Key Points
ATM-deficient T-cell lymphocytopenia is in part caused by defects in TCRδ rearrangements.
Aberrant TCRδ arrangement is required for the recurrent t(12;14) translocations, but not chromosome 14 amplification, in ATM-deficient thymic lymphomas.
Abstract
Ataxia telangiectasia mutated (ATM) is a protein kinase and a master regulator of DNA-damage responses. Germline ATM inactivation causes ataxia-telangiectasia (A-T) syndrome with severe lymphocytopenia and greatly increased risk for T-cell lymphomas/leukemia. Both A-T and T-cell prolymphoblastic leukemia patients with somatic mutations of ATM frequently carry inv(14;14) between the T-cell receptor α/δ (TCRα/δ) and immunoglobulin H loci, but the molecular origin of this translocation remains elusive. ATM−/− mice recapitulate lymphocytopenia of A-T patients and routinely succumb to thymic lymphomas with t(12;14) translocation, syntenic to inv(14;14) in humans. Here we report that deletion of the TCRδ enhancer (Eδ), which initiates TCRδ rearrangement, significantly improves αβ T cell output and effectively prevents t(12;14) translocations in ATM−/− mice. These findings identify the genomic instability associated with V(D)J recombination at the TCRδ locus as the molecular origin of both lymphocytopenia and the signature t(12;14) translocations associated with ATM deficiency.
Introduction
As a master regulator of the DNA damage response, ataxia telangiectasia mutated (ATM) kinase is rapidly activated by DNA double-strand breaks and phosphorylates substrates involved in both DNA repair and checkpoint control. Loss of ATM causes ataxia-telangiectasia (A-T) syndrome characterized by primary immunodeficiency and greatly increased risk for lymphoid malignancies, especially of T-cell lineage.1 Moreover, >50% of T-cell prolymphoblastic leukemia (T-PLL) patients carry a somatic mutation of ATM.2 Both T-PLL and A-T patients carry inv(14;14) that involves the T-cell receptor α/δ (TCRα/δ) and immunoglobulin H loci on human chromosome 14. However, the molecular origin of the seemingly T-cell–specific lymphocytopenia and inv(14;14) remains unknown.
ATM−/− mice recapitulate the T-cell lymphocytopenia of A-T patients and succumb to thymic lymphomas with TCRα/δ-related chromosome 14 amplification and t(12;14) translocations, syntenic with inv(14;14) in humans.3 Reduced αβ T-cell numbers and blockade at the double-positive (DP) to single-positive (SP) transition is characteristic of ATM-deficient T cells.4,5 Both human and mouse TCRδ loci reside between Vα and Jα segments.6 As such, TCRα rearrangement deletes the TCRδ gene, leading to irreversible commitment to the αβ T-cell lineage. This stage-specific rearrangement of the TCRδ in double-negative (DN) and TCRα in DP T cells is driven by the loci-specific enhancer elements Eδ and Eα, respectively.6,7 However, Eα deletion does not affect the kinetics or translocation patterns of ATM-deficient thymic lymphomas,3 suggesting that TCRδ might be involved. In this context, TCRδ-related translocations are found in the majority of T-ALL and >90% of TAL1 translocated cases.8 Using ATM−/−Eδ−/− mice, here we identified aberrant TCRδ rearrangements as the origin for both αβ T-cell development defects and the recurrent t(12;14) translocations in ATM-deficient mice.
Study design
All animal work was conducted with proof from the institutional Animal Care and Use Committee of Columbia University. ATM−/−9 and Eδ−/−10 mice were described previously. T-cell development was characterized in 4- to 6-week-old mice as previously described.11 Briefly, single-cell suspensions of thymus and spleen were counted and stained with fluorophore-conjugated antibodies against CD4, CD8, CD3, TCRβ, or TCRγ. For DN staining, thymocytes were counterstained with phycoerythrin-conjugated anti-CD4, CD8, B220, TCRγδ, Ter119 and CD19 antibodies, then with fluorophore-conjugated anti-CD44 and anti-CD25 antibodies. Flow cytometry data were collected on FACSCalibur (BD) and analyzed with FlowJo. For cytogenetic analyses, single cell suspensions were incubated with colcemid (10 µg/mL) for 4 to 10 hours and fixed and stained3 using ASI applied spectral imaging paints. Images were acquired and analyzed with Isis and Metafer 4 (MetaSystems, Newton, MA). Comparative genomic hybridization (CGH) was performed with the Agilent 244K mouse array. Reverse-transcription polymerase chain reaction (PCR) methods are detailed in Figure 2F and supplemental Table 1 (available on the Blood Web site).
Results and discussion
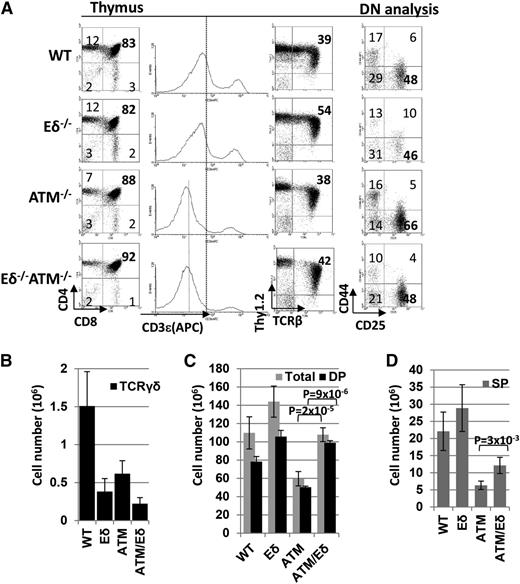
ATM−/−Eδ−/− mice were born at the expected ratio and undistinguishable from ATM−/− littermates. In thymuses of ATM−/−Eδ−/− mice, γδ T-cell frequency was reduced, but not abolished, consistent with the role of Eα in residual γδ T-cell development10 (Figure 1A-B). Although thymus cellularity was reduced by ∼50% in ATM−/− mice, both thymus cellularity and the number of mature SP T cells were significantly rescued in ATM−/−Eδ−/− mice (Figure 1C-D). This is unexpected, given that >90% of thymocytes are αβ T cells. Further analyses showed that this rescue was mostly due to moderate increases of DNIV (CD25−CD44−) percentage and marked increases of DP thymocyte number without changes in the SP to DP ratio (Figure 1C-D and supplemental Figure 1A-B). TCRδ undergoes V(D)J recombination in DNII-DNIII (CD25+).10 ATM is particularly important for inversional V(D)J recombination,12 and Vδ5 is located downstream of Cδ and requires inversional rearrangement.13 The configuration of the TCRα/δ locus also determines that if DNA breaks in the TCRδ loci are unrepaired, then Eα would separate from Vαs and disable TCRα V(D)J recombination (Figure 2C). These findings reveal an important role of genomic instability at the TCRδ locus in αβ T-cell development and T-cell lymphocytopenia associated with ATM deficiency, and they suggest that suppression of TCRδ rearrangement might improve T-cell counts in A-T patients.
Deletion of Eδ partially rescues lymphocytopenia in ATM-deficient mice. (A) Representative flow cytometric analyses of premalignant thymocytes from wild-type (WT), ATM−/−, Eδ−/−, and ATM−/−Eδ−/− mice (4-6 weeks). (B) Total thymic γδ cell number counts (×106). (C) Total number of thymocytes and DP cells (×106). (D) SP cell number counts (×106). Data represent the average ± standard deviation from at least 5 independent mice of each genotype (see supplemental Figure 1A for details). The P values between 2 genotypes were calculated based on the 2-tailed Student t test assuming unequal variance. Supplemental Figure 1B summarizes the P value from pairwise comparison for total, DP, and SP thymocytes between different genotypes.
Deletion of Eδ partially rescues lymphocytopenia in ATM-deficient mice. (A) Representative flow cytometric analyses of premalignant thymocytes from wild-type (WT), ATM−/−, Eδ−/−, and ATM−/−Eδ−/− mice (4-6 weeks). (B) Total thymic γδ cell number counts (×106). (C) Total number of thymocytes and DP cells (×106). (D) SP cell number counts (×106). Data represent the average ± standard deviation from at least 5 independent mice of each genotype (see supplemental Figure 1A for details). The P values between 2 genotypes were calculated based on the 2-tailed Student t test assuming unequal variance. Supplemental Figure 1B summarizes the P value from pairwise comparison for total, DP, and SP thymocytes between different genotypes.
Aberrant TCRδ rearrangement is required for t(12;14), but not chromosomal 14 amplifications, in murine ATM-deficient thymic lymphomas. (A) Survival curve for ATM−/− (n = 10), ATM−/−Eδ−/− (n = 11), and ATM−/−Eδ+/− (n = 8) mice with median survival at 99, 112, and 137 days, respectively. P values were calculated using the log-rank test. (B) Representative chromosome 12 (green) and chromosome 14 (red) paint analyses of a metaphase from no. 745 ATM−/−Eδ−/− lymphomas (without t(12;14)) and no. 900 ATM−/−Eδ+/− lymphomas with t(12;14) translocation. More than 20 metaphases were analyzed for each lymphoma, and t(12;14) in >50% metaphases was used as the criterion for clonal translocations. The results are summarized in panel E. (C) Southern blot mapping the rearrangements of Cδ and Jα. The diagram shows the TCRα/δ locus, EcoRI (black arrows), and probe locations (black dashed line for Southern probes and red dashes for adjacent CGH probes). A total of 10 µg EcoRI-digested genomic DNA from either tumor (T) or the corresponding kidney (K) was analyzed on each lane. Loading control probe detected a fragment of the single-copy DNA-PKcs gene. (D) CGH analyses of ATM−/−Eδ−/− thymic lymphomas 745 and 545. Each tumor DNA was analyzed with matched kidney control DNA on a 244K mouse CGH array from Agilent. The log ratio of tumor vs kidney control at each probe location is displayed in centromere-to-telomere orientation for each chromosome (from 1 to 19 then X/Y, from left to right). The red arrows and labels indicate recurrent amplification of Notch1, chromosome 14 upstream of TCRα/δ locus, and trisomy 15. Green arrows and labels indicate recurrent deletion of the telomeric portion of chromosome 12 and the Pten locus, as well as deletional rearrangement in TCR loci. (E) Summary of chromosome 12/14 paint analyses of ATM−/−Eδ−/− and ATM−/−Eδ+/− lymphomas. (F) Quantitative PCR results that measured the expression of IL7R, Jak1, Notch1, Pten, Rb1, Bcl11b, and Psmc6 genes from ATM−/−Eδ+/+ (n = 3), ATM−/−Eδ+/− (n = 4), ATM−/−Eδ−/− (n = 5) lymphomas. Psmc6 locates at the amplification area within chromosome 14 and is used as a marker for the amplification here.3 Bcl11b is within the region that is hemizygously deleted in chromosome 12.3 Briefly, total RNA was extracted using Trizol (Invitrogen, CA) from a single-cell suspension derived from ATM−/− and ATM−/−Eδ−/− thymic lymphomas as well as wild-type total thymus, and dissolved in 100 μL DEPC-H2O and verified to have A260 nm/280 nm between 1.8 and 2. Approximately 5 μg of total RNA was treated with RNase-free DNase I (Roche). The DNaseI activity was abolished by the addition of 2 µL of 25-mM EDTA and incubation at 65°C for 15 minutes. Reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen) and Oligod(T)18 (New England Biolabs) according to the manufacturer’s instructions. The resulted complementary DNA was dissolved in TE and quantified with NanoDrop (Thermo Scientific). Quantitative PCR was then performed with gene-specific primers (supplemental Table 1) for Notch1 (NM_008714.3, 138 bp across exons 25-26), Jak I (NM_146145.2, 162 bp across exons 18-20), IL7R (NM_008372.4, 149 bp across exons 5-7), Rb1 (NM_009029.2, 144 bp across exons 23-25), Bcl11b (NM_001079883.1, 126 bp across exons 3-4, within chromosome 12 telomeric deletion), Pten (NM_008960.2, 144 bp across exons 7-8), Psmc6 (NM_025959.3, 196 bp across exons 7-9, within chromosome 14 amplification), and β-actin (actb; NM_007393.3, 154 bp across exons 1-2). Reaction setups include 10 µL Sybr Select Master Mix (Applied Biosystems), 250 nM each primer, 10 ng complementary DNA, and water to a total of 20 µL. The PCR condition was 50°C (2 minutes), 95°C (10 minutes), and [95°C (15 seconds), 60°C (1 minute)] × 40, followed by the melt curve program provided by 7500 Real Time PCR System (Applied Biosystems). The results were analyzed with 7500 software package (v 2.0.6) and the ΔΔCT method (included in 7500 software) with the expression of β-actin as the reference and the expression of the specific gene in the wild-type samples as controls for normalization. Each gene in each sample was analyzed in triplicate together with H2O control. The H2O control samples always had CT beyond the range (>40) in all experiments.
Aberrant TCRδ rearrangement is required for t(12;14), but not chromosomal 14 amplifications, in murine ATM-deficient thymic lymphomas. (A) Survival curve for ATM−/− (n = 10), ATM−/−Eδ−/− (n = 11), and ATM−/−Eδ+/− (n = 8) mice with median survival at 99, 112, and 137 days, respectively. P values were calculated using the log-rank test. (B) Representative chromosome 12 (green) and chromosome 14 (red) paint analyses of a metaphase from no. 745 ATM−/−Eδ−/− lymphomas (without t(12;14)) and no. 900 ATM−/−Eδ+/− lymphomas with t(12;14) translocation. More than 20 metaphases were analyzed for each lymphoma, and t(12;14) in >50% metaphases was used as the criterion for clonal translocations. The results are summarized in panel E. (C) Southern blot mapping the rearrangements of Cδ and Jα. The diagram shows the TCRα/δ locus, EcoRI (black arrows), and probe locations (black dashed line for Southern probes and red dashes for adjacent CGH probes). A total of 10 µg EcoRI-digested genomic DNA from either tumor (T) or the corresponding kidney (K) was analyzed on each lane. Loading control probe detected a fragment of the single-copy DNA-PKcs gene. (D) CGH analyses of ATM−/−Eδ−/− thymic lymphomas 745 and 545. Each tumor DNA was analyzed with matched kidney control DNA on a 244K mouse CGH array from Agilent. The log ratio of tumor vs kidney control at each probe location is displayed in centromere-to-telomere orientation for each chromosome (from 1 to 19 then X/Y, from left to right). The red arrows and labels indicate recurrent amplification of Notch1, chromosome 14 upstream of TCRα/δ locus, and trisomy 15. Green arrows and labels indicate recurrent deletion of the telomeric portion of chromosome 12 and the Pten locus, as well as deletional rearrangement in TCR loci. (E) Summary of chromosome 12/14 paint analyses of ATM−/−Eδ−/− and ATM−/−Eδ+/− lymphomas. (F) Quantitative PCR results that measured the expression of IL7R, Jak1, Notch1, Pten, Rb1, Bcl11b, and Psmc6 genes from ATM−/−Eδ+/+ (n = 3), ATM−/−Eδ+/− (n = 4), ATM−/−Eδ−/− (n = 5) lymphomas. Psmc6 locates at the amplification area within chromosome 14 and is used as a marker for the amplification here.3 Bcl11b is within the region that is hemizygously deleted in chromosome 12.3 Briefly, total RNA was extracted using Trizol (Invitrogen, CA) from a single-cell suspension derived from ATM−/− and ATM−/−Eδ−/− thymic lymphomas as well as wild-type total thymus, and dissolved in 100 μL DEPC-H2O and verified to have A260 nm/280 nm between 1.8 and 2. Approximately 5 μg of total RNA was treated with RNase-free DNase I (Roche). The DNaseI activity was abolished by the addition of 2 µL of 25-mM EDTA and incubation at 65°C for 15 minutes. Reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen) and Oligod(T)18 (New England Biolabs) according to the manufacturer’s instructions. The resulted complementary DNA was dissolved in TE and quantified with NanoDrop (Thermo Scientific). Quantitative PCR was then performed with gene-specific primers (supplemental Table 1) for Notch1 (NM_008714.3, 138 bp across exons 25-26), Jak I (NM_146145.2, 162 bp across exons 18-20), IL7R (NM_008372.4, 149 bp across exons 5-7), Rb1 (NM_009029.2, 144 bp across exons 23-25), Bcl11b (NM_001079883.1, 126 bp across exons 3-4, within chromosome 12 telomeric deletion), Pten (NM_008960.2, 144 bp across exons 7-8), Psmc6 (NM_025959.3, 196 bp across exons 7-9, within chromosome 14 amplification), and β-actin (actb; NM_007393.3, 154 bp across exons 1-2). Reaction setups include 10 µL Sybr Select Master Mix (Applied Biosystems), 250 nM each primer, 10 ng complementary DNA, and water to a total of 20 µL. The PCR condition was 50°C (2 minutes), 95°C (10 minutes), and [95°C (15 seconds), 60°C (1 minute)] × 40, followed by the melt curve program provided by 7500 Real Time PCR System (Applied Biosystems). The results were analyzed with 7500 software package (v 2.0.6) and the ΔΔCT method (included in 7500 software) with the expression of β-actin as the reference and the expression of the specific gene in the wild-type samples as controls for normalization. Each gene in each sample was analyzed in triplicate together with H2O control. The H2O control samples always had CT beyond the range (>40) in all experiments.
Despite improved T-cell counts, the kinetics of succumbing to thymic lymphomas was similar in ATM−/−Eδ−/− mice and ATM−/− controls (P = .1), with a median survival of 112 days (vs 99 for ATM−/− controls) (Figure 2A). ATM−/−Eδ−/− thymic lymphomas consisted of immature (surface TCRβlow) DP T cells similar to ATM−/− controls (supplemental Figure 1C). Southern blot identified clonal TCRβ rearrangements and focal amplification centromeric to the TCRα/δ loci (chromosome 14) in all ATM−/−Eδ−/− (or ATM−/−Eδ+/−) lymphomas as documented for ATM−/− lymphomas (supplemental Figures 1D and 2A).3 ATM−/− thymic lymphomas were derived from immature T cells that had not yet undergone TCRα arrangement, evidenced by retention of the Cδ.3 All ATM−/−Eδ+/− and 2 of 5 ATM−/−Eδ−/− lymphomas (545 and 2280) retained at least 1 Cδ, suggesting that the chromosome 14 amplifications most likely occurred during TCRδ rearrangement in those tumors, consistent with the ability for Eα to drive residual TCRδ rearrangements in the absence of Eδ.10 Notably, the other 3 ATM−/−Eδ−/− lymphomas showed homozygous deletion of Cδ, indicative of biallelic TCRα rearrangements (Figure 2C). Southern blot further confirmed that all 3 tumors (497, 713, and 745) rearranged the proximal Jα segments (Figure 2C). Thus, TCRδ rearrangement is not required for chromosome 14 amplification and T-cell lymphomagenesis in ATM-deficient mice. In the absence of the TCRδ rearrangement, aberrant TCRα rearrangement is able to promote chromosome 14 amplifications and oncogenic transformation of ATM-deficient T cells.
We next investigated the origin of the t(12;14) translocations characteristic of ATM deficiency.3 Chromosome paint identified clonal t(12;14) translocations in all ATM−/−Eδ+/− lymphomas and the 2 Cδ-positive ATM−/−Eδ−/− lymphomas (545 and 2280), but not in the 3 ATM−/−Eδ−/− lymphomas (497, 713, and 745) with biallelic TCRα rearrangements (Figure 2B,E). CGH analyses of 2 Cδ-positive (2280 and 545) and 2 Cδ-negative (497 and 745) ATM−/−Eδ−/− lymphomas confirmed focal amplification upstream of the TCRα/δ locus (Figure 2D and supplemental Figure 2B) and also revealed a unique hemizygous deletion of chromosome 14 downstream of the TCRα/δ locus only in Cδ-negative (497 and 745) tumors (Figure 2D and supplemental Figure 2B). CGH probe mapping showed that the deletion started at the proximal Jα (Figure 2C-D). Together with the absence of the t(12;14) translocation, this result suggests that when chromosome 14 amplification occurs in DP T cells during TCRα arrangement, the break in chromosome 12 is no longer available to form the t(12;14) translocation, causing the telomeric portion of chromosome 14 to be lost and pointing to concurrent immunoglobulin H and TCRδ rearrangement in DN T cells as the molecular origin for t(12;14). This further implied that t(12;14) and the syntenic inv(14,14) are not essential for T-cell transformation. Despite the lack of t(12;14), both Cδ-negative (497 and 745) tumors displayed hemizygous deletion of chromosome 12 telomeric regions, supporting the existence of potential tumor-suppressor genes (eg, Bcl11b).3,14-16 In this context, SKY analyses identified t(12;15) translocations involving chromosome 15 in tumor 745 (Figure 2B and supplemental Figure 2C). Finally, CGH also indicated that murine ATM−/− thymic lymphomas, regardless of Eδ status, display genetic changes that have been associated with human T-cell acute lymphoblastic leukemia (T-ALL), including trisomy of chromosome 15 containing c-Myc, amplification and overexpression of Notch1, deletion of Pten, and overexpression of IL7R (Figure 2F), highlighting ATM-deficient murine thymic lymphomas as a bona fide animal model for human T-ALL. In summary, our study identifies aberrant TCRδ rearrangement in the absence of ATM together with the unique configurations of the TCRα/δ locus as one of the major causes to both T-cell–specific lymphocytopenia and the t(12;14) and related inv(14;14) associated with ATM deficiency and potentially in other T-ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) National Cancer Institute grants 5R01CA158073, 1R01CA184187, and 1P01CA174653-01 and American Cancer Society Research Scholar Grant RSG-13-038-01 DMC (S.Z.) and by NIH National Cancer Institute grant P01CA109901 (F.W.A.). S.Z. was a St Baldrick’s Scholar for Pediatric Cancer and is a Leukemia Lymphoma Society Scholar. W.J. was supported in part by NIH National Cancer Institute grant T32-CA09503. F.W.A. is a Howard Hughes Medical Institute investigator.
Authorship
Contributions: W.J., F.W.A., and S.Z. designed experiments; S.Z. wrote the paper; M.G. performed the SKY analyses for tumor 745; and W.J., B.J.L., C.L., R.L.D., and S.Z. performed the rest of the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shan Zha, Institute for Cancer Genetics, Columbia University Medical Center, 1130 St. Nicholas Ave, Room 503B, New York, NY 10032; e-mail: sz2296@columbia.edu.

![Figure 2. Aberrant TCRδ rearrangement is required for t(12;14), but not chromosomal 14 amplifications, in murine ATM-deficient thymic lymphomas. (A) Survival curve for ATM−/− (n = 10), ATM−/−Eδ−/− (n = 11), and ATM−/−Eδ+/− (n = 8) mice with median survival at 99, 112, and 137 days, respectively. P values were calculated using the log-rank test. (B) Representative chromosome 12 (green) and chromosome 14 (red) paint analyses of a metaphase from no. 745 ATM−/−Eδ−/− lymphomas (without t(12;14)) and no. 900 ATM−/−Eδ+/− lymphomas with t(12;14) translocation. More than 20 metaphases were analyzed for each lymphoma, and t(12;14) in >50% metaphases was used as the criterion for clonal translocations. The results are summarized in panel E. (C) Southern blot mapping the rearrangements of Cδ and Jα. The diagram shows the TCRα/δ locus, EcoRI (black arrows), and probe locations (black dashed line for Southern probes and red dashes for adjacent CGH probes). A total of 10 µg EcoRI-digested genomic DNA from either tumor (T) or the corresponding kidney (K) was analyzed on each lane. Loading control probe detected a fragment of the single-copy DNA-PKcs gene. (D) CGH analyses of ATM−/−Eδ−/− thymic lymphomas 745 and 545. Each tumor DNA was analyzed with matched kidney control DNA on a 244K mouse CGH array from Agilent. The log ratio of tumor vs kidney control at each probe location is displayed in centromere-to-telomere orientation for each chromosome (from 1 to 19 then X/Y, from left to right). The red arrows and labels indicate recurrent amplification of Notch1, chromosome 14 upstream of TCRα/δ locus, and trisomy 15. Green arrows and labels indicate recurrent deletion of the telomeric portion of chromosome 12 and the Pten locus, as well as deletional rearrangement in TCR loci. (E) Summary of chromosome 12/14 paint analyses of ATM−/−Eδ−/− and ATM−/−Eδ+/− lymphomas. (F) Quantitative PCR results that measured the expression of IL7R, Jak1, Notch1, Pten, Rb1, Bcl11b, and Psmc6 genes from ATM−/−Eδ+/+ (n = 3), ATM−/−Eδ+/− (n = 4), ATM−/−Eδ−/− (n = 5) lymphomas. Psmc6 locates at the amplification area within chromosome 14 and is used as a marker for the amplification here.3 Bcl11b is within the region that is hemizygously deleted in chromosome 12.3 Briefly, total RNA was extracted using Trizol (Invitrogen, CA) from a single-cell suspension derived from ATM−/− and ATM−/−Eδ−/− thymic lymphomas as well as wild-type total thymus, and dissolved in 100 μL DEPC-H2O and verified to have A260 nm/280 nm between 1.8 and 2. Approximately 5 μg of total RNA was treated with RNase-free DNase I (Roche). The DNaseI activity was abolished by the addition of 2 µL of 25-mM EDTA and incubation at 65°C for 15 minutes. Reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen) and Oligod(T)18 (New England Biolabs) according to the manufacturer’s instructions. The resulted complementary DNA was dissolved in TE and quantified with NanoDrop (Thermo Scientific). Quantitative PCR was then performed with gene-specific primers (supplemental Table 1) for Notch1 (NM_008714.3, 138 bp across exons 25-26), Jak I (NM_146145.2, 162 bp across exons 18-20), IL7R (NM_008372.4, 149 bp across exons 5-7), Rb1 (NM_009029.2, 144 bp across exons 23-25), Bcl11b (NM_001079883.1, 126 bp across exons 3-4, within chromosome 12 telomeric deletion), Pten (NM_008960.2, 144 bp across exons 7-8), Psmc6 (NM_025959.3, 196 bp across exons 7-9, within chromosome 14 amplification), and β-actin (actb; NM_007393.3, 154 bp across exons 1-2). Reaction setups include 10 µL Sybr Select Master Mix (Applied Biosystems), 250 nM each primer, 10 ng complementary DNA, and water to a total of 20 µL. The PCR condition was 50°C (2 minutes), 95°C (10 minutes), and [95°C (15 seconds), 60°C (1 minute)] × 40, followed by the melt curve program provided by 7500 Real Time PCR System (Applied Biosystems). The results were analyzed with 7500 software package (v 2.0.6) and the ΔΔCT method (included in 7500 software) with the expression of β-actin as the reference and the expression of the specific gene in the wild-type samples as controls for normalization. Each gene in each sample was analyzed in triplicate together with H2O control. The H2O control samples always had CT beyond the range (>40) in all experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/17/10.1182_blood-2015-01-622621/4/m_2665f2.jpeg?Expires=1767731489&Signature=hWEC3WmH0pj5V3DxcYBuglz~sVlwzYz4QBMZ5apem29ydrP5U4KSQqQ5dE7y1U4iY8mkl7WsuzsP-s9XybNz-5ZCkEDPehMWXCpY3KESpl8pOOguuazfJlMl6q4UeFi7OiUb4Lkcb5-kAEmniPbML5rDLsrkJrFhKUbGxA6Hoh37cJTzn2yyKGIou~HCEJmmvkzyUhnaQvNSYDXiwpH8a1LxDmNHLF-B-l8QkLZ~5Xn7K6YrvZytDCdrglp47Qt73RS9iVgfbY4qLwQAm5h5bL4-LH66aPdEQa2m0xt1REugMQJ-UwT1edrXpxJZMGOTuWSY3elfackQOchY0lyOoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal