In this issue of Blood, Nguyen et al show that mycophenolic acid (MPA) induces GTP depletion, which inhibits the function of transcription initiation factor I (TIF-IA) and impacts the interaction of TIF-IA with ErbB3-binding protein 1 (Ebp1), a key in regulating proliferating cell nuclear antigen (PCNA) expression and ribosomal RNA (rRNA) synthesis in T cells during activation.1
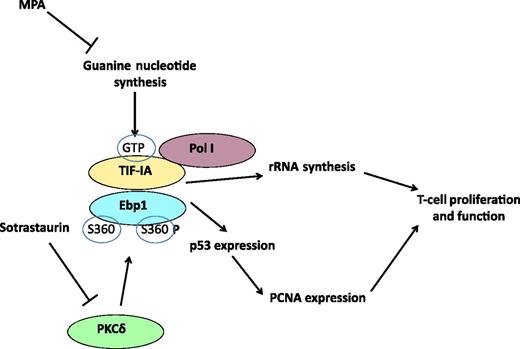
MPA inhibits guanine nucleotide synthesis and depletes GTP. This impacts the function of TIF-IA and in particular the capacity of TIF-IA to interact with Ebp1, an interaction that regulates rRNA synthesis via the interaction of TIF-IA with Pol I and transcription of PCNA, thus impacting T-cell proliferation and function. In addition, sotrastaurin inhibits PKCδ, which phosphorylates Ebp1 at position S360, a step required for PCNA expression. MPA and sotrastaurin synergize and inhibit T-cell activation and proliferation very potently by inhibiting the functional interaction between TIF-IA and Ebp1.
MPA inhibits guanine nucleotide synthesis and depletes GTP. This impacts the function of TIF-IA and in particular the capacity of TIF-IA to interact with Ebp1, an interaction that regulates rRNA synthesis via the interaction of TIF-IA with Pol I and transcription of PCNA, thus impacting T-cell proliferation and function. In addition, sotrastaurin inhibits PKCδ, which phosphorylates Ebp1 at position S360, a step required for PCNA expression. MPA and sotrastaurin synergize and inhibit T-cell activation and proliferation very potently by inhibiting the functional interaction between TIF-IA and Ebp1.
The figure summarizes the main findings of the report and shows how MPA can regulate T-cell proliferation and activation at different levels by targeting the interaction between TIF-IA and Ebp1, thus inhibiting rRNA synthesis and regulating the expression of key factors involved in cell proliferation, such as PCNA and p53. Moreover, this study suggests that the use of MPA together with sotrastaurin suppresses T-cell activation even more potently than each drug alone, by also inhibiting the TIF-IA–Ebp1 interaction.
Mycophenolate mofetil (MMF) is an immunosuppressive drug that has been used more frequently over the past 20 years. Currently, MMF is used in combination with other drugs to treat certain autoimmune diseases, is part of the immunosuppressive regimen to modulate graft-versus-host disease following hematopoietic stem cell transplantation, and is used to prevent graft rejection post–organ transplantation. MMF is a prodrug of MPA, which is a purine analog. MPA inhibits specifically T-cell and B-cell activation and function by inhibiting the type II isoform of inosine 5′-monophosphate dehydrogenase, a rate-limiting enzyme in the de novo synthesis of guanosine, thus depleting guanine nucleotides.2 Notably, previous studies by Huang et al, Dayton et al, and others have shown that MPA could also inhibit the synthesis of rRNA3,4 ; however, so far, the MPA mechanisms that could mediate this effect remain to be understood.
TIF-IA is involved in the regulation of rRNA synthesis and is expressed by all mammalian cells.5,6 In their recent work, Nguyen et al tested whether TIF-IA requires GTP binding in order to regulate RNA synthesis in T cells while interacting with other proteins. A systematic approach was used by the authors to assess the effects of MPA on cell lines, primary T cells, or cells from patients when possible, using chromatin immunoprecipitation assays and RNA analysis, and by knocking down or overexpressing the factor of interest.
Interestingly, they found that TIF-IA is a GTP-binding protein and that MPA treatment induced GTP depletion that then modified TIF-IA function and localization to the periphery of nucleolus into T cells, while blocking RNA polymerase I (Pol I) binding to the rDNA promoter. In addition, MPA treatment also led to increased p53 expression while decreasing PCNA expression, explaining how MPA can inhibit rRNA synthesis as well as proliferation in T cells. The investigators then went on to assess the role of Ebp1, which negatively regulates p53,7 and found that Ebp1 is upregulated in proliferating T cells and binds to TIF-IA. They showed that the interaction between TIF-IA and Ebp1 plays a key role in the regulation of cell proliferation and PCNA expression. In addition, they found that MPA reduced the interaction between TIF-IA and Ebp1 while upregulating p53 but decreasing PCNA expression, which suggests that the interaction between TIF-IA and Ebp1 might depend on TIF-IA binding to GTP.
It has been reported that Ebp1 can interfere with rRNA processing; however, whether Ebp1 regulates RNA synthesis was unknown. In the present study, the authors demonstrate for the first time that Ebp1 is key for TIF-IA retention where RNA synthesis occurs, and that both TIF-IA and Ebp1 play key roles in the regulation of rRNA. Moreover, phosphorylation at S360 of Ebp1 by protein kinase C δ (PKCδ) was necessary for TIF-IA–mediated regulation of RNA synthesis. Interestingly, inhibiting phosphorylation of Ebp1 by using sotrastaurin, a specific inhibitor of PKCδ, together with MPA led to a very potent inhibition of RNA synthesis, PCNA expression, T-cell proliferation, and reduced interleukin-2 production by T cells.
Sotrastaurin is a drug that is currently being used to inhibit graft rejection in renal transplantation and to treat certain inflammatory diseases such as psoriasis.8,9 Therefore, these results are of great interest because they suggest that the combination of MPA and sotrastaurin could be used in the future as a highly potent regimen to modulate lymphocyte activation (see figure) in the context of transplantation or to treat certain autoimmune diseases. However, sotrastaurin is a paninhibitor and more studies are warranted to assess whether other immune cells will be affected. In addition, sotrastaurin may affect more than just rRNA synthesis or growth within 1 cell and this needs to be addressed as well to truly evaluate the possibility of using this drug combination safely in patients. These studies will be highly relevant because therapies such as transplantation rely heavily on successful and effective immunosuppression.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal