In this issue of Blood, Dhédin et al from the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) show that young to middle-aged adults who receive a pediatric-intensive chemotherapy regimen for treatment of Philadelphia chromosome-negative acute lymphoblastic leukemia (Ph-neg ALL) do not appear to require an allogeneic stem cell transplant (alloSCT) if they achieve a good response on minimal residual disease (MRD) testing after induction therapy. Patients who are not good MRD responders however, achieve better outcomes with alloSCT than their counterparts who do not receive alloSCT.1
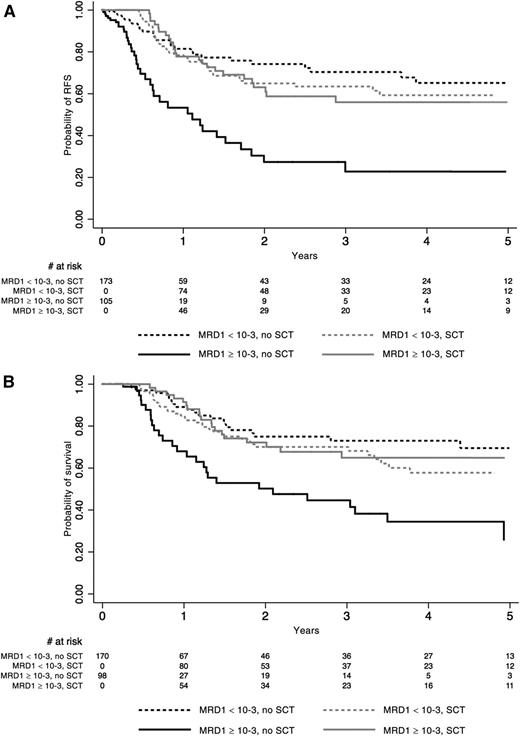
Outcome according to MRD response in the whole population of 278 patients tested. See Figure 3 in the article by Dhédin et al that begins on page 2486.
Outcome according to MRD response in the whole population of 278 patients tested. See Figure 3 in the article by Dhédin et al that begins on page 2486.
After years of stagnation and poor outcomes, the treatment of adults with ALL has seen a sea of change in outcomes that relate to multiple factors. The most prominent factor began with the observation by Stock et al in the Alliance Cooperative Group (former Cancer and Leukemia Group B) and the Children’s Oncology Group that adolescents and young adults aged 16 to 20 years had superior survival when treated with a pediatric-intensive multiagent chemotherapy regimen compared with those who receive a more traditional adult intensive chemotherapy regimen.2 The GRAALL was one of the first groups to apply this approach prospectively to a group of young and middle-aged adults and demonstrated in a phase 2 study that they could achieve an overall survival of 61% at 42 months compared with 41% on a previous study (LALA-94) using an adult-intensive regimen.3 As recently reported at the American Society of Hematology meeting in December 2014, the US intergroup study led by Dr Stock demonstrated remarkably similar results in a group of patients between the ages of 18 and 40 years treated with a pediatric-intensive regimen.4
The GRAALL has made further observations from its studies and looked at whether or not traditional prognostic factors such as age, leukocyte count, and conventional cytogenetic factors remain relevant as prognostic factors in the era of MRD assessment and molecular analyses. They have shown that patients with postinduction MRD levels ≥10−4 and unfavorable genetic characteristic including MLL gene rearrangement or focal IKZF1 gene deletion in B-cell ALL and no NOTCH1/FBXW7 mutation and/or N-KRAS mutation and/or PTEN gene alteration in T-cell ALL are the key negative prognostic factors in assessment of relapse risk when using pediatric-intensive chemotherapy and that traditional risk factors such as leukocyte count and immunophenotype were no longer significant.5,6
A central precept in the treatment of adult patients with ALL has been that most, if not all, patients should be considered for alloSCT given their poor outcomes with traditional chemotherapy regimens. However, the improved results with pediatric-intensive chemotherapy regimens have brought this long-standing precept into question. Dhédin et al look at the role of alloSCT in patients treated in the GRAALL-2003/2005 trials. They assessed 522 patients between the ages of 15 and 55 years who presented with ≥1 conventional high-risk disease factor and assessed them for candidacy for alloSCT in first complete remission (CR). Of these, 282 (54%) were actually transplanted in first CR. They were compared with the 240 patients who were not transplanted. No significant differences in baseline characteristics or early response were noted between these 2 groups, except that more patients with t(4;11) abnormalities received alloSCT. No difference in relapse-free survival or overall survival was found overall between those who were transplanted and those who were not. Patients with the IKZF1 deletion did benefit from SCT compared with those who were not transplanted. The most significant finding from their study was that patients with an MRD level <10−3 at the end of induction therapy had equivalent outcomes whether they were transplanted or treated with chemotherapy, whereas patients with an MRD level ≥10−3 clearly benefitted from alloSCT compared with those poor MRD responders who were treated with chemotherapy. The patients with poor MRD response who had an alloSCT had equivalent outcomes to the good MRD responders (see figure).1
In a recently presented analysis from the Center for International Blood and Marrow Transplant Research, where outcomes with alloSCT in Ph-neg ALL were compared with a cohort of young adults treated with the Dana Farber Cancer Institute ALL Consortium pediatric regimen, superior outcomes were demonstrated with the chemotherapy regimen compared with alloSCT. A high transplant-related mortality diminished the benefit of alloSCT.7 These data provide further confirmation of the results presented here by Dhédin et al.
In conclusion, the use of pediatric-intensive regimens in young and middle-aged adults has significantly improved outcomes in adult ALL and has abrogated the need for alloSCT in many of these patients. New oncogenetic risk factors combined with assessment of MRD are providing new refined tools to assess risk and help further define who needs alloSCT and who does not. These advances in combination with the new paradigms in the application of immunologic therapy to adult ALL, including new monoclonal antibody constructs and chimeric antigen receptor T-cell therapy, promise to bring continued improvements in therapy for children and adults with ALL.8 After years of stagnation, we are finally moving forward in the treatment of this dreaded disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.