In this issue of Blood, Bowers et al report that osteoblasts maintain a subset of quiescent stem cells and that osteoblast ablation converts bone marrow into a proliferation-promoting environment for both normal and malignant stem cells.1
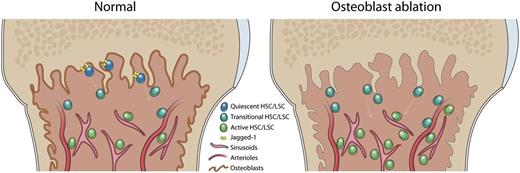
Different stem cells are maintained in different niche zones in bone marrow. After osteoblast ablation, the quiescent HSC subset is lost, and bone marrow is converted into a proliferation-promoting microenvironment.
Different stem cells are maintained in different niche zones in bone marrow. After osteoblast ablation, the quiescent HSC subset is lost, and bone marrow is converted into a proliferation-promoting microenvironment.
Hematopoietic stem cells (HSCs) are thought to localize in a discrete bone marrow microenvironment, or niche, which is critical for their maintenance and regulation. In the long-standing search for HSC supporting stromal cells, osteoblasts and bone-lining cells in the endosteal zone of trabecular bone were initially identified through functional genetic studies.2,3 Subsequent studies have shown that large numbers of HSCs often localize near endothelial and/or perivascular cells in the central marrow, which together constitute the perivascular niche.4-6 Functionally, deleting critical HSC regulation factors from the perivascular niche dramatically reduces HSC abundance in the bone marrow; however, significant HSC function is still maintained, as determined by a bone marrow transplantation assay.7 Other studies have reported that a rare HSC subpopulation with deep quiescence and long-term self-renewal potential is located in the trabecular bone area and has a robust capacity for recovering hematopoiesis.8,9 This indicates the possible existence of a reserve HSC population, which is maintained in the endosteal zone instead of the perivascular niche.
By using a collagen α1 type 1 promoter-mediated long-term ablation system, Bowers et al investigated the role of osteoblasts from the endosteal zone in HSC maintenance and in leukemia development. Osteoblast ablation did not lead to a significant reduction of the HSC (defined by CD150+FLk2–CD48–LSK) pool size. Instead, there was a slight increase in HSC and progenitor numbers. Although this is largely consistent with a previous study which found that the bulk of the HSC pool localizes in the perivascular niche, deleting HSC regulation factors from osteoblasts may not have significant effects on overall HSC number.7 The increase in HSCs and progenitors can be explained by osteoblast ablation causing a subset of HSCs to lose quiescence and enter into cell cycle for proliferation and differentiation. Indeed, through careful and detailed characterization of HSC subpopulations, Bowers et al observed that a rare CD49b–CD229–-marked quiescent HSC subpopulation was significantly reduced after osteoblast ablation. Losing this subset of HSCs resulted in reduced stem cell engraftment, particularly over the long term, which is consistent with the concept that a reserve HSC subpopulation is critical for long-term hematopoiesis maintenance.10 Overall, this study sheds light on a controversy in HSC niche studies: whether and how the osteoblastic niche regulates HSCs. Feasibly, the osteoblasts in the endosteal zone either directly or indirectly maintain a reserve HSC subpopulation, which is low in number but significantly contributes to HSC long-term function.
Bowers et al further investigated the consequences of osteoblast ablation in the context of leukemogenesis. In the tested chronic myelogenous leukemia model, osteoblast ablation resulted in increased malignant proliferation and accelerated leukemia development, suggesting a conversion in bone marrow into a proliferation-promoting microenvironment. However, leukemic cells from this converted microenvironment had impaired leukemogenesis capacity in the secondary transplantation recipients, which suggests the osteoblastic niche may also contribute to preserving leukemia stem cells (LSCs). In the future, both whether and how the endosteal zone directly contributes to LSC maintenance and drug resistance will need to be studied. Consistent with a previous study,9 Bowers et al also show that Jagged-1, which is highly expressed in the endosteal zone of trabecular bone area, facilitates the maintenance of both normal and malignant stem cells in vitro.
In conclusion, Bowers et al provide strong evidence to verify the role of the osteoblastic niche in the endosteal zone in maintaining the quiescence and long-term self-renewal potential of normal HSCs and preserving LSCs in the leukemia model. This study also supports the concept that different HSC subpopulations are maintained in different niches in bone marrow (see figure).
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal