Key Points
Normal IgG and IgG2 differentially inhibit HIT antibody-dependent platelet activation according to the FcγRIIA H131R polymorphism.
This variable effect of IgG and IgG2 probably explains the higher risk of thrombosis in patients homozygous for the FcγRIIA 131R allele.
Abstract
Thrombosis results in heparin-induced thrombocytopenia (HIT) from cellular activation involving Fc receptors. In this study, the FcγRIIA 131RR genotype was found to increase the risk of thrombosis in HIT patients (odds ratio: 5.9; 95% confidence interval: 1.7-20). When platelet aggregation tests (PATs) were performed with platelet-rich plasma (PRP), a shorter lag time was measured in 131RR donors compared to individuals with the HR and HH genotypes in response to HIT plasma or 5B9, a recently developed humanized monoclonal antibody to PF4/heparin. Importantly, this difference was no longer detectable when PATs were performed with washed platelets or immunoglobulin (Ig)G-depleted PRP. Moreover, polyclonal IgG or monoclonal IgG1 added to IgG-depleted PRP increased the lag time in response to 5B9. HH platelets were also sensitive to IgG2, which in contrast, failed to inhibit the response of 131RR platelets to 5B9. Finally, higher tissue factor messenger RNA levels were measured in the whole blood of 131RR donors after activation by HIT antibodies, with increased phospholipid procoagulant activity. These results demonstrate that HIT patients homozygous for the FcγRIIA 131R allele have a higher risk of thrombosis, probably due to increased cell activation by antibodies to PF4/heparin, with a lower inhibitory effect of endogenous IgG, especially from the IgG2 subclass.
Introduction
Heparin-induced thrombocytopenia (HIT) is an atypical immune complication of heparin treatment due to antibodies specific to platelet factor 4/heparin (PF4/H) complexes.1 Despite having thrombocytopenia, affected patients usually develop thrombosis, and this particular clinical presentation is explained by the central role of Fcγ receptors in the pathophysiology of HIT, particularly FcγRIIA, which mediates platelet activation induced by immunoglobulin (Ig)G-PF4/H immune complexes (ICs). In addition, platelets from healthy donors exhibit wide variability in their response to HIT antibodies,2-4 and many patients who synthesize significant levels of antibodies to PF4/H while being treated with heparin do not develop HIT.5-7
The FCGR2A gene, which encodes FcγRIIA, displays a functional allelic dimorphism generating 2 codominantly expressed allotypes with either a histidine (H) or an arginine (R) at amino acid position 131 in the second Ig-like extracellular domain. It has been reported that although neither allotype binds monomeric IgG,8 they differ substantially in their ability to bind IgG-containing ICs: the 131H allotype efficiently binds human IgG1 and IgG2, whereas the 131R allotype binds human IgG1 but poorly binds IgG2.9 The consequences of this differential binding may be important. For instance, individuals homozygous for the 131R allele are at higher risk of serious infection with encapsulated organisms, which are cleared via a predominant IgG2 response.10
An influence of platelet FcγRIIA H131R polymorphism on the risk of HIT has also been proposed, but with discordant results,11-16 and no association was confirmed.17 One study, however, showed an increased frequency of the 131R variant in HIT in patients who had thrombosis,16 and we had previously found that platelet counts were lower in FcγRIIA 131RR patients with antibodies to PF4/H after cardiac surgery.6 Carlson et al assumed that PF4/heparin antibody ICs were less efficiently removed from the circulation in FcγRIIA 131RR patients who had subsequent prolonged IC-dependent activation of platelets. This hypothesis, however, is unlikely because HIT antibodies to PF4/H are predominantly IgG1,14 and the 2 FcγRIIA allotypes have identical affinity for IgG1 ICs.
The aims of the present study were therefore to elucidate the mechanisms explaining why homozygous FcγRIIA 131RR HIT patients have a higher risk of thrombosis, and especially to investigate whether IgG subclasses differentially modulate the platelet response to HIT antibodies according to the FcγRIIA polymorphism.
Patients and methods
Patients
The HIT patients and control groups have previously been described by Rollin et al.18 The patient group (HIT) comprised 89 individuals, including 35 with thrombotic complications, and the 2 antibody control groups, Abpos and Abneg, comprised 160 and 174 non-HIT patients, having or not having developed significant levels of PF4-specific antibodies after cardiac surgery, respectively.
Two groups of patients, who had presented with ischemic stroke (n = 113) or venous thromboembolism (n = 97) unrelated to HIT, were investigated. In addition, a previously analyzed19 control population including 206 healthy subjects was studied.
All patients and controls were white, and blood samples were collected after obtaining informed consent according to the Helsinki Declaration principles. Moreover, our institutional Ethics Committee and the Ministry of Research had previously approved collection of DNA from patients with HIT or thrombosis and from healthy controls for genetic studies (agreement numbers 2011-N7, 2002-13, DC2008-308, respectively).
Materials
5B9 is a chimeric humanized anti-PF4/H monoclonal IgG1 antibody that we recently developed and that fully mimics human HIT antibodies (see supplemental Figure 1 on the Blood Web site).
Monoclonal anti-CD32 antibody (clone IV.3) was purchased from Stemcell Technologies. Polyclonal IgG (Privigen) was obtained from CSL Behring. Cetuximab, a chimeric mouse-human IgG1 anti-EGFR (epidermal growth factor receptor), and panitumumab, a human IgG2 anti-EGFR, was obtained from Merck Serono and Amgen, respectively. Both IgG1 and IgG2 antibodies are specific for EGFR, which is not expressed on platelets, and were shown to be monomeric (>98%) with size-exclusion chromatography analysis. Horm type I collagen from equine tendon was obtained from Nycomed, and unfractionated heparin (Heparin Choay) from Sanofi.
Genotyping
Genomic DNA was extracted from citrated whole blood using the FlexiGene DNA kit (Qiagen) according to the manufacturer’s instructions. Genotypes of FcγRIIA H131R polymorphism (rs1801274) were defined by a polymerase chain (PCR)–restriction fragment length polymorphism method as previously described.6
Assays of TF mRNA levels after whole blood stimulation by HIT antibodies
Whole blood collected from healthy donors on 0.129 M sodium citrate was incubated with HIT plasma (1:5 dilution) or 5B9 (10 μg/mL) in the presence or absence of heparin (0.5 IU/mL). After 60 minutes of incubation at 37°C, total RNA was isolated using the QIAamp RNA Blood Mini kit (Qiagen). PCR was performed using Platinum Quantitative PCR SuperMix (Invitrogen) and a TaqMan probe (Hs00175225_m1; Applied Biosystems). In addition, the expression level of the CD14 gene was evaluated using Platinum SYBR Green qPCR SuperMix (Invitrogen) and 3 pmol of forward (5′-GGTTCCTGCTCAGCTACTGG-3′)-specific and reverse (5′-TAGGTCCTCGAGCGTCAGTT-3′)-specific primers. The relative increase in tissue factor (TF) messenger (m)RNA levels was quantified after incubation of HIT antibodies and heparin, as compared to the condition without heparin, and using the 2−ΔΔCt method.
Assay of plasma procoagulant activity after whole blood stimulation by 5B9
In selected experiments, platelet-depleted plasma was obtained after 2 centrifugations (2250g, 15 minutes) following incubation of whole blood with 5B9 (10 μg/mL) with or without heparin (0.5 IU/mL). The STA-Procoag-PPL assay (Diagnostica Stago), evaluating the procoagulant activity of phospholipid microparticles,20 was then performed. Briefly, 25 μL of test plasma was mixed with 25 μL of phospholipid-depleted human plasma and prewarmed for 2 minutes at 37°C. Coagulation was then triggered by adding 100 μL of factor Xa/calcium reagent (containing 0.01 U/mL bovine factor Xa in a buffered calcium solution), followed by the measurement of clotting time using a KC4 instrument (Diagnostica Stago). The relative decrease in clotting time was calculated for each donor after stimulation with 5B9 and heparin, as compared to the experimental condition without heparin.
Platelet aggregation tests and serotonin release assays
Whole blood from 70 healthy aspirin-free donors (30 men and 40 women) was collected according to the recommendations of the International Society on Thrombosis and Haemostasis21 in acid-citrate-dextrose supplemented with prostaglandin E1 (0.1 mM). Platelets were washed and suspended at a final count adjusted to 350 × 106/mL.22 In addition, whole blood from healthy donors was collected on 0.129 M sodium citrate, and platelet-rich plasma (PRP) was isolated (mean platelet count: 352 ± 82 × 106/mL). All platelet aggregation tests (PATs) were performed in an APACT 4 aggregometer (ELITech Group). PATs were performed with 15 μL of HIT plasma, or with 18 or 36 μg/mL of 5B9, each in the presence of 0.5 IU/mL of heparin. In addition, a PAT was also performed with 0.5 μg/mL of collagen.
In selected experiments, citrated plasma of a healthy donor was depleted of endogenous IgG using a HiTrap Protein G column (GE Healthcare Life Sciences). Washed platelets were then suspended in autologous IgG-depleted plasma at a final count adjusted to 350 × 106/mL to obtain IgG-depleted PRP. PATs were performed using 5B9 (18 μg/mL) and heparin (0.5 IU/mL) with IgG-depleted PRP in the absence or presence of different concentrations of polyclonal IgG (1, 2, 3, 4, 5, and 10 mg/mL), or monoclonal IgG1 or IgG2 (1, 2, and 4 mg/mL).
Serotonin release assays were performed as previously described.22
Flow cytometry assay of monomeric IgG binding to platelet FcγRIIA
Monoclonal antibody IV.3 is a mouse IgG2b that blocks the binding of IgG to FcγRIIA.23 We therefore evaluated the ability of monomeric IgG to inhibit the binding of fluorescein isothiocyanate–conjugated IV.3 to FcγRIIA of platelets from HH or RR donors, using a procedure similar to an assay we had developed to evaluate IgG/FcγIIIA interactions.24 Briefly, platelets (5 × 104 in 10 μL) were incubated with varying concentrations of polyclonal IgG, IgG1, or IgG2 (30 minutes). Platelets were then incubated with fluorescein isothiocyanate–conjugated IV.3 (1 μg/mL, 30 minutes) and analyzed by flow cytometry after adding 300 μL of phosphate-buffered saline containing 1% bovine serum albumin. The results were expressed as the percentage of inhibition of IV.3 binding: (% of IV.3-positive platelets in the absence of IgG − % of IV.3-positive platelets in the presence of IgG) × 100 ÷ % of IV.3-positive platelets in the absence of IgG.
Statistical analysis
The χ2 test was used to compare the genotypes and allele frequencies in HIT patients and controls. Kruskal-Wallis, Mann-Whitney U, and Student t tests were performed to analyze the aggregation parameters and the data obtained after whole blood stimulation in relation to the FcγRIIA H131R polymorphism. P values <.05 were considered significant.
Results
The frequency of the FcγRIIA 131R isoform is higher in HIT patients with thrombosis
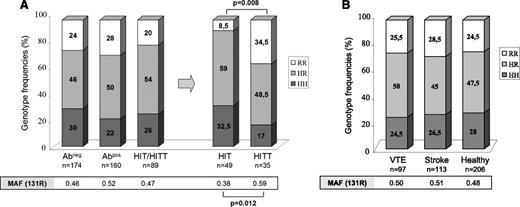
Genotypes and allele frequencies were similar in HIT patients and the 2 groups of controls (Abneg and Abpos) (Figure 1A). However, the 131R allele was more frequent in HIT patients with thrombotic complications compared to those without thrombosis (59% vs 38%, respectively, P = .012). Moreover, homozygous RR subjects were more numerous in HIT patients with thrombosis than in those without (34.5% vs 8.5%, respectively, P = .008). The FcγRIIA 131RR genotype, therefore, appeared to significantly increase the risk of thromboembolic complications in HIT (odds ratio: 5.9; 95% confidence interval: 1.7-20).
Genotype and allele frequencies of FcγRIIA H131R polymorphism. (A) Analysis of the 3 groups of patients (Abneg, Abpos, and HIT). HIT patients were also stratified according to the presence (HITT) or absence (HIT) of thrombotic complications. No information was available about thrombotic events in 5 HIT patients. (B) Analysis of H131R polymorphism in patients with venous thromboembolism (VTE) or stroke and in healthy subjects. P value was calculated using the χ2 test. MAF, minor allele frequency.
Genotype and allele frequencies of FcγRIIA H131R polymorphism. (A) Analysis of the 3 groups of patients (Abneg, Abpos, and HIT). HIT patients were also stratified according to the presence (HITT) or absence (HIT) of thrombotic complications. No information was available about thrombotic events in 5 HIT patients. (B) Analysis of H131R polymorphism in patients with venous thromboembolism (VTE) or stroke and in healthy subjects. P value was calculated using the χ2 test. MAF, minor allele frequency.
Notably, FcγRIIA genotypes and allele frequencies were similar in patients with venous thromboembolism or ischemic stroke and in healthy subjects (Figure 1B), supporting the hypothesis that the effect of the FcγRIIA H131R polymorphism on the risk of thrombosis in HIT was specific to HIT.
The potency of HIT antibodies to stimulate TF synthesis and induce procoagulant activity is higher in individuals homozygous for the FcγRIIA 131R isoform
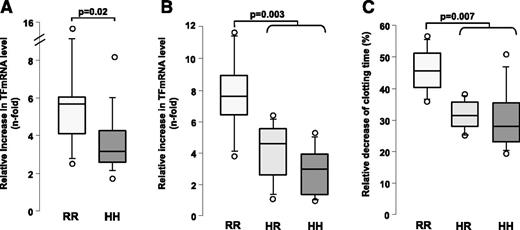
A significant increase in TF mRNA levels was found for all donors tested after HIT plasma samples had been added to whole blood with heparin (0.5 IU/mL), as compared to the experimental conditions without heparin (4.2-fold median increase in TF mRNA level). Interestingly, this relative increase in TF mRNA level was greater after stimulation of whole blood from homozygous FcγRIIA 131R donors (median value: 5.6-fold vs 3.1-fold for HH donors, P = .02; Figure 2A). This result was also confirmed after addition to whole blood of 5B9 and heparin, with a 7.5-fold increase in TF mRNA levels in RR individuals compared to 4.5- and 2.9-fold increases in the HR and HH groups, respectively (P = .003; Figure 2B). Noticeably, TF gene expression was also strongly inhibited when HIT plasma was co-incubated with IV.3 (supplemental Figure 2).
Influence of FcγRIIA H131R polymorphism on TF gene expression and procoagulant activity induced by HIT antibodies in whole blood. (A) Relative increase in TF levels measured by quantitative PCR (see “Patients and methods” section) after addition of HIT plasma and heparin (0.5 IU/mL) to the whole blood from 24 donors (HH: n = 12; RR: n = 12), as compared to the condition without heparin. Plasma samples from 5 HIT patients, all positive in PF4-specific enzyme-linked immunosorbent assay (A405nm values between 1.6 and 3) and serotonin release assay, were tested. P values were calculated using the Mann-Whitney U test. Relative increase in TF mRNA levels (B) and relative shortening of plasma clotting time (C) were measured after addition to the whole blood of 20 donors (HH: n = 8; HR: n = 6; RR: n = 6) of 5B9 (10 μg/mL) with heparin (0.5 IU/mL), as compared to the condition without heparin. P values were calculated using the Mann-Whitney U test.
Influence of FcγRIIA H131R polymorphism on TF gene expression and procoagulant activity induced by HIT antibodies in whole blood. (A) Relative increase in TF levels measured by quantitative PCR (see “Patients and methods” section) after addition of HIT plasma and heparin (0.5 IU/mL) to the whole blood from 24 donors (HH: n = 12; RR: n = 12), as compared to the condition without heparin. Plasma samples from 5 HIT patients, all positive in PF4-specific enzyme-linked immunosorbent assay (A405nm values between 1.6 and 3) and serotonin release assay, were tested. P values were calculated using the Mann-Whitney U test. Relative increase in TF mRNA levels (B) and relative shortening of plasma clotting time (C) were measured after addition to the whole blood of 20 donors (HH: n = 8; HR: n = 6; RR: n = 6) of 5B9 (10 μg/mL) with heparin (0.5 IU/mL), as compared to the condition without heparin. P values were calculated using the Mann-Whitney U test.
5B9 plus heparin also induced a procoagulant activity dependent on phospholipid microparticles that was variable according to the FcγRIIA polymorphism. Therefore, the relative shortening of plasma clotting time measured was greater in RR subjects than in the other genotypes (median decrease in clotting time: 46.2% vs 31.7% and 28.1% in RR vs HR and HH groups, respectively, P = .007; Figure 2C).
The FcγRIIA H131R polymorphism influences platelet activation induced by HIT antibodies only in the presence of plasma
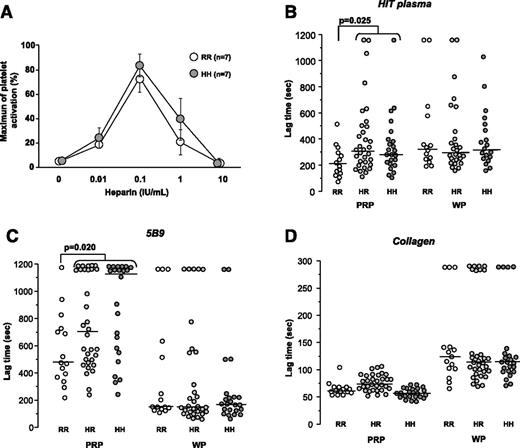
To investigate the influence of FcγRIIA H131R polymorphism on the platelet response to HIT antibodies, we first performed a serotonin release assay, and the release measured was identical when washed platelets from healthy donors homozygous for the 131R and 131H FcγRIIA isoforms were incubated with HIT plasma and heparin (Figure 3A). PATs were then performed with 70 healthy donors, and similar results were obtained according to the FcγRIIA H131R polymorphism after activation of washed platelets by HIT antibodies (Figure 3B). In contrast, when PATs were performed with PRP, the lag time measured was shorter in healthy donors homozygous for the FcγRIIA 131R isoform compared to that of HH and HR individuals (median: 201 vs 287 and 310 seconds, respectively, P = .025).
Influence of FcγRIIA H131R polymorphism on platelet activation induced by HIT antibodies. (A) Serotonin release assay performed with washed platelets from FcγRIIA 131HH (n = 5) and 131RR donors (n = 5) incubated with HIT plasma sample containing a high titer of activating PF4-specific antibodies (A405nm = 2.7 in enzyme-linked immunosorbent assay), and different concentrations of unfractionated heparin. (B-D) Lag times measured with PRP and washed platelets (WP) from 70 healthy donors according to FcγRIIA H131R genotype (RR: n = 15; HR: n = 32; HH: n = 23). The median values are indicated as solid lines. Aggregation tests were performed with unfractionated heparin (0.5 IU/mL). HIT plasma was tested using the serotonin release assay as described above (B), 5B9 was tested at 18 μg/mL (C), and collagen was tested at 0.5 μg/mL (D). The Mann-Whitney U test was performed to compare lag times in relation to FcγRIIA H131R polymorphism; P values are displayed.
Influence of FcγRIIA H131R polymorphism on platelet activation induced by HIT antibodies. (A) Serotonin release assay performed with washed platelets from FcγRIIA 131HH (n = 5) and 131RR donors (n = 5) incubated with HIT plasma sample containing a high titer of activating PF4-specific antibodies (A405nm = 2.7 in enzyme-linked immunosorbent assay), and different concentrations of unfractionated heparin. (B-D) Lag times measured with PRP and washed platelets (WP) from 70 healthy donors according to FcγRIIA H131R genotype (RR: n = 15; HR: n = 32; HH: n = 23). The median values are indicated as solid lines. Aggregation tests were performed with unfractionated heparin (0.5 IU/mL). HIT plasma was tested using the serotonin release assay as described above (B), 5B9 was tested at 18 μg/mL (C), and collagen was tested at 0.5 μg/mL (D). The Mann-Whitney U test was performed to compare lag times in relation to FcγRIIA H131R polymorphism; P values are displayed.
A wide variability in platelet response to 5B9 and heparin was also evidenced in PRP, particularly when the lower concentration of antibody (18 μg/mL) was tested, with an absence of aggregation in 23 of the 70 donors studied. Importantly, 22 of the nonresponders to 5B9 were carriers of the FcγRIIA H allele, and only 1 was an RR homozygote (Figure 3C). In addition, the lag time measured with 5B9 was shorter when the PRP from RR subjects was tested (median: 471 vs 750 seconds in carriers of the H allele, P = .02). This apparent influence of the FcγRIIA H131R polymorphism in PRP was no longer visible when a higher concentration of 5B9 (36 μg/mL) was used (data not shown). However, the lag times measured in response to 18 μg/mL of 5B9 were similar when PATs were performed with washed platelets regardless of the FcγRIIA H131R genotype (Figure 3C). Finally, and as expected, no differences among the genotypes were found when PATs were performed with collagen (0.5 μg/mL), either with PRP or washed platelets (Figure 3D).
Together, these results strongly support the hypothesis that the FcγRIIA H131R polymorphism influences the platelet response to HIT antibodies, but because this effect required the presence of plasma components, an influence of endogenous IgG was hypothesized.
Endogenous immunoglobulin subclasses differentially modulate platelet activation induced by HIT antibodies according to the FcγRIIA H131R polymorphism
No differences were found regarding total IgG and IgG subclass levels in any subgroup compared with others, either in healthy donors or in HIT patients (supplemental Figure 3). In particular, levels of IgG2 that corresponded to about 35% of total IgG (vs approximately 48% of IgG1) were similar in homozygous donors for the H and R alleles.
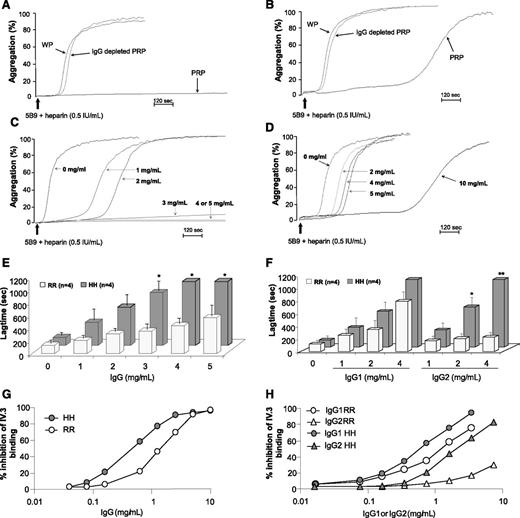
When the platelet response to 5B9 of IgG-depleted PRP from FcγRIIA HH and RR donors was evaluated, the lag times obtained in both groups were shorter as compared to native PRP and identical to those measured with washed platelets (Figure 4A-B). As expected, no effect of IgG depletion on the platelet response to collagen in PRP was observed (supplemental Figure 4A). The addition of polyclonal immunoglobulins to IgG-depleted PRP before performing PATs resulted in a dose-dependent increase in the lag time, and the platelet response to 5B9 was similar to that obtained with PRP when 5 mg/mL or 10 mg/mL of IgG was present (Figure 4C-D). In addition, this effect of polyclonal IgG, which we also confirmed with PATs performed using human HIT plasma (supplemental Figure 5), was more obvious with HH platelets than with RR platelets. Indeed, the lag times were significantly longer with HH platelets compared to RR platelets as soon as 3 mg/mL of polyclonal IgG was added to IgG-depleted PRP (mean value: 996 vs 412 seconds, P = .015; Figure 4E). Moreover, the platelet response to 5B9 was fully abolished with 4 or 5 mg/mL of polyclonal IgG only in HH donors.
Influence of normal IgG and monoclonal IgG1 or IgG2 on platelet response induced by 5B9 with heparin, and relationship with the FcγRIIA H131R polymorphism. Representative aggregation profiles obtained after addition of 5B9 (18 μg/mL) and heparin (0.5 IU/mL) to washed platelets (WP), PRP, and IgG-depleted PRP from homozygous 131HH (A) and 131RR donors (B). Representative aggregation profiles (C-D) and mean lag times (±1 standard error of the mean) (E) obtained after addition of 5B9 (18 μg/mL) and heparin (0.5 IU/mL) with varying concentrations of polyclonal IgG to IgG-depleted PRP from homozygous 131HH (C) and 131RR (D) donors. The Student t test was used to compare the data obtained in the 2 groups of donors. (F) Mean lag times (±1 standard error of the mean) measured after addition of 5B9 (18 μg/mL) and heparin (0.5 IU/mL) with varying concentrations of human monoclonal IgG1 (cetuximab) or IgG2 (panitumumab) to IgG-depleted PRP from homozygous donors FcγRIIA 131RR and 131HH. The Student t test was used to compare the values obtained in the 2 groups of donors; *P < .05 and **P < .01. Inhibition of monoclonal antibody IV.3 binding (expressed in %) on HH and RR platelets by polyclonal IgG (G) and human monoclonal IgG1 or IgG2 (H). The binding of fluorescein isothiocyanate–conjugated IV.3 on platelets from HH and RR donors in the presence of increasing concentrations of polyclonal human IgG or monomeric IgG1 (cetuximab) or IgG2 (panitimumab) was assessed by flow cytometry. The data are representative of flow cytometry analyses performed with 3 different pairs of HH and RR donors.
Influence of normal IgG and monoclonal IgG1 or IgG2 on platelet response induced by 5B9 with heparin, and relationship with the FcγRIIA H131R polymorphism. Representative aggregation profiles obtained after addition of 5B9 (18 μg/mL) and heparin (0.5 IU/mL) to washed platelets (WP), PRP, and IgG-depleted PRP from homozygous 131HH (A) and 131RR donors (B). Representative aggregation profiles (C-D) and mean lag times (±1 standard error of the mean) (E) obtained after addition of 5B9 (18 μg/mL) and heparin (0.5 IU/mL) with varying concentrations of polyclonal IgG to IgG-depleted PRP from homozygous 131HH (C) and 131RR (D) donors. The Student t test was used to compare the data obtained in the 2 groups of donors. (F) Mean lag times (±1 standard error of the mean) measured after addition of 5B9 (18 μg/mL) and heparin (0.5 IU/mL) with varying concentrations of human monoclonal IgG1 (cetuximab) or IgG2 (panitumumab) to IgG-depleted PRP from homozygous donors FcγRIIA 131RR and 131HH. The Student t test was used to compare the values obtained in the 2 groups of donors; *P < .05 and **P < .01. Inhibition of monoclonal antibody IV.3 binding (expressed in %) on HH and RR platelets by polyclonal IgG (G) and human monoclonal IgG1 or IgG2 (H). The binding of fluorescein isothiocyanate–conjugated IV.3 on platelets from HH and RR donors in the presence of increasing concentrations of polyclonal human IgG or monomeric IgG1 (cetuximab) or IgG2 (panitimumab) was assessed by flow cytometry. The data are representative of flow cytometry analyses performed with 3 different pairs of HH and RR donors.
The addition of monoclonal IgG1 to IgG-depleted PRP at concentrations similar to those usually present in normal plasma resulted in a similar prolongation in the lag time of HH and RR platelets in response to 5B9 (Figure 4F). In contrast, IgG2 strongly inhibited the response to 5B9 of platelets expressing the H isoform, with a dose-dependent increase in lag time, whereas no effect was observed on RR platelets (Figure 4F).
As expected, neither polyclonal IgG nor monoclonal IgG1 or IgG2 modified the platelet response to collagen according to the FcγRIIA H131R polymorphism (supplemental Figure S4).
Lastly, competitive flow cytometry assays were performed to evaluate the ability of monomeric IgG1 and IgG2 to bind FcγRIIA receptors. As shown in Fig 4G-H, a dramatic inhibition of monoclonal antibody IV.3 binding to both HH and RR platelets (∼90% and ∼70%, respectively) was achieved by 2.5 mg/mL of either polyclonal human IgG or monoclonal IgG1. By contrast, when monoclonal IgG2 was added at similar concentration, a significant inhibition of IV.3 binding to platelets was obtained with HH platelets (∼60%) but not with RR platelets (<15%; Figure 4H). These findings indicate that HH platelets bind both monomeric IgG1 and IgG2 when present at physiological concentrations, whereas RR platelets exclusively bind monomeric IgG1.
Discussion
Given the central role of FcγRIIA in the pathogenesis of HIT, several studies have investigated the influence of the H131R polymorphism on the risk of HIT, but no association was demonstrated.17 The present study confirms this conclusion, but also shows that the frequencies of the R allele and of the homozygous RR genotype are significantly higher in HIT patients with thromboembolic complications than in those with thrombocytopenia alone. Only 3 studies previously investigated the influence of the FcγRIIA H131R polymorphism on the risk of thromboembolic complications in HIT. In the first, 36 patients (including 23 with thrombosis) were studied, and no association was found.14 Conversely, Carlsson et al found in a larger study that the frequency of the 131RR genotype was overrepresented in patients with thrombotic events16 (with a rate of 37%, similar to the 34.5% found in the present study). Scarparo et al had also reported an increased frequency of the FcγRIIA RR genotype in HIT patients with thrombosis, but this association was significant only when combined with the HPA-1a/b or PECAM1-V/V125 gene polymorphism.25
The H131R substitution is located in the extracellular domain of FcγRIIA, which interacts with the Fc fragment of IgG and does not influence the expression level of this receptor on the platelet surface.12 FcγRIIA appears to be physiologically important for αIIbβ3-mediated outside-in signaling and thrombus formation.26 However, our results failed to suggest any prothrombotic effect of the R allele in homozygous patients with stroke or venous thromboembolism, and therefore the impact of the FcγRIIA H131R polymorphism on the risk of thrombosis seems to be very specific to HIT.
HIT is a particular clinical prothrombotic syndrome, and Carlsson et al hypothesized that IgG-PF4/heparin ICs might be less efficiently removed from the circulation of patients with the FcγRIIA 131RR genotype, thereby prolonging cell activation and increasing the risk of thrombosis.16 However, HIT antibodies to PF4/H are predominantly IgG1,14 and only marginal differences in the affinity of human IgG1 ICs to the H and R allotypes of FcγRIIA have previously been reported.8 Moreover, no significant difference in circulating anti-PF4/H antibody levels in relation to the FcγRIIA H131R polymorphism was evidenced in our cohort (data not shown). We therefore postulated that the greater risk of thrombosis in HIT patients with the RR genotype was related to more efficient cellular activation by HIT antibodies, independent of the clearance of ICs.
Thrombotic complications in HIT result from multicellular activation that involves platelets, monocytes, neutrophils, and endothelial cells, with an important role of TF and procoagulant microparticles in triggering and amplifying the coagulation cascade.27-29 Accordingly, a significant increase in TF gene expression was measured when plasma samples from HIT patients were incubated in whole blood from healthy donors with heparin, and this effect was stronger with 5B9, a chimeric IgG1 antibody to PF4/H complexes that mimics human HIT antibodies. More importantly, 5B9 and human HIT antibodies stimulated TF gene activity more effectively when added to the whole blood of donors homozygous for the FcγRIIA R allele. In addition, the phospholipid procoagulant activity evaluated by measuring the plasma clotting time of these individuals was greater after activation by 5B9 and heparin, an effect probably related to the microparticles produced by activated platelets and monocytes. The monocyte activation by IgG-PF4/H ICs can be direct or indirect, involving platelet-monocyte interactions, but Kasthuri et al demonstrated that direct activation of monocytes by HIT antibodies mainly depended on FcγRI receptors, without a significant role of FcγRIIA.29 However, our results showing that TF gene expression after stimulation in whole blood was inhibited by IV.3 strongly support a major contribution of FcγRIIA receptors in cell activation induced by HIT antibodies. It has been recently shown that monocytes preferentially bound HIT ICs because of the higher affinity of monocyte surface glycosaminoglycans for PF4 compared with platelet chondroitin sulfate molecules.28 However, monocytes express only 5% of the total amount of FcγRIIA receptors in whole blood,30 whereas 80% are present in circulating platelets31 and ∼5% are present in neutrophils.32 The activation of monocytes in HIT might therefore also depend in vivo on platelet activation induced by IgG-PF4/H ICs. In this regard, PATs clearly demonstrated that the FcγRIIA H131R polymorphism influences the platelet response to HIT antibodies. However, significant differences with shorter lag times were visible only when PATs were performed with PRP, not with washed platelets. Moreover, this variation in lag time, likely reflecting a variable capability of HIT ICs to cross-link FcγRIIA receptors and activate platelets, disappeared when the PRP tested was IgG depleted, or when PATs were performed with a higher concentration of 5B9 (ie, 36 μg/mL). These results, therefore, suggest that normal plasma IgG was competing with 5B9, with an influence of the FcγRIIA dimorphism that was no longer present when the amount of HIT antibodies was too high. Furthermore, the lag time obtained with IgG-depleted PRP was always similar to that measured with washed platelets from the same donor after activation by 5B9. Finally, the addition of polyclonal IgG to IgG-depleted samples partly inhibited the platelet response to 5B9 and HIT human antibodies, but this effect was more pronounced with platelets expressing the 131H isoform. These findings are also in agreement with previous studies showing that addition of high concentrations of polyclonal IgG to washed platelets inhibited platelet activation and aggregation induced by HIT plasma,33,34 and with the fact that few HIT patients have been successfully treated by IV infusion of immunoglobulins.35,36 The plasma IgG fraction, therefore, appears to be critical in contributing to the variations in platelet response to HIT antibodies according to the FcγRIIA H131R polymorphism. Moreover, this effect was probably independent of the plasma levels of total IgG and IgG subclasses, which were similar in the healthy donors tested, regardless of their FcγRIIA genotype.
The H and R allotypes of FcγRIIA differentially bind IgG2, which represents ∼35% to 40% of total IgG present in polyclonal IgG and plasma samples used in our study, but they interact similarly with IgG1 and IgG3 ICs.8 We therefore assumed that the difference in platelet response in PRP according to the FcγRIIA H131R polymorphism was mainly related to the plasma IgG2 subclass. However, this hypothesis presupposed that monomeric IgG present in the plasma can bind efficiently to FcγRIIA, although this interaction has been considered to be minimal.8 Our results obtained with aggregation tests and flow cytometry analysis unambiguously demonstrate that monomeric IgG2 efficiently binds to the H allotype at physiological concentrations and decreases the platelet activation induced by HIT antibodies. We also determined that monomeric IgG1 efficiently binds to both H and R allotypes, with similar inhibition of HIT antibody–mediated platelet activation.
These results therefore strongly support the concept that, in addition to IgG1, endogenous IgG2 (these 2 classes correspond to >90% of total plasma IgG) may reduce the avidity of HIT ICs on the surface of platelets (and maybe of monocytes) and subsequent cell activation in patients expressing the 131H isoform, thereby protecting them from thrombosis. However, because IgG2 is unable to bind to the FcγRIIA R allotype, the modulation of platelet activation by HIT antibodies in RR patients can only depend on IgG1 (corresponding to about 55% of total plasma IgG), therefore contributing to greater pathogenicity of PF4-specific antibodies, with an increased risk of thrombosis (Figure 5). It can be postulated that, in addition to the variable intrinsic sensitivity of platelets to HIT IgG, other parameters such as the specificity and the concentration of anti-PF4/H antibodies or PF4 levels on the platelet surface, the plasma concentrations of IgG1 and IgG2, and FcγRIIA H131R polymorphism may be critical in modifying the risk of thrombosis in HIT patients. It would therefore be of interest to study these parameters in a large population of HIT patients but also in other medical situations associated with immune-mediated thrombocytopenia and thrombosis such as primary antiphospholipid syndrome, in which the FcγRIIA H131R polymorphism may also influence the pathogenic effects of IgG2 antibodies.37
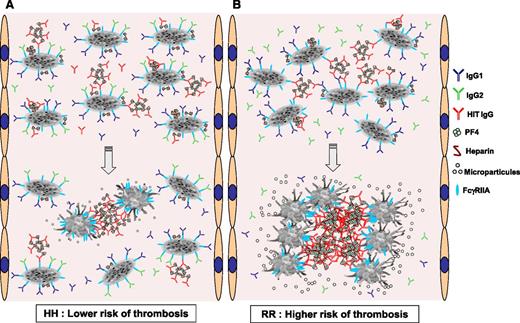
Proposed schematic representation of the interaction of HIT IgG and normal IgG subclasses with platelets according to the FcγRIIA H131R polymorphism. (A) In FcγRIIA HH–immunized patients, IgG2 (green) and IgG1 (dark blue) may bind to FcγRIIA receptors (blue). This interaction thereby reduces the ability of HIT IgG (red) associated with PF4/H complexes to bind via their Fc fragment in either cis (intraplatelet) or trans (interplatelet) FcγRIIA receptors at a sufficient extent to activate platelets. (B) In FcγRIIA RR–immunized patients, IgG2 are not able to bind platelet FcγRIIA receptors, which therefore remain available to cross-link with a larger number of HIT IgG-PF4/H ICs, inducing more potent platelet activation with the release of larger amounts of phospholipid procoagulant microparticles. Together with enhanced synthesis of TF, this effect probably explains the higher risk of thrombosis in patients homozygous for the FcγRIIA R allele.
Proposed schematic representation of the interaction of HIT IgG and normal IgG subclasses with platelets according to the FcγRIIA H131R polymorphism. (A) In FcγRIIA HH–immunized patients, IgG2 (green) and IgG1 (dark blue) may bind to FcγRIIA receptors (blue). This interaction thereby reduces the ability of HIT IgG (red) associated with PF4/H complexes to bind via their Fc fragment in either cis (intraplatelet) or trans (interplatelet) FcγRIIA receptors at a sufficient extent to activate platelets. (B) In FcγRIIA RR–immunized patients, IgG2 are not able to bind platelet FcγRIIA receptors, which therefore remain available to cross-link with a larger number of HIT IgG-PF4/H ICs, inducing more potent platelet activation with the release of larger amounts of phospholipid procoagulant microparticles. Together with enhanced synthesis of TF, this effect probably explains the higher risk of thrombosis in patients homozygous for the FcγRIIA R allele.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Marc-Antoine May who contributed to the collection of samples; and Ms Doreen Raine for editing the English language.
This study was supported by the Institut pour la Recherche sur la Thrombose et l’Hémostase and by the “Investissements d’avenir” Grant Agreement LabEx MAbImprove: ANR-10-LABX-53 program.
Authorship
Contribution: J.R. performed and designed the research, analyzed the data, and wrote the paper; C.P., G.T., and Y.G. designed the research, analyzed the data, and wrote the paper; V.G.-G. performed the research, analyzed the data, and wrote the paper; and H.C.S., D.L., and A.S. performed the research and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yves Gruel, Department of Hematology-Hemostasis, CHRU Tours, 37044 Tours Cedex, France; e-mail: gruel@med.univ-tours.fr.
References
Author notes
G.T. and Y.G. contributed equally to the design of this study.