Key Points
Bone marrow OB ablation leads to reduced quiescence, long-term engraftment, and self-renewal capacity of hematopoietic stem cells.
Significantly accelerated leukemia development and reduced survival are seen in transgenic BCR-ABL mice following OB ablation.
Abstract
Hematopoietic stem cells (HSCs) reside in regulatory niches in the bone marrow (BM). Although HSC niches have been extensively characterized, the role of endosteal osteoblasts (OBs) in HSC regulation requires further clarification, and the role of OBs in regulating leukemic stem cells (LSCs) is not well studied. We used an OB visualization and ablation mouse model to study the role of OBs in regulating normal HSCs and chronic myelogenous leukemia (CML) LSCs. OB ablation resulted in increase in cells with a LSK Flt3−CD150+CD48− long-term HSC (LTHSC) phenotype but reduction of a more highly selected LSK Flt3−CD34−CD49b−CD229− LTHSC subpopulation. LTHSCs from OB-ablated mice demonstrated loss of quiescence and reduced long-term engraftment and self-renewal capacity. Ablation of OB in a transgenic CML mouse model resulted in accelerated leukemia development with reduced survival compared with control mice. The notch ligand Jagged-1 was overexpressed on CML OBs. Normal and CML LTHSCs cultured with Jagged-1 demonstrated reduced cell cycling, consistent with a possible role for loss of Jagged-1 signals in altered HSC and LSC function after OB ablation. These studies support an important role for OBs in regulating quiescence and self-renewal of LTHSCs and a previously unrecognized role in modulating leukemia development in CML.
Introduction
Stem cells are located in specific niches, which regulate their maintenance, proliferation, self-renewal, and differentiation. Hematopoietic stem cells (HSCs) are located primarily in the bone marrow (BM) cavity, with HSC niche function residing in nonhematopoietic cells within the BM microenvironment. BM niches maintain a quiescent pool of HSCs that can be recruited to generate new blood cells as needed. The nature of the HSC niche within the BM microenvironment has been the subject of much investigation. Several cell types, including sinusoidal and arteriolar vascular endothelial cells, subendothelial cells, and osteoblastic cells, have been proposed as HSC niches.1
Osteoblasts (OB) are bone-forming cells that secrete calcium and synthesize the bone matrix. OBs cover the endosteal bone surface, forming an interface between calcified bone and marrow cells. OBs are reported to provide signals required for HSC quiescence, long-term maintenance, and BM retention.2 Visnjic et al showed that ablation of OBs leads to loss of BM cellularity, decreased numbers of HSCs and progenitors in the BM, and increased extramedullary hematopoiesis.3 Calvi et al reported that OBs are a regulatory component of the HSC niche in vivo that influences HSC function through Notch activation.4 OBs in the trabecular bone area were shown to express high levels of Jagged-1 and support a higher frequency of HSCs in these regions.5 Increased numbers of spindle-shaped N-cadherin+CD45− osteoblastic cells following conditional inactivation of bone morphogenetic protein correlated with an increase in HSC numbers.6 Similarly, CD166 expressing OBs were reported to play an important role in supporting quiescent long-term repopulating cells.7,8 Other studies, however, have not supported an essential role for OBs in HSC maintenance. Calvi et al reported in a follow up study that in vivo parathyroid hormone-induced expansion of mature OBs and osteocytes did not lead to increased HSCs.9 Deletion of stem cell factor and CXCL12 from OB did not affect HSC maintenance.10,11 However, these studies, while excluding a role for OB in contributing these specific factors required for HSC regulation, do not rule out a role for other OB-mediated mechanisms in HSC regulation. Indeed, it is possible that the interaction of several BM microenvironmental populations may be required for HSC maintenance.12,13
Several leukemias, including chronic myelogenous leukemia (CML), are maintained by a pool of leukemia stem cells (LSCs) within the BM.14-16 As with normal HSCs, LSCs are also thought to localize to specific niches in the BM microenvironment that support their growth and maintenance and may protect them from therapeutic exposures. In addition, leukemic-induced alterations in the BM microenvironment may lead to altered niche function,17-19 potentially contributing to a growth advantage of LSCs over normal HSCs.20 At present, little is known about LSC niches, and there is considerable interest in understanding the role of different microenvironmental niche populations in regulating LSCs and signals involved in this regulation. Better understanding of the LSC niche may yield new strategies to enhance therapeutic targeting and elimination of LSCs.
In this study, we examined the role of OBs in the regulation of both normal and leukemic hematopoiesis, using transgenic mice in which OB can be conditionally ablated.3,21 To carefully validate OB ablation and determine effects of OB ablation on normal and CML hematopoiesis, we crossbred these mice with transgenic mice in which OBs can be visualized based on green fluorescent protein (GFP) expression.22
Materials and methods
Mouse strains
Transgenic mice in B6 background expressing a truncated version of the herpes simplex virus thymidine kinase (Δtk) gene (HSV-TK) under control of an OB-specific 2.3-kb fragment of the rat collagen α1 type 1 promoter (Col2.3Δtk),21 were crossed with mice expressing GFP under control of the same promoter (Col2.3GFP).22 The resulting Col2.3GFP/Col2.3Δtk mice showed lineage-specific expression of both GFP and HSV-TK genes in OBs, facilitating documentation of presence or elimination of OBs. To achieve OB ablation, control and Δtk mice were injected intraperitoneally with ganciclovir (GCV; 10 mg/kg/day; Genentech USA, South San Francisco, CA) for 28 to 40 days. To generate a mouse model of CML in which OBs could be ablated, Col2.3GFPΔtk mice were crossed with SCL-tTA/breakpoint cluster region-Abelson murine leukemia viral oncogene (BCR-ABL) mice backcrossed to the B6 background to generate Col2.3GFP/Col2.3Δtk/SCL-tTA/BCR-ABL mice. Transgenic BCR-ABL mice were maintained on tetracycline water at 0.5 g/L. Withdrawal of tetracycline results in expression of BCR-ABL and generation of a CML-like disease in these mice. All experimental procedures were carried out in accordance with federal guidelines and protocols approved by City of Hope’s Institutional Animal Care and Use Committee. Polymerase chain reaction primers used for genotyping are shown in supplemental Table 1, available on the Blood Web site.
Bone marrow morphology and immunohistochemistry
Following GCV treatment, femurs were collected, incubated in 10% formalin for 24 to 48 hours, decalcified for 3 hours, and washed under running water for 1 hour. Following additional formalin incubation, femurs were dehydrated, incubated, and blocked in paraffin for microtome sectioning. Representative sections were baked to remove excess paraffin, stained with hematoxylin and eosin, and imaged. Immunohistochemistry was used to visualize GFP-expressing cells, following deparaffinization, antigen retrieval, and anti-GFP antibody labeling. Endogenous peroxidase activity was quenched followed by blocking using Block Aid Blocking Solution (Invitrogen B10710 and Triton X-100). Slides were incubated with primary mouse anti-GFP antibody (Abcam; ab290, 1:200) overnight at 4°C, followed by secondary biotinylated anti-rabbit antibody (Vector; 1:1000) for 1 hour at room temperature. The Vectastain ABC Elite kit (Vector; PK-6100) was used for antigen visualization. Slides were counterstained with hematoxylin and coverslipped in Cytoseal 60 (Richard-Allan Scientific; 8310-16). Bright field microscopy was performed using a Nikon TE2000-U microscope. Images were captured and processed using a SPOT RT Slider digital camera and software (Diagnostic Instruments, Sterling Heights, MI).
Analysis of hematopoietic cells by flow cytometry
BM (femurs and tibias), spleens (SP), and peripheral blood (PB) were collected from GCV-treated mice. For analysis of OB numbers, pelvic bones were also collected. Bones were crushed and digested with collagenase for 45 minutes at 37°C, and cells were isolated and counted prior to staining with fluorescent antibodies for flow cytometry. All analyses were performed on a LSRII flow cytometer (BD Biosciences). Stem and progenitor populations were identified as long-term hematopoietic stem cells (LTHSCs; Lin−Sca1+cKit+Flt3−CD150+CD48−), Multipotent progenitor cells (MPPs; Lin−Sca1+cKit+Flt3−CD150−CD48−, Lin−Sca1+cKit+Flt3−CD150+CD48+, Lin−Sca1+cKit+Flt3−CD150−CD48+), lymphoid-primed MPPs (LMPPs; Lin−Sca1+cKit+Flt3+CD150–), common myeloid progenitors (CMPs; Lin−Sca1−cKit+CD16/32−CD34+), granulocyte macrophage progenitors (GMPs; Lin−Sca1−cKit+CD16/32+CD34+), and megakaryocyte erythroid progenitors (MEPs; Lin−Sca1−cKit+CD16/32−CD34−). OBs were identified as CD45−Ter119−CD31−GFP+ cells. The following antibodies were used for flow cytometry: lineage markers-biotin (Ter119, CD3, NK1.1, immunoglobulin (Ig)M, CD4, CD8a, CD19, B220, Gr-1, CD11b, IL7Rα), anti-streptavidin-phycoerythrin (PE)-Texas Red, PerCP-Cy5.5 or Pacific blue, Flt3-PE or PerCP-cy5.5, Sca-1-Alexa700 or fluorescein isothiocyanate (FITC) or PE-cy7, CD117 (cKit)-allophycocyanin (APC)-eFluor780, CD150-PerCP-Cy5.5 or PE, CD48-APC or Pacific blue (Biolegend), CD229-PE, CD49b-FITC (BD Pharmingen), CD34-Alexa647, CD16/32-PE-Cy7, CD11b-PE-Cy7 or PE, Gr-1-Alexa700, CD19-Alexa647, B220-eFluor 450, CD4-PE-Cy7, CD8-PerCP-Cy5.5, CD45-PE, Ter119-APC-eFluor 780, CD31-APC, CD45.1-biotin or PE-Cy7, and CD45.2-FITC (eBioscience, San Diego, CA).
Transplantation studies
Following 28 days of GCV treatment, total BM cells (CD45.2; 8000, 40 000, or 200 000 cells per mouse) or LTHSCs (CD45.2; 50 cells per mouse, together with 200 000 wild-type CD45.1 BM cells), from control or ablated mice, were transplanted into 8-week-old lethally irradiated CD45.1 mice (B6-LY5.2/Cr, NCI). For LTHSC selection, the Lin− fraction was enriched via magnetic column separation (Miltenyi/MACS), stained with fluorescent antibodies for LTHSC markers (Lin: PE-TxRed, Sca1: FITC, cKit: APC-Cy7, Flt3: PE, CD150: PerCP-Cy5.5, CD48: APC), and sorted on a BD Aria III instrument (Becton Dickinson). Donor cell engraftment in PB was monitored after transplantation by flow cytometry examination of CD45.2 and lineage marker expression. At 15 to 16 weeks after transplantation, mice were euthanized, and BM and SP were collected for engraftment analysis and secondary transplantation. Engraftment was defined as >1% CD45.2 cells. The percentage of CD45.2 cells within the B-, T-, and myeloid cell populations was also analyzed (B cells: B220 + Pacific blue, CD19 + APC; T cells: CD4 + PE-Cy7; myeloid cells: CD11b + PE). For secondary transplantation, 500 000 whole BM cells from primary recipient mice were transplanted into 8-week-old lethally irradiated CD45.1 mice (B6-LY5.2/Cr). PB engraftment was analyzed after transplantation. At 16 weeks after transplantation, mice were euthanized, and engraftment in BM and SP was analyzed.
Cell cycle analysis
Lin− BM cells from GCV-treated mice were stained with antibodies for LTHSC markers, washed, fixed, permeabilized, and labeled with Ki67-FITC (BD Pharmingen) and 4,6 diamidino-2-phenylindole (DAPI) prior to flow cytometry analysis of cell cycle.
Analysis of CML mice
Col2.3GFP/Col2.3Δtk/Scl-tTA/BCR-ABL mice were treated with GCV for 1 week prior to induction of BCR-ABL expression via tetracycline withdrawal. GCV was administered at 10 mg/kg/day for the duration of the experiment. At 2, 4, and 6 weeks after BCR-ABL induction, leukemia development was monitored via PB analysis. Cells were labeled with fluorescent antibodies to Gr-1 (PE), Mac1/CD11b (APC-Cy7), and B220 (APC). Mice were monitored daily for survival, and the study was discontinued when all mice from the Col2.3GFP+/Col2.3Δtk+ group were deceased. In other experiments, CD45.2 LTHSCs (400 cells per mouse) selected from the BM of control or ablated BCR/ABL mice (28 days of GCV treatment and 3 weeks of BCR-ABL induction) were transplanted into lethally irradiated CD45.1 mice together with 200 000 wild-type CD45.1 BM cells. One set of recipients was followed for survival, and another was monitored for donor cell engraftment and WBC counts.
Jagged 1 expression in OBs from normal and BCR/ABL mice
OBs (CD45−Ter119−CD31−GFP+ cells) from the BM of normal and BCR-ABL mice (induced 3 weeks) were sorted and RNA was extracted using the RNeasy plus micro kit (Qiagen, Valencia, CA). Quantitative polymerase chain reaction analysis for detection of Jagged 1 transcripts was performed using a real-time TaqMan assay and the ABI Prism 7900 sequence detector (Applied Biosystems, Foster City, CA). Results were expressed as a ratio to actin.
In vitro effect of Jagged 1 on normal and BCR/ABL LTHSCs
LTHSCs from the BM of normal and BCR/ABL mice were cultured on 96-well plates with OP9 or OP9-Jagged-1 (OP9-Jag1) cells or coated with Jagged-1 Fc (Jag1/fc) or control IgG. For Jag1/Fc immobilization, plates were incubated overnight with goat anti-human IgG (Jackson ImmunoResearch), followed by addition of Jag1/Fc (10 μg/mL; R&D Systems) for 4 hours. OP9 or OP9-Jag1 were cultured in α-minimal essential medium (Invitrogen) with 20% fetal bovine serum (Hyclone). Confluent cells were irradiated with 2000 rads. LTHSCs were cultured on Jag1/Fc or OP9 plates for 48 hours, and cell numbers were measured by Lumino Glo (Promega). Apoptosis was analyzed by CD45 and Annexin V (BD, San Diego, CA) labeling of CD45+ cells. Cell cycle was analyzed by anti-Ki-67 (BD) and DAPI labeling.
Homing assay
Lin− donor cells from the BM of control mice (CD45.1) were transplanted into CD45.2 TK− and TK+ recipient mice that had been treated with GCV for 28 days (irradiated 900 rads, 1 × 106 Lin− cells per mouse). Engraftment of CD45.1+ donor nucleated cells, LSK, and LTHSCs in the BM of recipient mice was analyzed at 16 hours after transplantation by flow cytometry.
Statistics
Results are displayed as means ± standard errors of the means (SEM). Significance values for differences between groups were calculated using GraphPad Prism software using unpaired, nonparametric t tests (Mann-Whitney test) or 2-way analysis of variance. Survival was analyzed using Kaplan-Meyer curve analysis.
Results
Confirmation of OB ablation
The Col2.3Δtk mouse model allows OB-specific expression of HSV Δtk. Administration of GCV leads to formation of a thymidine base analog that incorporates into DNA, leading to OB cell death.21 Col2.3GFPΔtk mice were derived by crossing Col2.3Δtk mice with Col2.3GFP mice, with OB-specific expression of GFP,22 resulting in mice in which OBs could both be ablated and readily visualized (Figure 1A). To confirm OB elimination following GCV treatment, Col2.3GFP+Δtk+ (TK+) mice and control Col2.3GFP+Δtk− mice (TK−) mice were treated with GCV either at 8 mg/kg twice per day or 10 mg/kg once per day for a range of 21 to 40 days (Figure 1B). No adverse effects of GCV treatment were detected at any time point. Hematoxylin and eosin staining showed the presence of OBs lining the endosteal surface between the calcified bone and bone marrow in control TK− mice and their absence in TK+ mice following GCV treatment (Figure 1C). Immunohistochemical labeling of sections for GFP after antigen retrieval also demonstrated the absence of GFP+ OBs in the TK+ but not TK− mice treated with GCV (Figure 1C). Together these results provided visual confirmation of OB ablation. Treating mice once or twice per day did not produce significantly different results for OB ablation, nor did treating for 28 to 40 days (data not shown). In addition, flow cytometry analysis confirmed that GFP-expressing cells in the nonhematopoietic, nonendothelial, mesenchymal (CD45−Ter119−CD31−) cell fraction were significantly reduced in GCV-treated TK+ compared with TK− mice (Figure 1D).
Ablation of BM OBs in Col2.3GFP/Col2.3Δtk mice. (A) Strategy for generation of GFP-expressing OB ablation mice. (B) Experimental procedure for OB ablation and detection. (C) (Upper) Hematoxylin and eosin staining and (lower) GFP and DAPI staining of femur sections demonstrating the presence and absence of OB following GCV treatment in control (TK−) and ablation (TK+) mice, respectively. (D) Percentage (left) and number (right) of OBs in BM following GCV treatment as detected by flow cytometry (n = 19). Error bars represent mean ± SEM. Significance values: ***P < .001.
Ablation of BM OBs in Col2.3GFP/Col2.3Δtk mice. (A) Strategy for generation of GFP-expressing OB ablation mice. (B) Experimental procedure for OB ablation and detection. (C) (Upper) Hematoxylin and eosin staining and (lower) GFP and DAPI staining of femur sections demonstrating the presence and absence of OB following GCV treatment in control (TK−) and ablation (TK+) mice, respectively. (D) Percentage (left) and number (right) of OBs in BM following GCV treatment as detected by flow cytometry (n = 19). Error bars represent mean ± SEM. Significance values: ***P < .001.
Effects of OB ablation on BM hematopoietic stem and progenitor cell populations
GCV treatment resulted in significant decrease in overall BM cellularity in TK+ mice compared with TK− mice (Figure 2A), whereas SP cellularity remained unchanged (Figure 2B). The percentage and numbers of mature hematopoietic cell populations in BM and SP were not significantly altered (supplemental Figures 1 and 2). Analysis of committed progenitor populations in BM by flow cytometry (Figure 2C) showed a trend toward an increase in the percentage of GMP in OB-ablated mice (Figure 2D), without significant alteration of CMP and other progenitors (supplemental Figure 3A-H). The percentage and number of GMPs (Figure 2E) and percentage of CMPs (supplemental Figure 4A-H) were significantly increased in SP of GCV-treated TK+ mice compared with TK− mice, whereas other progenitor populations were not significantly altered (supplemental Figure 4). BM LSK cells, LSK Flt3−CD34−, or LSK Flt3−CD34−CD49b− HSC subpopulations were not significantly different in TK+ vs TK− mice (supplemental Figure 5A-C). On the other hand, the percentage of cells with the LTHSC phenotype (LSK Flt3−CD150+CD48−) was significantly increased in BM (Figure 2F) and SP (Figure 2G) of GCV-treated TK+ mice. Importantly, a highly selected LTHSC subpopulation lacking CD229 expression, LSK Flt3−CD34−CD49b−CD229−, was significantly reduced in TK+ mice (Figure 2H).
Effects of OB ablation on BM cellularity and stem and progenitor cell populations. (A) Total BM cellularity and (B) SP cellularity in control (TK−) and OB ablated (TK+) mice detected by flow cytometry. (C) Representative flow cytometry gating for LTHSCs. Percentage (left) and total cell number (right) of GMP in (D) BM and (E) SP of OB ablated and control mice. (F) Percentage and (G) total number of LTHSCs of OB ablated and control mice. (H) Percentage (left) and total cell number (right) of LSK Flt3−CD34−CD49b−CD229− cells in MB and SP of OB ablated and control mice. Error bars represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
Effects of OB ablation on BM cellularity and stem and progenitor cell populations. (A) Total BM cellularity and (B) SP cellularity in control (TK−) and OB ablated (TK+) mice detected by flow cytometry. (C) Representative flow cytometry gating for LTHSCs. Percentage (left) and total cell number (right) of GMP in (D) BM and (E) SP of OB ablated and control mice. (F) Percentage and (G) total number of LTHSCs of OB ablated and control mice. (H) Percentage (left) and total cell number (right) of LSK Flt3−CD34−CD49b−CD229− cells in MB and SP of OB ablated and control mice. Error bars represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
Effects of OB ablation on BM hematopoietic stem cell function
To evaluate BM hematopoietic function, whole BM cells (8000, 40 000, or 200 000 cells, CD45.2) obtained from TK+ and TK− mice treated with GCV for 28 days were transplanted into lethally irradiated recipient mice (CD45.1). Donor engraftment in blood was checked 5, 10, and 15 weeks after transplant. We did not observe significant overall differences in engraftment of BM cells from TK+ compared with TK− mice (supplemental Figure 6A). Given the increased frequency of cells with the LTHSC phenotype in BM of TK+ mice, these results suggest a possible reduction in long-term engraftment capacity of LTHSCs from OB-ablated mice. We further evaluated the effect of OB ablation on LTHSC function by examining the long-term engraftment capacity of purified LTHSCs (CD45.2, 50 cells per mouse) from GCV-treated TK− and TK+ mice transplanted into lethally irradiated CD45.1 mice (Figure 3A, primary transplant). At 16 weeks after transplant, engraftment of CD45.2 cells was significantly decreased in mice receiving LTHSCs from OB ablated compared with nonablated mice (Figure 3B). Analysis of myeloid, B, and T cells (supplemental Figure 6B, representative plots) revealed that LTHSCs from OB ablated mice demonstrated decreased multilineage potential (Figure 3C). To evaluate the cell cycle status of LTHSCs from BM of TK− and TK+ mice treated with GCV for 4 weeks, cells were labeled with Ki67 and DAPI and analyzed for G0, G1, and S/G2/M populations. OB ablation was associated with reduction in LTHSCs in G0 (TK−, 74 ± 6%; TK+, 69 ± 7%; n = 12), indicating their increased entry into cycle (Figure 3D). To examine secondary repopulation capacity, 500 000 whole BM cells, obtained at 16 weeks from primary transplant recipient mice, were transplanted into lethally irradiated CD45.1 secondary recipient mice, and engraftment of CD45.2 cells in PB was examined at 4, 8, 12, and 16 weeks (Figure 3A, secondary transplant). BM cells from OB ablated mice generated significantly reduced engraftment in secondary recipients in all 3 lineages (Figure 4A-B). CD45.2 and multilineage engraftment in BM were also significantly decreased (Figure 4C-D). These results indicate that LTHSCs from OB ablated mice demonstrate reduced quiescence and impaired long-term regenerative and self-renewing capacity.
Engraftment of LTHSC from OB-ablated mice after transplantation. (A) Schematic representation of experimental procedure for transplantation of LTHSCs (CD45.2) selected from BM of OB ablated or nonablated mice into lethally irradiated CD45.1 wild-type recipient mice and secondary transplantation of whole BM cells from primary recipient mice into secondary recipient mice. (B) Engraftment of CD45.2 cells following transplantation of LTHSCs from control (TK−) or OB ablated (TK+) mice. (C) Engraftment of CD45.2 myeloid, B-cell, and T-cell lineages following transplantation of LTHSCs from control (TK−) or OB ablated (TK+) mice. (D) Lin− cells selected from BM of control (TK−) or OB ablated (TK+) mice were stained with LTHSC markers, fixed, permeabilized, labeled with Ki-67 and DAPI, and analyzed for cell cycle by flow cytometry. Error bars represent mean ± SEM. Significance values: *P < .05, **P < .01.
Engraftment of LTHSC from OB-ablated mice after transplantation. (A) Schematic representation of experimental procedure for transplantation of LTHSCs (CD45.2) selected from BM of OB ablated or nonablated mice into lethally irradiated CD45.1 wild-type recipient mice and secondary transplantation of whole BM cells from primary recipient mice into secondary recipient mice. (B) Engraftment of CD45.2 cells following transplantation of LTHSCs from control (TK−) or OB ablated (TK+) mice. (C) Engraftment of CD45.2 myeloid, B-cell, and T-cell lineages following transplantation of LTHSCs from control (TK−) or OB ablated (TK+) mice. (D) Lin− cells selected from BM of control (TK−) or OB ablated (TK+) mice were stained with LTHSC markers, fixed, permeabilized, labeled with Ki-67 and DAPI, and analyzed for cell cycle by flow cytometry. Error bars represent mean ± SEM. Significance values: *P < .05, **P < .01.
LTHSC functional analysis: secondary transplant. (A) CD45.2 engraftment analysis of LTHSCs derived from control (TK−) or ablation (TK+) mice in BM 16 weeks after secondary transplant. (B) Multilineage engraftment analysis of LTHSCs derived from control (TK−) or ablation (TK+) mice in BM 16 weeks after secondary transplant. (C) CD45.2 and multilineage (D) engraftment analysis of whole BM derived from secondary transplant recipients at 16 weeks. Engraftment is defined as >1% CD45.2, detected by flow cytometry. Error bars represent mean ± SEM. ***P < .0001 by 2-way analysis of variance.
LTHSC functional analysis: secondary transplant. (A) CD45.2 engraftment analysis of LTHSCs derived from control (TK−) or ablation (TK+) mice in BM 16 weeks after secondary transplant. (B) Multilineage engraftment analysis of LTHSCs derived from control (TK−) or ablation (TK+) mice in BM 16 weeks after secondary transplant. (C) CD45.2 and multilineage (D) engraftment analysis of whole BM derived from secondary transplant recipients at 16 weeks. Engraftment is defined as >1% CD45.2, detected by flow cytometry. Error bars represent mean ± SEM. ***P < .0001 by 2-way analysis of variance.
We also evaluated homing of normal HSCs to the BM of OB ablated mice. BM cells from normal mice (CD45.1) were transplanted intravenously into TK− and TK+ mice (CD45.2) that were treated with GCV for 4 weeks. Engraftment of donor cells was analyzed after 16 hours. No significant differences were seen in Lin−, LSK, or LTHSC engraftment in BM of TK+ or TK− mice, indicating that OB ablation does not affect homing of LTHSCs in BM (supplemental Figure 6C).
Effect of OB ablation on leukemic hematopoiesis
To examine how OB ablation may affect leukemic hematopoiesis, we used transgenic SCL-tTA/BCR-ABL mice, which provide a representative model of human chronic phase CML. This model has previously proven useful for in vivo characterization of CML LTHSCs and their microenvironmental interactions. Tetracycline withdrawal results in induction of BCR-ABL expression in LTHSCs and development of a CML-like myeloproliferative disorder. An OB ablation CML mouse model was developed by crossing the Col2.3GFP/Col2.3Δtk mice with SCLtTA/BCR-ABL (BCR-ABL) mice (Figure 5A). To examine the effect of OB ablation on CML development, BCR-ABL TK+ and TK− mice were treated with GCV for 1 week to initiate OB ablation, followed by tetracycline withdrawal to induce BCR-ABL expression, with continuing daily GCV administration. Leukemia development was monitored by examining blood counts every 2 weeks (Figure 5B). OB ablated mice demonstrated enhanced neutrophilic leukocytosis at 6 weeks after induction compared with nonablated CML mice (Figure 5C). Mice were followed for survival until all GCV-treated BCR-ABL+ TK+ mice were deceased. Kaplan-Meyer analysis demonstrated significantly decreased survival of OB ablated CML mice compared with controls (Figure 5D). These results indicate that OB ablation results in accelerated leukemia development.
CML induction in OB ablation model. (A) Schematic representation of generation of OB ablation CML induction mice. (B) Schematic representation of CML induction. (C) WBC counts, percentage of neutrophils, and Gr-1+CD11b+ cells in the PB of control and OB ablated BCR/ABL mice at 6 weeks after BCR/ABL induction. (D) Survival curve for control or OB ablated BCR/ABL mice. (E-F) After 28 days of GCV treatment and 3 weeks of BCR-ABL induction, CD45.2 LTHSCs (400 cells per mouse) were sorted from control or OB ablated BCR/ABL mice and transplanted into CD45.1 recipient mice. (E) Survival curves for transplanted mice. (F) Donor cell engraftment, WBC counts, and percentage of Gr-1+CD11b+ cells in PB was monitored. *P < .05. Error bars represent mean ± SEM.
CML induction in OB ablation model. (A) Schematic representation of generation of OB ablation CML induction mice. (B) Schematic representation of CML induction. (C) WBC counts, percentage of neutrophils, and Gr-1+CD11b+ cells in the PB of control and OB ablated BCR/ABL mice at 6 weeks after BCR/ABL induction. (D) Survival curve for control or OB ablated BCR/ABL mice. (E-F) After 28 days of GCV treatment and 3 weeks of BCR-ABL induction, CD45.2 LTHSCs (400 cells per mouse) were sorted from control or OB ablated BCR/ABL mice and transplanted into CD45.1 recipient mice. (E) Survival curves for transplanted mice. (F) Donor cell engraftment, WBC counts, and percentage of Gr-1+CD11b+ cells in PB was monitored. *P < .05. Error bars represent mean ± SEM.
We compared the ability of LSC from OB ablated and nonablated CML mice to engraft and generate leukemia after transplantation. LTHSC populations isolated from BCR-ABL+ TK+ and TK− mice (CD45.2) treated with GCV for 4 weeks were transplanted into CD45.1 recipient mice (400 cells per mouse). We observed increased survival of mice receiving LTHSC from OB ablated BCR-ABL mice compared with control mice (Figure 5E), with reduced neutrophilic leukocytosis (Figure 5F). These results indicate that LTHSCs from OB ablated BCR-ABL mice show reduced long-term engraftment and leukemogenic capacity after transplantation.
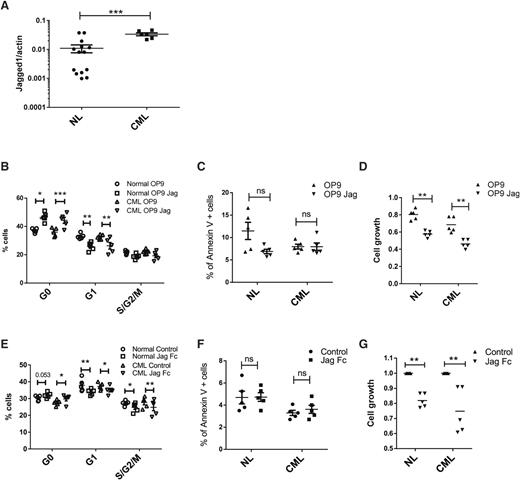
Role of Jagged-1 in regulating normal and CML LTHSC growth
Jagged-1 expressed on OBs has been reported to contribute to maintenance of HSC repopulating capacity.5 Recently, expression of an activated β-catenin mutant in OB was reported to induce expression of Jagged-1 in OB, activate Notch signaling in HSC, and promote development of AML.23 We therefore analyzed expression of Jagged-1 in OBs from normal and CML mice and its role in regulating normal and BCR-ABL+ LTHSC growth. Jagged-1 mRNA expression was increased in OB purified from CML mice compared with normal mice (Figure 6A). To evaluate response to Jagged-1 exposure, normal and BCR-ABL+ LTHSCs were cultured with OP9 cells engineered to express Jagged-1 (OP9-Jag) compared with control OP9 cells. Both normal and BCR-ABL+ LTHSCs cultured with OP9-Jag cells showed a significant increase in G0 and reduction in G1 and S/G2/M cells (Figure 6B), but no difference in cell survival (Figure 6C). BCR-ABL+ and normal LTHSCs generated reduced numbers of cells in culture with OP9-Jag compared with control OP9 cells (Figure 6D). Similarly, culture of BCR-ABL+ and normal LTHSCs on plates coated with Jagged-1-Fc also led to reduced cell cycle (Figure 6E), did not affect apoptosis (Figure 6F), and resulted in generation of reduced numbers of cells (Figure 6G) compared with control plates. These results support a role for Jagged-1 in inhibiting cell cycling of both normal and CML LTHSCs. Loss of Jagged-1 signals following OB ablation could potentially contribute to loss of quiescence and long-term self-renewing capacity of normal LTHSCs, as well as increased cycling of BCR-ABL+ LTHSCs.
Role of Jagged-1 in regulating normal and CML LTHSC growth. (A) Jagged 1 expression in OBs from normal and BCR/ABL mice. (B) Cell cycle, (C) apoptosis, and (D) cell growth of LTHSCs from control and BCR/ABL mice after coculture with OP9 or OP9-Jag1 for 48 hours. (E) Cell cycle, (F) apoptosis, and (G) cell growth of LTHSCs from control and BCR/ABL mice after culture with or without Jag1/Fc immobilized wells. NL, normal.
Role of Jagged-1 in regulating normal and CML LTHSC growth. (A) Jagged 1 expression in OBs from normal and BCR/ABL mice. (B) Cell cycle, (C) apoptosis, and (D) cell growth of LTHSCs from control and BCR/ABL mice after coculture with OP9 or OP9-Jag1 for 48 hours. (E) Cell cycle, (F) apoptosis, and (G) cell growth of LTHSCs from control and BCR/ABL mice after culture with or without Jag1/Fc immobilized wells. NL, normal.
Discussion
In the current study, we generated mouse models allowing for both OB ablation and OB visualization, allowing us to establish conditions under which OBs are effectively and consistently ablated. OB ablation was associated with an increase in cells with LTHSC phenotype. However, LTHSCs from OB-ablated mice exhibited reduced quiescence, long-term engraftment, and secondary repopulating capacity, suggesting that OBs contribute to preservation of long-term self-renewing HSCs. By crossing the OB ablation and visualization model with a transgenic BCR-ABL mouse model, we demonstrated that OB ablation resulted in accelerated development of leukemia, consistent with increased LSC proliferation. These results enhance our understanding of the role of OBs in regulation of normal and leukemic hematopoiesis.
Our results support a role for OBs in regulating the long-term self-renewal potential of LTHSCs. Previous reports indicated a more severe phenotype of GCV-treated TK+ mice with loss of lymphoid, erythroid, and myeloid progenitors in the BM, decrease in HSC numbers, and increased extramedullary hematopoiesis.3,21 These studies were performed in CD-1/ICR mice, and it is possible the severity of the phenotype observed is strain dependent. The milder phenotype observed here is consistent with reports indicating the importance of other BM niches in HSC maintenance. Deletion of stem cell factor or CXCL12 from OBs did not affect LTHSC maintenance and instead supported a critical role for endothelial and perivascular mesenchymal cells in HSC maintenance.10,11 However, other OB-mediated regulatory mechanisms could contribute to HSC maintenance. Indeed, multiple niche cells may cooperate to provide signals required for HSC maintenance.12,13
Reduced HSC self-renewal in OB ablated mice may be related to loss of LTHSC quiescence. It has been reported that OBs in trabecular bone area express high levels of Jagged-1, which interacts with Notch ligand on HSCs. HSCs that are not bound to Jagged-1 have reduced repopulating capacity.5 Our studies indicate that Jagged-1 inhibits LTHSC entry into cell cycle. Loss of Jagged-1-induced signaling may contribute to loss of LTHSC quiescence following OB ablation. However, other mechanisms could also contribute. CD166 or activated leukocyte cell adhesion molecule receptor is expressed on both HSCs and OBs and may support self-renewal of long-term repopulating cells.7,8,24 OB ablation could also indirectly affect HSC function by affecting other hematopoietic niche components.1 The physical change to the endosteal region on OB ablation could impact the way LTHSCs are able to interact with other niche components. Finally, the GCV used to induce OB ablation generates a toxic thymidine analog through interaction with ΔTK. Although we cannot rule out the possibility that the toxic thymidine analog could have affected surrounding cells, this appears unlikely because total LTHSC numbers were not reduced and instead were increased after OB ablation.
The enhanced leukocytosis and decreased overall survival observed following OB ablation in CML mice supports a role for OBs in regulating CML LSC proliferation, inhibiting leukemia development in primary CML mice. These observations are consistent with studies using a transduction-transplantation model of CML that indicate that increased OB development and bone remodeling following parathyroid hormone administration was associated with decreased LSC proliferation.25 Interestingly, Jagged-1 was overexpressed in OB from BCR-ABL compared with normal mice. As with normal LTHSCs, Jagged-1 exposure inhibited cell cycling of BCR-ABL LTHSCs, suggesting that loss of Jagged-1 signaling could contribute to accelerated leukemia development following OB ablation. The role of OB-mediated Jagged-1 signaling in CML LSC regulation and persistence warrants further investigation in future studies. We also observed that LSCs from OB ablated mice demonstrated impaired capacity to generate leukemia in secondary recipients after transplantation. Although the underlying mechanisms are unclear, one possible explanation could be reduced LSC self-renewal related to increased proliferation. However, reduced leukemogenicity in secondary recipients could be related to functional alterations in LSC homing and engraftment properties following OB ablation. It will be of interest to determine whether OB signaling is important for LSC maintenance and preventing their exhaustion over time.
In conclusion, our studies provide new insights into the role of the OB niche in maintaining long-term self-renewal of normal HSCs and in regulating CML LSCs to modulate leukemia evolution. These studies support further efforts to elucidate OB induced signals modulating HSC self-renewal and OB alterations and signaling in leukemia.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the analytical cytometry and surgical pathology cores for assistance and the laboratory of Dr Karen Aboody.
This work was supported by National Institutes of Health, National Cancer Institute grants R01 CA172447 and P30CA033572 and the California Institute for Regenerative Medicine.
Authorship
Contribution: M.B. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; B.Z. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; Y.H. performed experiments; P.A. performed experiments; C.-C.C. designed experiments and interpreted data; and R.B. designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.A. and R.B. is Division of Hematology-Oncology, University of Alabama at Birmingham, Birmingham, AL.
Correspondence: Ravi Bhatia, Division of Hematology-Oncology, Department of Medicine, University of Alabama Birmingham, 1802 6th Ave, South, North Pavilion, Room 2555C, Birmingham, AL 35223; e-mail: rbhatia@uabmc.edu.
References
Author notes
M.B. and B.Z. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal