To the editor:
Langerhans cell histiocytosis (LCH) can be a multisystem disease with an unpredictable outcome, from autoregressive to vital organ damage and sometimes fatal evolution. In 2010, Badalian-Very et al reported the BRAFV600E somatic activating mutation was present in 57% of LCH cases.1 In LCH without BRAFV600E mutation, ARAF mutation and MAP2K1 mutation have been reported recently within the mitogen-activated protein kinase (MAPK) signaling pathway.2,3 However, BRAF status is not enough to clearly determine the clinical course of the disease, and other somatic molecular events may drive the pathogenesis of the disease. The phosphatidylinositol 3-kinase (PI3K)–mammalian target of rapamycin (mTOR) pathway is another master mechanism used to control cell survival, differentiation, and proliferation, and it has been shown to interact with the MAPK signaling pathway.4 Somatic mutation of PIK3CA has recently been reported in Erdheim-Chester disease,5 a close histiocytic disorder with the same oncogenic pathway as LCH,6 containing overlapping mutations in the MAPK signaling pathway. Thus, we conducted a study to identify such mutations in LCH.
Eighty-six LCH patients were included in this study after giving informed written consent; 81 were children and 5 were adults. The median age at diagnosis was 4.6 years (range, 0-87 years). There were 54 cases of single-system LCH (SS-LCH) (63%), 14 cases of multisystem LCH (MS-LCH) (16%) without risk organ involvement, and 18 cases of MS-LCH with risk organ involvement (21%). BRAFV600E mutation was found in 33 out of 86 patients.
Genomic DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) LCH samples after histologic review. Enrichment by macrodissection to obtain >20% histiocytes was done when necessary. Detection of BRAFV600E mutations was performed by pyrosequencing, and screening for the 5 most common activating mutations of the PI3KCA gene (codons 542, 545, 546, 1044, and 1047) was performed with iPLEX mass-spectrometric–based genotyping technology as previously described (Sequenom-Agena Bioscience).7 Other mutations involving BRAF, KRAS, and NRAS were tested in the same Sequenom experiment (see supplemental Data available on the Blood Web site). Each run included PI3KCA positive controls from commercial FFPE DNA (Horizon Diagnostics).
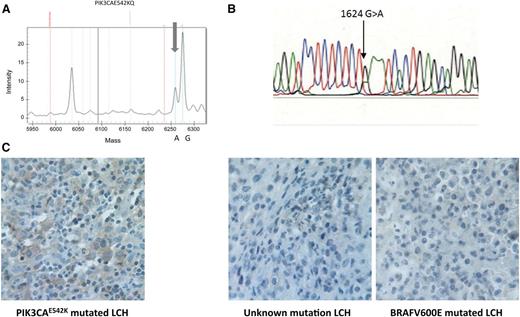
The somatic missense mutation p.E542K in the PIK3CA gene was identified in only 1 of 86 (1.2%) LCH patients (Figure 1A). In this case, the mutation was confirmed by Sanger sequencing (Figure 1B) and was not associated with the BRAFV600E mutation. The patient was a 10-year-old girl with SS-LCH who presented with left thoracic swelling following a limited injury. Extension investigation showed only a lytic lesion of the 11th left rib that appeared to be a single lesion after the complete workup. The diagnosis of LCH was confirmed by biopsy. The lesion regressed spontaneously in a few months without recurrence during a follow-up period of 6 years.
Detection of PIK3CAE542K mutation. (A) Sequenom mass-spectrometric–based genotyping assays. Mutants are detectable with the appearance of a new peak (gray arrows) with an allele frequency ∼25% in a lesion with 50% tumor infiltration. (B) Sanger sequencing. (C) Anti–phospho-AKT staining in the PIK3CAE542K mutated LCH. Histiocytes from an LCH lesion of the patient with PIK3CA542K mutation show positive cytoplasmic staining in comparison with histiocytes from 2 LCH lesions from patients without the PIK3CA542K mutation (1 patient with the BRAFV600E mutation and 1 patient without a known mutation).
Detection of PIK3CAE542K mutation. (A) Sequenom mass-spectrometric–based genotyping assays. Mutants are detectable with the appearance of a new peak (gray arrows) with an allele frequency ∼25% in a lesion with 50% tumor infiltration. (B) Sanger sequencing. (C) Anti–phospho-AKT staining in the PIK3CAE542K mutated LCH. Histiocytes from an LCH lesion of the patient with PIK3CA542K mutation show positive cytoplasmic staining in comparison with histiocytes from 2 LCH lesions from patients without the PIK3CA542K mutation (1 patient with the BRAFV600E mutation and 1 patient without a known mutation).
To confirm activation of the PI3K-AKT pathway, we studied the phosphorylation of the PI3K downstream kinase AKT by immunohistochemistry. Anti–phospho-AKT (Ser473) (clone D9E; 1:10; Cell Signaling) was applied to a 4-µm FFPE biopsy section; biopsy specimens from PIK3CAwt LCH were used as a control (Figure 1C). We observed cytoplasmic staining in the larger cells morphologically corresponding to pathological Langerhans cells, which was not observed in the control cases.
This observation shows that mutation in the PIK3CA gene could be implied in LCH as recently reported in Erdheim-Chester disease but at a lower frequency (1.2% vs 12%).5 However, this study is limited to 5 hotspots accounting for only two-thirds of activating mutations of PIK3CA.
Interestingly, we showed by anti–phospho-ERK immunohistochemistry analysis that activation of the PI3K-AKT pathway was associated with activation of the MAPK pathway, as observed in Erdheim-Chester disease and other malignancies in which implication of these 2 pathways is intricate. We did not identify KRAS, NRAS, or BRAF hotspot somatic mutations, which could be responsible for constitutive activation of MAPK in this case.
In cells, the catalytic subunit p110α of PI3Kα forms a heterodimer with the p85 subunit, which stabilizes p110α but also inhibits its kinase activity. The hotspot-activating somatic mutation E542K in the helical domain p110α interferes with this p85-p110α interaction and thus relieves the inhibition,8 leading to the constitutive activation of the PI3K-mTOR pathway.9
In conclusion, despite the rare prevalence of the 5 most common PIK3CA mutations in LCH, this report highlights that the PI3K-AKT pathway could be implied in the pathogenesis of LCH and should be considered to explain the diversity of the clinical course of the disease and response to targeted therapy.10 Also, PI3K inhibitors developed for cancer therapy would be a potential treatment of such cases.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Mr M. Barkaoui for collecting clinical data, the pathologists who provided access to LCH biopsy specimens, and the clinicians from the Société Française des Cancers de l'Enfant who participated in this study.
This work was supported by grants from the Société Française des Cancers de L’Enfant, the Fédération Enfants et Santé, the Association Histiocytose France, the Association 111 les Arts, and the Recherche Maladie Hématologiques de l'Enfant.
Contribution: S.H. designed and coordinated the research, collected the data, and wrote the manuscript; R.S. performed the Sequenom mass-spectrometric–based genotyping assay and Sanger sequencing and critically read the manuscript; N.R.-R. performed the immunohistochemistry study and critically read the manuscript; Y.P. performed experiments; M.P. performed pathological diagnosis of the patient and critically read the manuscript; H.P. participated in the clinical care of the patient and critically read the manuscript; A.L. critically read the manuscript; J.H. designed the research, participated in the clinical care of the patient, and critically read the manuscript; J.D. designed the research, participated in the clinical care of the patient, and critically read the manuscript; and J.-F.E. designed the research, performed the histologic review and the BRAF pyrosequencing study, and critically read the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sébastien Héritier, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital, 26 Avenue du Dr Netter, 75012 Paris, France; e-mail: sebastien.heritier@trs.aphp.fr.