressed less surface β2GPI (Figure 1B). Similar results were obtained by using N-terminus FLAG-tagged β2GPI or C-terminus His-tagged β2GPI detected with anti-FLAG or anti-His mAb, respectively (supplemental Figure 1, available on the Blood Web site). Furthermore, β2GPI of the predicted size was co-precipitated together with HLA-DR7 from cells expressing both β2GPI and HLA-DR7 (Figure 1C). Similarly, HLA-DR was co-precipitated together with β2GPI from the transfectants. Because association of full-length IgG heavy chain with HLA-DR was detected in the ER,33 full-length β2GPI, but not fragmented β2GPI, also seems to be associated with HLA-DR in the ER and transported to the cell surface by HLA-DR (Figure 1A).
β2GPI complexed with HLA class II molecules is recognized by aPL antibody. (A) Possible conformations of β2GPI. Free β2GPI in serum shows a closed circular conformation, whereas β2GPI associated with phospholipids (PLs) shows a linear conformation. Because cellular misfolded proteins are presented by HLA class II molecules,32 β2GPI with a unique conformation might also be presented by them. (B) β2GPI is displayed on the cell surface in the presence of HLA-DR. β2GPI was transfected into 293T cells together with GFP in the presence or absence of HLA-DR7 or HLA-DR8, and the transfectants were stained with anti-β2GPI, anti-HLA-DR, or aPL antibody (EY2C9) (red line). Antibody (Ab) binding to GFP-expressing cells is shown. Cells transfected with GFP alone were stained as a control (shaded histogram). (C) Direct association of β2GPI with HLA-DR. β2GPI and HLA-DR were cotransfected, and HLA-DR or β2GPI was precipitated. β2GPI and HLA-DR in the precipitates were detected by western blotting. HLA-DR or β2GPI in total cell lysates was also detected. (D) HLA-DR4 containing a covalently attached Cw4 peptide (blue lines) or wild-type HLA-DR4 (red lines) was cotransfected into 293T cells together with β2GPI and GFP. Cells transfected with β2GPI and GFP alone were used as a control (black line). β2GPI expression and aPL antibody binding to GFP-expressing cells were analyzed. Data are representative of at least 3 independent experiments.
β2GPI complexed with HLA class II molecules is recognized by aPL antibody. (A) Possible conformations of β2GPI. Free β2GPI in serum shows a closed circular conformation, whereas β2GPI associated with phospholipids (PLs) shows a linear conformation. Because cellular misfolded proteins are presented by HLA class II molecules,32 β2GPI with a unique conformation might also be presented by them. (B) β2GPI is displayed on the cell surface in the presence of HLA-DR. β2GPI was transfected into 293T cells together with GFP in the presence or absence of HLA-DR7 or HLA-DR8, and the transfectants were stained with anti-β2GPI, anti-HLA-DR, or aPL antibody (EY2C9) (red line). Antibody (Ab) binding to GFP-expressing cells is shown. Cells transfected with GFP alone were stained as a control (shaded histogram). (C) Direct association of β2GPI with HLA-DR. β2GPI and HLA-DR were cotransfected, and HLA-DR or β2GPI was precipitated. β2GPI and HLA-DR in the precipitates were detected by western blotting. HLA-DR or β2GPI in total cell lysates was also detected. (D) HLA-DR4 containing a covalently attached Cw4 peptide (blue lines) or wild-type HLA-DR4 (red lines) was cotransfected into 293T cells together with β2GPI and GFP. Cells transfected with β2GPI and GFP alone were used as a control (black line). β2GPI expression and aPL antibody binding to GFP-expressing cells were analyzed. Data are representative of at least 3 independent experiments.
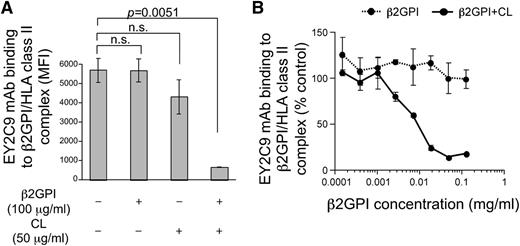
APS patients possess autoantibodies that bind to cryptic epitopes on β2GPI revealed by conformational changes induced by association with phospholipids.14-18 We tested the possibility that conformation of β2GPI bound to HLA-DR7 is similar to β2GPI complexed with phospholipids and thus is recognized by aPL antibodies from APS patients. EY2C9, a well-characterized human aPL mAb derived from an APS patient, represents binding and procoagulant properties of anti-β2GPI found in APS patients. EY2C9 mAb binds to β2GPI complexed with phospholipids but not to β2GPI or phospholipids alone.45 We found that EY2C9 mAb bound well to cells expressing both β2GPI and HLA-DR7, but not β2GPI alone, in the absence of phospholipids. EY2C9 mAb bound weakly to cells expressing β2GPI together with HLA-DR8 (Figure 1B). Because EY2C9 recognizes β2GPI associated with phosphatidylserine, we analyzed cell surface phosphatidylserine on HLA-DR7 and HLA-DR8 transfectants by using annexin V, which binds to phosphatidylserine. There was no difference in annexin V binding to HLA-DR7 and HLA-DR8 transfectants, suggesting that preferential binding of EY2C9 to β2GPI and HLA-DR7 transfectants is not due to an increase of cell surface phosphatidylserine on HLA-DR7 transfectants (supplemental Figure 2). In addition, an HLA-Cw4 peptide (a peptide naturally bound to HLA-DR447 ) covalently attached to HLA-DR4 significantly blocked transport of β2GPI to the cell surface and inhibited the binding of aPL antibody without affecting cell surface expression of HLA-DR (Figure 1D). These findings indicated that aPL antibody recognizes β2GPI bound to the peptide-binding groove of HLA-DR. Furthermore, the EY2C9 mAb binding to β2GPI complexed with HLA-DR7 was blocked by β2GPI complexed with CL, but not by β2GPI alone or CL alone (Figure 2A-B). These data indicate that EY2C9 mAb recognizes an epitope conserved between β2GPI/HLA-DR7 complexes and β2GPI/phospholipid complexes. It has been suggested that domains I, IV, and V of β2GPI are involved in EY2C9 mAb binding.48-50 Indeed, when domain I-deleted β2GPI and HLA-DR were cotransfected, EY2C9 mAb failed to recognize the domain I-deleted β2GPI complexed with HLA-DR7, although the mutant β2GPI complexed with HLA-DR7 was well recognized by anti-β2GPI domain IV-V antibody (supplemental Figure 3). These data suggested that domain I plays a critical role for EY2C9 mAb recognition of the β2GPI/HLA-DR7 complexes and that domain I is not involved in the association of β2GPI with HLA-DR7.
β2GPI complexed with HLA class II molecules shares aPL antibody epitopes with β2GPI complexed with CL. EY2C9 mAb was incubated with β2GPI in the presence or absence of CL at the concentrations indicated and was used for staining cells transfected with β2GPI and HLA-DR7. (A) MFI of the stained cells and (B) relative MFI compared with staining with EY2C9 mAb alone is shown as mean ± standard deviation (SD) of triplicates. Data are representative of at least 3 independent experiments.
β2GPI complexed with HLA class II molecules shares aPL antibody epitopes with β2GPI complexed with CL. EY2C9 mAb was incubated with β2GPI in the presence or absence of CL at the concentrations indicated and was used for staining cells transfected with β2GPI and HLA-DR7. (A) MFI of the stained cells and (B) relative MFI compared with staining with EY2C9 mAb alone is shown as mean ± standard deviation (SD) of triplicates. Data are representative of at least 3 independent experiments.
Autoantibodies against β2GPI complexed with HLA class II molecules are present in most APS patients
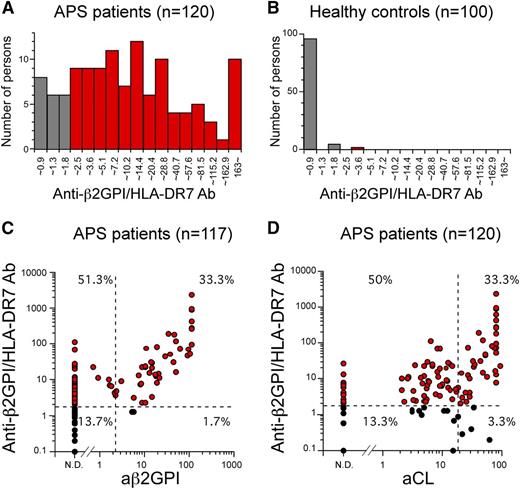
We examined whether β2GPI complexed with HLA-DR is recognized by autoantibodies from APS patients whose clinical characteristics are provided in Table 1. We found that sera from APS patients, including aCL-IgG antibody–negative and anti-β2GPI-IgG antibody–negative patients, contained IgG autoantibodies against β2GPI bound to HLA-DR7, whereas sera from almost all healthy individuals did not (Figure 3). Cells transfected with HLA-DR7 alone were not recognized by serum IgG from either APS patients or healthy individuals (supplemental Figure 4). In addition, autoantibodies from APS patients bound more effectively to cells co-transfected with β2GPI and HLA-DR7 than to those with β2GPI and HLA-DR8, similar to EY2C9 mAb (supplemental Figure 5). Strikingly, anti-β2GPI/HLA-DR7 complex IgG antibody titers in 100 (83.3%) of the 120 APS patients examined were above the normal range (<1.8 U) established by using 100 healthy controls with 99th percentile cutoff values (Figure 4A-B). Anti-β2GPI/HLA-DR7 complex antibody titers between APS patients and healthy controls were significantly different (P = 3.3 × 10−33). It is noteworthy that 60 (51.3%) of the 117 APS patients possessed autoantibodies that bind to β2GPI/HLA-DR7 complexes but not β2GPI bound to negatively charged plates (Figure 4C). Similarly, 60 APS patients (50%) whose aCL-IgG antibody titers were within normal range possessed autoantibodies to β2GPI/HLA-DR7 complexes (Figure 4D). Therefore, β2GPI/HLA-DR7 complexes appear to possess unique epitopes that are frequently recognized by autoantibodies in APS, but such epitopes are not present on plate-bound β2GPI or β2GPI/CL complexes. Conversely, a significant correlation between anti-β2GPI/HLA-DR7 complex antibody titers and anti-β2GPI-IgG antibody titers was observed when 52 APS patients (44.4%) with detectable anti-β2GPI-IgG antibody titers were analyzed (r = 0.57; P = 1.13 × 10−5) (Figure 4C). A similar significant correlation between anti-β2GPI/HLA-DR7 complex antibody titers and aCL-IgG antibody titers was also observed (r = 0.44; P = .330 × 10−6) (Figure 4D). This suggests that β2GPI/HLA-DR complexes also possess autoantibody epitopes shared by β2GPI bound to negatively charged plates or β2GPI/CL complexes.
Patient characteristics
| Patient . | Age, y . | Sex . | Primary or secondary APS (complicated by) . | Clinical manifestation . |
|---|---|---|---|---|
| APS1 | 68 | F | Secondary (SLE) | Lacunar infarction |
| APS2 | 77 | M | Secondary (RA) | Lacunar infarction |
| APS3 | 33 | F | Secondary (SLE) | Deep venous thrombosis |
| APS4 | 39 | F | Secondary (SLE) | Lacunar infarction |
| APS5 | 69 | F | Secondary (SLE) | Deep venous thrombosis, pulmonary embolism |
| APS6 | 42 | F | Primary | Recurrent spontaneous abortion |
| Patient . | Age, y . | Sex . | Primary or secondary APS (complicated by) . | Clinical manifestation . |
|---|---|---|---|---|
| APS1 | 68 | F | Secondary (SLE) | Lacunar infarction |
| APS2 | 77 | M | Secondary (RA) | Lacunar infarction |
| APS3 | 33 | F | Secondary (SLE) | Deep venous thrombosis |
| APS4 | 39 | F | Secondary (SLE) | Lacunar infarction |
| APS5 | 69 | F | Secondary (SLE) | Deep venous thrombosis, pulmonary embolism |
| APS6 | 42 | F | Primary | Recurrent spontaneous abortion |
F, female; M, male; SLE, systemic lupus erythematosus.
β2GPI complexed with HLA class II molecules is recognized by autoantibodies in APS patients. Autoantibody binding to β2GPI bound to HLA class II in aPL antibody-positive and -negative APS patients and healthy controls. Diluted sera were mixed with cells transfected with β2GPI and HLA-DR7, and IgG Ab binding to the cells was assessed (open histogram). Cells transfected with GFP alone were stained as a control (shaded histogram). Levels of aCL antibody-IgG, anti-β2GPI antibody-IgG, and lupus anticoagulant (LA) of each sample are indicated. Levels above the normal ranges (aCL antibody-IgG, 18.5 IgG phospholipid; anti-β2GPI antibody-IgG, 2.2 U; LA, 1.3) are indicated in bold underlined numbers. N.D., not detected. Data are representative of at least 3 independent experiments.
β2GPI complexed with HLA class II molecules is recognized by autoantibodies in APS patients. Autoantibody binding to β2GPI bound to HLA class II in aPL antibody-positive and -negative APS patients and healthy controls. Diluted sera were mixed with cells transfected with β2GPI and HLA-DR7, and IgG Ab binding to the cells was assessed (open histogram). Cells transfected with GFP alone were stained as a control (shaded histogram). Levels of aCL antibody-IgG, anti-β2GPI antibody-IgG, and lupus anticoagulant (LA) of each sample are indicated. Levels above the normal ranges (aCL antibody-IgG, 18.5 IgG phospholipid; anti-β2GPI antibody-IgG, 2.2 U; LA, 1.3) are indicated in bold underlined numbers. N.D., not detected. Data are representative of at least 3 independent experiments.
Autoantibodies against β2GPI/HLA class II complex are detected in most APS patients. (A-B) Distribution of serum anti-β2GPI/HLA-DR7 complex antibody titers in APS patients and healthy controls. Anti-β2GPI/HLA-DR7 complex antibody titers higher than the normal upper limit for anti-β2GPI/HLA-DR7 complex antibody titers established by using 100 healthy controls (1.8 U) are indicated as red bars. (C-D) Correlations between serum anti-β2GPI/HLA-DR7 complex antibody titers and serum anti-β2GPI antibody or aCL antibody titers in APS patients. The normal upper limits for anti-β2GPI antibody, aCL antibody, and anti-β2GPI/HLA-DR7 complex antibody titers are shown as dashed lines. Patients whose anti-β2GPI/HLA-DR7 complex antibody titers are higher than the normal upper limit are indicated as red circles. Data are representative of at least 3 independent experiments.
Autoantibodies against β2GPI/HLA class II complex are detected in most APS patients. (A-B) Distribution of serum anti-β2GPI/HLA-DR7 complex antibody titers in APS patients and healthy controls. Anti-β2GPI/HLA-DR7 complex antibody titers higher than the normal upper limit for anti-β2GPI/HLA-DR7 complex antibody titers established by using 100 healthy controls (1.8 U) are indicated as red bars. (C-D) Correlations between serum anti-β2GPI/HLA-DR7 complex antibody titers and serum anti-β2GPI antibody or aCL antibody titers in APS patients. The normal upper limits for anti-β2GPI antibody, aCL antibody, and anti-β2GPI/HLA-DR7 complex antibody titers are shown as dashed lines. Patients whose anti-β2GPI/HLA-DR7 complex antibody titers are higher than the normal upper limit are indicated as red circles. Data are representative of at least 3 independent experiments.
HLA-DR allele differences influence aPL antibody binding to β2GPI/HLA-DR complexes
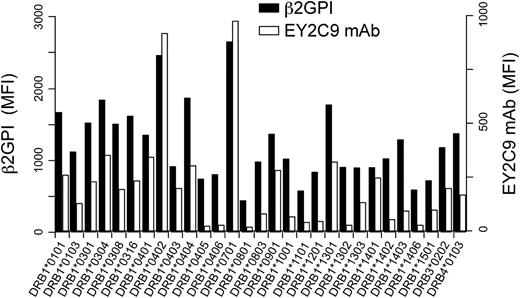
Specific HLA-DR alleles are associated with susceptibility to APS.24-27 We analyzed the ability of different HLA-DR alleles to transport β2GPI to the cell surface (Figure 5). In addition to HLA-DR7, HLA-DR4 (HLA-DRA*01:01 and DRB1*04:02), another APS susceptibility allele, was found to be particularly effective at transporting high levels of β2GPI to the cell surface, as recognized by the EY2C9 aPL mAb. In contrast, very little β2GPI was transported to the cell surface by several other HLA-DR alleles. The Ii associates with nascent HLA class II molecules and blocks their association with other ER proteins. Similarly, we found that Ii blocked β2GPI binding by most HLA-DR alleles, whereas HLA-DR7 and HLA-DR4 still bound significant amounts of β2GPI, even in the presence of Ii (supplemental Figure 6). These data suggested that interactions between β2GPI or Ii and HLA-DR might differ among different HLA-DR alleles. APS susceptibility conferred by certain HLA-DR alleles might be partially explained by these differences.
aPL antibody binds to β2GPI complexed with different HLA-DR alleles. N-terminal His-tagged β2GPI and GFP were cotransfected into 293T cells together with different HLA-DR alleles. The transfectants were stained with anti-His mAb (β2GPI, solid bars) or aPL antibody (EY2C9 mAb, open bars), followed by APC-labeled anti-mouse IgG or anti-human IgM Ab, respectively. MFIs of APC on GFP-positive cells are shown. Data are representative of 3 independent experiments.
aPL antibody binds to β2GPI complexed with different HLA-DR alleles. N-terminal His-tagged β2GPI and GFP were cotransfected into 293T cells together with different HLA-DR alleles. The transfectants were stained with anti-His mAb (β2GPI, solid bars) or aPL antibody (EY2C9 mAb, open bars), followed by APC-labeled anti-mouse IgG or anti-human IgM Ab, respectively. MFIs of APC on GFP-positive cells are shown. Data are representative of 3 independent experiments.
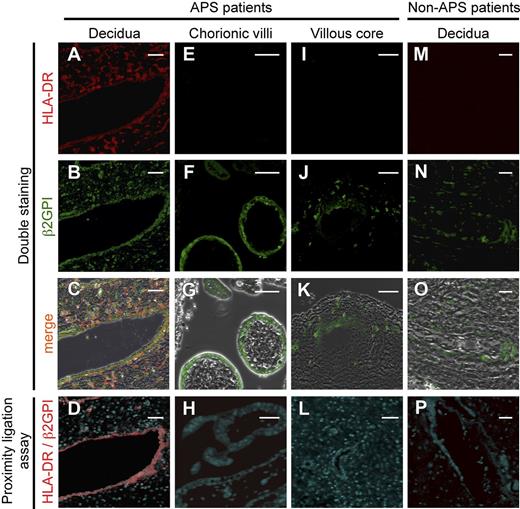
β2GPI is complexed with HLA-DR on endothelial cells of vessels in uterine decidual tissues from APS patients
Because β2GPI bound to HLA-DR is a target for autoantibodies in APS patients, we tested the hypothesis that β2GPI is bound to HLA-DR in diseased tissues of APS patients. Accordingly, we analyzed β2GPI and HLA-DR expression on placental tissues obtained from APS patients with spontaneous abortion (n = 6) by immunofluorescence staining and PLAs, which detect close proximity (less than 40 nm) between two molecules.51 β2GPI and HLA-DR were found to colocalize in endothelial cells of vessels and in stromal cells in the placental decidua of 4 of the 6 APS patients examined (Figure 6A-C). Furthermore, PLA signals between β2GPI and HLA-DR were detected in endothelial cells of vessels in decidua, but not in stromal cells (Figure 6D). In villous tissues, β2GPI was expressed in syncytiotrophoblasts (Figure 6F) and stromal cells around placental stem vessels (Figure 6J), but no colocalization of β2GPI and HLA-DR was observed (Figure 6G-H, K-L). Conversely, β2GPI, but not HLA-DR, was present in endothelial cells of vessels in decidua of placental tissues from patients without APS (n = 6) (Figure 6M-O), and no PLA signal was detected (Figure 6P). These results suggest that HLA class II expression is induced in uterine decidua of APS patients, and thus β2GPI forms complexes with HLA-DR, which can be targeted by autoantibodies in APS patients.
β2GPI is complexed with HLA-DR in endothelial cells of vessels in uterine decidual tissues obtained from APS patients. (A-C, E-G, I-K, M-O) β2GPI and HLA-DR are coexpressed in endothelial cells of vessels in uterine decidual tissues of APS patients, but not patients without APS. Tissue sections of (A-D) uterine decidua, (E-H) chorionic villi, and (I-L) villous core from APS patients and (M-P) decidua from APS-free patients were costained with anti-HLA-DR Ab (red, A, E, I, M) and anti-β2GPI Ab (green, B, F J, N). The images were merged to show colocalization of β2GPI and HLA-DR (C, G, K, O). PLA signals (red) between HLA-DR and β2GPI were analyzed in tissues of APS patients and those without APS (D, H, L, P). Scale bars, 50 μm. Data are representative of 3 independent experiments.
β2GPI is complexed with HLA-DR in endothelial cells of vessels in uterine decidual tissues obtained from APS patients. (A-C, E-G, I-K, M-O) β2GPI and HLA-DR are coexpressed in endothelial cells of vessels in uterine decidual tissues of APS patients, but not patients without APS. Tissue sections of (A-D) uterine decidua, (E-H) chorionic villi, and (I-L) villous core from APS patients and (M-P) decidua from APS-free patients were costained with anti-HLA-DR Ab (red, A, E, I, M) and anti-β2GPI Ab (green, B, F J, N). The images were merged to show colocalization of β2GPI and HLA-DR (C, G, K, O). PLA signals (red) between HLA-DR and β2GPI were analyzed in tissues of APS patients and those without APS (D, H, L, P). Scale bars, 50 μm. Data are representative of 3 independent experiments.
We analyzed whether β2GPI is complexed with HLA class II molecules on primary endothelial cells (supplemental Figure 7A). Because it has been reported that endothelial cells express β2GPI,12,13 and that IFN-γ and TNF-α upregulate HLA class II expression,52,53 we stimulated primary endothelial cell lines (human dermal microvascular endothelial cells) with these cytokines and analyzed EY2C9 mAb binding. HLA class II expression was induced on endothelial cells upon stimulation with IFN-γ and TNF-α. However, endothelial cells stimulated with IFN-γ and TNF-α were not recognized by EY2C9 mAb. Conversely, when endothelial cells were stimulated with IFN-γ and TNF-α in the presence of exogenous β2GPI at the concentration found in serum (200 μg/mL), EY2C9 mAb bound to the endothelial cells. To analyze the role of HLA class II molecules on EY2C9 mAb binding, we examined 293T cells transfected with HLA-DR (supplemental Figure 7B). HLA-DR 293T transfectants were also recognized by EY2C9 mAb in the presence of exogenous β2GPI. These results suggested that not only intracellular β2GPI but also extracellular β2GPI can be a target for aPL antibodies upon association with HLA class II molecules.
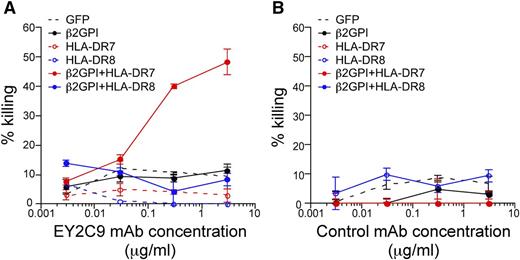
Complement-mediated cytotoxicity of aPL antibody against cells expressing β2GPI and HLA class II molecules
Because β2GPI associated with HLA class II molecules is a target for autoantibodies in APS patients, we analyzed whether aPL antibodies are cytotoxic for cells expressing β2GPI and HLA-DR (Figure 7A-B). aPL mAb EY2C9, but not control mAb, exhibited complement-mediated cytotoxicity against cells expressing β2GPI together with the APS susceptibility allele HLA-DR7, but not HLA-DR8. Cells expressing β2GPI alone were not killed. These results suggest that expression of HLA class II on β2GPI–expressing cells might play a crucial role in the pathogenesis of APS (supplemental Figure 8).
APL antibodies exert complement-mediated cytotoxicity against cells expressing β2GPI/HLA-DR7 complexes. Complement-mediated cytotoxicity by (A) aPL antibody (EY2C9 mAb) or (B) control mAb against cells transfected with β2GPI and HLA-DR7 or HLA-DR8. Percentage specific cytotoxicity is shown as mean ± SD of triplicates. Data are representative of 3 independent experiments.
APL antibodies exert complement-mediated cytotoxicity against cells expressing β2GPI/HLA-DR7 complexes. Complement-mediated cytotoxicity by (A) aPL antibody (EY2C9 mAb) or (B) control mAb against cells transfected with β2GPI and HLA-DR7 or HLA-DR8. Percentage specific cytotoxicity is shown as mean ± SD of triplicates. Data are representative of 3 independent experiments.
Discussion
Although HLA class II molecules are well recognized to present peptide antigens to T cells, recently we found that ER misfolded proteins are transported to the cell surface by HLA class II molecules when they are associated with the peptide-binding groove of HLA class II molecules.32 Furthermore, intact IgG heavy chain is transported to the cell surface by HLA class II molecules via association with the peptide-binding groove, and IgG heavy chain/HLA class II complexes are recognized by autoantibodies in rheumatoid factor–positive sera from RA patients.33 In contrast, autoantibodies in rheumatoid factor–positive sera from non-RA individuals did not bind to IgG heavy chain/HLA class II complexes, suggesting that IgG heavy chain complexed with HLA-DR is a specific target for autoantibodies from RA patients. Of note, a strong correlation between autoantibody binding to IgG complexed with certain HLA-DR alleles and the odds ratio for association of these alleles with RA was observed.33 These findings suggested that misfolded ER proteins complexed with certain HLA class II alleles might affect susceptibility to other autoimmune diseases as a specific target for autoantibodies.
Here, we found that intact β2GPI protein, not peptide, is also transported to the cell surface by HLA class II molecules. Because both ends of the peptide-binding groove of HLA class II molecules are open, it is structurally possible that large proteins, including β2GPI, associate with the peptide-binding groove of HLA class II molecules. Although the structure of β2GPI complexed with HLA class II molecules has not yet been determined, it is likely that linear epitopes exposed on misfolded or structurally altered β2GPI proteins associate with HLA class II molecules, because all of the proteins that we have shown to associate with HLA class II molecules are not correctly folded proteins.32,33 It is noteworthy that 83.3% of APS patients, including aCL-IgG antibody–negative and anti-β2GPI-IgG antibody–negative patients, were found to possess autoantibodies against β2GPI complexed with HLA class II molecules in the absence of phospholipids. In contrast, autoantibodies against β2GPI complexed with HLA class II molecules were rarely detected in healthy individuals. These results suggest that β2GPI/HLA-DR complexes are a major target in APS and that anti-β2GPI/HLA-DR complex autoantibodies might be a novel and useful diagnostic marker for APS.
β2GPI is a serum lipoprotein produced mainly by hepatocytes, although some endothelial cells of blood vessels and placental villous tissue also express it.12,13 A circular conformation of β2GPI in plasma is changed to linear conformation by association with negatively charged phospholipids or negatively charged plates, resulting in the exposure of an epitope visible to aPL antibodies.14-18 Because misfolded cellular proteins, but not correctly folded proteins, are transported to the cell surface by HLA class II molecules,32 β2GPI complexed with HLA class II molecules appears to exhibit a similar linear conformation and is thus recognizable by autoantibodies from APS patients. The significant correlation between anti-β2GPI/HLA-DR antibody titers and anti-CL antibody or anti-β2GPI antibody titers suggests that there are common autoantibody epitopes shared between β2GPI/HLA-DR complexes and β2GPI bound to phospholipid on negatively charged plates. However, about half of APS patients (whose autoantibody titers against β2GPI bound to phospholipid or negatively charged plates were within normal range) possess autoantibodies that bind to β2GPI/HLA-DR7 complexes. These results indicate that β2GPI/HLA-DR7 complexes also possess unique autoantibody epitopes for APS that are not present on phospholipid-bound β2GPI or plate-bound β2GPI. It has been reported that domains IV and V of β2GPI are involved in EY2C9 mAb binding.48,49 Conversely, mutational analyses of domain I suggested that domain I is also involved in EY2C9 mAb recognition of β2GPI.50 Our analyses of domain I deletion mutants also suggest that domain I plays an important role in recognition of β2GPI/HLA-DR complexes by EY2C9 mAb. These observations support our hypothesis that pathogenic epitopes on β2GPI that are recognized by autoantibodies in APS are exposed by association with HLA-DR.
Specific HLA-DR alleles are associated with susceptibility to APS24-27 and DQB1*06:04/5/6/7/9-DRB1*13:02 haplotypes are also reported to be involved in APS susceptibility.54 However, DRA1*01:01/DRB1*13:02 transported β2GPI to the cell surface less efficiently than HLA-DR7 and HLA-DR4. Therefore, certain HLA-DP or HLA-DQ alleles that are closely linked to the DRB1*13:02 haplotype or certain autoantigens for APS other than β2GPI, such as prothrombin,54 might be involved in APS susceptibility caused by the DQB1*06:04/5/6/7/9-DRB1*13:02 haplotypes.
The mechanisms of thrombosis production in patients with APS are not completely defined. aPL antibodies are thought to induce perturbation and/or damage in endothelial cells, which results in a prothrombotic and proinflammatory response and subsequently thrombosis.7,8 It has been suggested that anti-β2GPI autoantibodies mediate nuclear factor κB–dependent activation of endothelial cells via annexin A2 and toll-like receptor 4, which form multiprotein complexes with β2GPI.55,56 Conversely, a recent study suggested that activation of platelets by aPL antibodies is associated with thrombosis formation.57 In these ways, aPL antibodies seem to be a major contributor to the pathogenesis of APS.6-9 HLA class II expression is induced on endothelial cells after exposure to cytokines such as IFN-γ and TNF-α.40,41 Although expression of β2GPI on endothelial cells has remained controversial, EY2C9 mAb bound to IFN-γ and TNF-α stimulated endothelial cells in the presence of exogenous β2GPI. Similarly, HLA-DR transfected cells were recognized by EY2C9 mAb in the presence of exogenous β2GPI. Therefore, cytokines produced in response to inflammatory stimuli, such as those resulting from viral infection, might induce the formation of β2GPI and HLA class II complexes on endothelial cells. Complement deposition on decidual endothelial cells is detected in APS patients and thus complement activation is suggested to be involved in the production of thrombosis and pregnancy morbidity in patients with aPL antibodies.58-60 Therefore, complement-mediated cytotoxicity of endothelial cells expressing both β2GPI and HLA class II by autoantibodies, in addition to nuclear factor κB–dependent activation of endothelial cells, might target tissues affected by APS. This may explain why symptoms in some patients are mainly thrombosis, whereas others mainly suffer recurrent spontaneous abortion despite the presence of aPL antibodies in both pathologies.
Because misfolded proteins associated with MHC class II molecules efficiently stimulate antigen-specific B cells,32 β2GPI complexed with HLA-DR might be involved in autoantibody production in APS patients. Furthermore, autoantibodies against β2GPI/HLA class II complexes are detectable in some patients with unexplained recurrent pregnancy loss who are negative for both aPL antibodies and lupus anticoagulant (K. Tanimura, H.Y., and H.A., unpublished observation). Therefore, the presence of autoantibodies against β2GPI/HLA-DR complexes might help to understand as yet uncharacterized immune disorders. Conversely, it is well known that aPL antibodies are often detected in patients with leprosy regardless of the absence of clinical manifestations of APS. Further studies are needed to determine whether these aPL antibodies are different from autoantibodies against β2GPI/HLA-DR complexes. In addition to those in APS and RA, autoantibodies in Graves’ disease and Hashimoto thyroiditis also recognize self-antigens complexed with disease-susceptible HLA class II molecules (H.J., L.L.L., and H.A. unpublished observation), suggesting that self-antigens complexed with HLA class II molecules might be general targets for autoantibodies produced in many autoimmune diseases. Further analyses of misfolded protein transport by HLA class II molecules will help us better understand autoimmune diseases.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ryosuke Hiwa for critical reading of our manuscript, K. Shida, S. Matsuoka, and M. Matsumoto for technical assistance, and C. Kita for secretarial assistance.
This work was supported by grants from Japan Science and Technology Agency, Core Research for Evolutional Science and Technology, a Grant-in-Aid for Scientific Research on Innovative Areas “HLA disease and evolution” (22133009 [T. Sasazuki], 22133003 [K.Y.], 25133705 [H.A.]) from Ministry of Education, Culture, Sports, Science & Technology Japan, and a Grant-in-Aid for Scientific Research (B) (23390112 [H.A.]), (C) (24590584 [M.K.], 25460565 [T. Suenaga]) and Young Scientists (B) (26870334 [K.H.]) from the Japan Society for the Promotion of Science. L.L.L. is an American Cancer Society Professor and is supported by grant AI068129 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Authorship
Contribution: K. Tanimura, H.J., N.A., S.M., and K.K. performed experiments and analyzed data; K.H. performed statistical analysis; Y.E., S.Y., T.H., K. Takasugi, K.O., I.K., T.A., and H.Y. collected and analyzed clinical samples; T. Suenaga, N.A., K.H., M.K., I.K., K.Y., T. Sasazuki, T.A., H.Y., and L.L.L. helped design the study and write the manuscript; H.A. designed the study and wrote the manuscript; and all authors discussed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.Y. is Department of Medical Chemistry, Kurume University School of Medicine, Kurume, Fukuoka, Japan.
Correspondence: Hisashi Arase, Immunology Frontier Research Center, Osaka University, 3-1 Yamadaoka, Suita, Osaka, Japan; e-mail: arase@biken.osaka-u.ac.jp.
References
Author notes
K. Tanimura and H.J. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal