In this issue of Blood, Hijazi et al challenge the view that consumptive coagulopathy that accompanies traumatic brain injury (TBI) results in a sequence of events that lead to intracranial hemorrhage (ICH).1
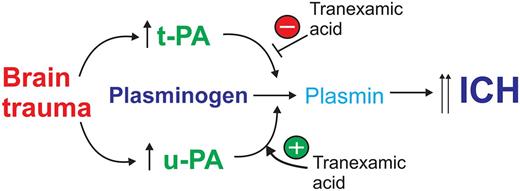
Following TBI, there is an increase in brain-derived tPA- and uPA-mediated fibrinolysis that promotes ICH. TXA blocks tPA-mediated fibrinolysis and ICH, but potentiates uPA-mediated plasminogen activation promoting ICH.
Following TBI, there is an increase in brain-derived tPA- and uPA-mediated fibrinolysis that promotes ICH. TXA blocks tPA-mediated fibrinolysis and ICH, but potentiates uPA-mediated plasminogen activation promoting ICH.
Why is it that an antifibrinolytic drug that is given to reduce bleeding in severe trauma patients can sometimes cause the very thing it is intending to stop? The paper highlighted in this commentary provides an interesting insight that goes a considerable way toward explaining this paradox and also how the fibrinolytic system can be inadvertently turned on when it should be turned off.
One of the greatest causes of mortality associated with TBI is ICH. Consumptive coagulopathy that accompanies TBI is widely assumed to underlie the sequence of events leading to ICH expansion. Not only do Hijazi et al challenge this view, but they imply that coagulopathy is not a cause of ICH at all but rather occurs as a consequence of the fibrinolytic system being activated within the brain.
To explore the role of the fibrinolytic system in the promotion of ICH following TBI, wild-type (WT) mice and mice deficient in tissue-type plasminogen activator (tPA) or urokinase plasminogen activator (uPA) were subjected to a closed head injury model of TBI, and the degrees of ICH and coagulopathy were evaluated. WT mice had an increase in plasma D-dimer levels and a drop in platelet count within 2 hours, but there was no change in the international normalization ratio (INR), indicating normal clotting parameters. Hence, this model of TBI causes only a mild coagulopathy. Nonetheless, the extent of ICH steadily increased over time but did not correlate with D-dimer levels or platelet count. Instead, the extent of ICH correlated with an increase in fibrinolysis, but the interest here is in the detail. Fibrinolytic activity increased in the cerebrospinal fluid but not in the blood of injured mice. However, both tPA−/− and uPA−/− mice developed less ICH after TBI, but only tPA−/− mice became coagulopathic; D-dimer levels and platelet counts were essentially unchanged in uPA−/− mice. Urokinase was therefore causing a pronounced effect on the hemostatic system.
To further support the role of the fibrinolytic system in promoting ICH, the authors forced coagulopathy by anticoagulating mice with warfarin. Warfarin increased the INR to a similar extent in WT, tPA−/−, and uPA−/− mice, but only WT mice developed a greater degree of ICH following TBI. The lack of ICH expansion in warfarin-treated tPA−/− or uPA−/− mice strengthens the idea that both uPA and tPA were driving ICH. The capacity of both u-PA and t-PA to promote ICH and the increase in ICH seen in anticoagulated WT mice were inhibited by an inactive t-PA variant (tPA-S481A).
Together, these findings are relevant considering current efforts to attenuate consumptive coagulopathy in severe trauma patients using antifibrinolytic drugs. Most prominent here is the use of the lysine analog tranexamic acid (TXA). The CRASH-2 trial2 indicated a net benefit of TXA if administered within 3 hours of injury. However, the administration of TXA after 3 hours resulted in more deaths due to bleeding. So, as highlighted at the outset, there appears to be a paradox: why would an antifibrinolytic agent promote the very thing it was intended to block? The authors then showed that tPA and uPA levels transiently increase in the brain soon after TBI, but the temporal profile of each was different: tPA levels peaked at 3 hours post-TBI, consistent with published reports,3 whereas uPA levels began to rise after tPA levels had subsided, reaching a peak at 8 hours.
Although TXA effectively blocks tPA-mediated fibrinolysis, it is well known that TXA actually promotes the ability of uPA to activate plasminogen.4,5 This is because the binding of TXA to plasminogen causes a conformational change allowing it to be more efficiently cleaved by uPA.4 Could the selective increase in uPA at the later time point explain the “TXA paradox”? The authors treated WT-mice with TXA and observed reduced ICH at 2 hours post-TBI, consistent with blocking the effect of tPA. However, when TXA was administered to mice 8 hours post-injury, at a time when uPA levels were at the highest level and tPA levels had subsided, an increase in ICH was seen, reminiscent of the findings of the CRASH-2 trial (see figure).
This evidence points to the possibility that the increase in ICH by TXA was due to the known effect of TXA in increasing the efficiency of uPA-mediated plasminogen activation. However, the authors did not determine if fibrinolytic activity was actually increased in the brain after TXA treatment, nor did they perform this experiment in uPA−/− mice to formally associate uPA with this effect. Although the evidence supports a causal role for tPA and uPA in promoting ICH after TBI, what remains to be determined is the mechanism for how this occurs.
The fibrinolytic system is well known to have a major effect on the neurovascular unit.6 Although most of this work has been in the context of ischemic stroke, endogenous brain-derived tPA has also been shown to enhance edema7 and promote protein extravasation into the brain following TBI.8 The delayed increase in uPA levels following TBI is consistent with earlier findings, where uPA was shown to increase in the hours following cerebral ischemia subsequent to the rise in tPA activity.9,10
Nonetheless, this paper has a bearing on the clinical management of TBI in humans, in particular, the capacity of TXA to worsen ICH. Clearly, any new antifibrinolytic approaches aimed at reducing ICH following TBI should be tailored to annul the effects of both tPA and uPA. So, at the end, there is really no paradox. What appears to be happening is actually consistent with what was already known about the differential effects of TXA on tPA- and uPA-mediated plasminogen activation. We must now wait with interest to see if tPA481A or other antifibrinolytic approaches can be harnessed more effectively in patients with severe trauma.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal