Key Points
Stimulation of Toll-like receptors 2 and 6 reduces ferroportin expression in mouse macrophages by hepcidin-independent mechanism(s).
Reduced expression of ferroportin in macrophages that recycle iron from red cells is sufficient to rapidly induce hypoferremia in mice.
Abstract
Regulation of iron metabolism and innate immunity are tightly interlinked. The acute phase response to infection and inflammation induces alterations in iron homeostasis that reduce iron supplies to pathogens. The iron hormone hepcidin is activated by such stimuli causing degradation of the iron exporter ferroportin and reduced iron release from macrophages, suggesting that hepcidin is the crucial effector of inflammatory hypoferremia. Here, we report the discovery of an acute inflammatory condition that is mediated by Toll-like receptors 2 and 6 (TLR2 and TLR6) and which induces hypoferremia in mice injected with TLR ligands. Stimulation of TLR2/TLR6 triggers profound decreases in ferroportin messenger RNA and protein expression in bone marrow–derived macrophages, liver, and spleen of mice without changing hepcidin expression. Furthermore, C326S ferroportin mutant mice with a disrupted hepcidin/ferroportin regulatory circuitry respond to injection of the TLR2/6 ligands FSL1 or PAM3CSK4 by ferroportin downregulation and a reduction of serum iron levels. Our findings challenge the prevailing role of hepcidin in hypoferremia and suggest that rapid hepcidin-independent ferroportin downregulation in the major sites of iron recycling may represent a first-line response to restrict iron access for numerous pathogens.
Introduction
Iron plays a central role in host-pathogen interactions.1 Most pathogens require iron for proliferation and full virulence. The innate immune system fights infections by sequestration of iron in macrophages of the reticuloendothelial system. The resulting hypoferremia represents a major host defense strategy and promotes anemia of inflammation, a form of anemia commonly associated with infectious and inflammatory conditions as well as cancer.2
Toll-like receptors (TLRs) are key sensors of the innate immune system.3 They recognize pathogen-associated molecular patterns (PAMPs) and control the hypoferremic host response. Lipopolysaccharide (LPS) is a cell wall component of gram-negative bacteria recognized by TLR4. LPS injection into mice causes the release of proinflammatory cytokines and triggers a well-characterized acute phase response including induction of the hepatic iron hormone hepcidin4-6 by interleukin-6 (IL-6).7,8 Hepcidin binds to and causes internalization and degradation of the iron exporter ferroportin, which limits iron release from iron-exporting cells such as macrophages, hepatocytes, and duodenal enterocytes.9 Diminished iron export from macrophages that recycle iron from senescent red cells rapidly induces hypoferremia due to the high iron demand of erythropoiesis.10,11 Excess levels of hepcidin have been recognized so far as the main cause of anemia of inflammation.12-14
Here, we present TLR6 as an effective regulator of ferroportin expression in macrophages and show that the stimulation of the TLR2/6 pathway through FSL1 or PAM3CSK4 triggers a profound decrease in ferroportin messenger RNA (mRNA) and protein expression in bone marrow–derived macrophages as well as in the liver and the spleen of mice. Unexpectedly, hepcidin expression remains unchanged. These findings are further confirmed in C326S ferroportin mutant mice with a disrupted hepcidin/ferroportin regulatory circuitry challenging the prevailing role of hepcidin in inflammatory hypoferremia and suggesting that an alternative mechanism, that may have preceded the evolution of hepcidin in early vertebrates, is responsible for the induction of hypoferremia during infection.
Materials and methods
siRNA screening
A stable and inducible HeLa cell line was established by applying the Flp-In-T-Rex system (Life Technologies) according to the manufacturer’s instructions. The pcDNA5/FRT/TO plasmid contained the coding sequence of human ferroportin fused to Renilla luciferase (Fpn-Rluc) or only Renilla luciferase (Rluc) under the control of a tetracycline-regulated, hybrid human cytomegalovirus/TetO2 promoter. For the small interfering RNA (siRNA) screen, the cell-based Renilla luciferase assay was adapted to the 384-well plate format and high-throughput conditions. The Protein Kinase siRNA library Thermo Fisher siGenome (Dharmacon) targeting protein kinases and other related genes (779 genes) was used. The library was arrayed in 384-well white plates (Greiner Bio-One), each well containing 1.25 pmol of a pool of 4 synthetic siRNA duplexes (final concentration in wells, 25 nM). Viability controls included a siRNA pool directed against PLK1 and COPB2 (Dharmacon). As a negative control, 3 scrambled siRNA pools (Dharmacon and Ambion) were used. Reverse transfection of HeLa cells was performed by dispensing 15 μL of RPMI 1640–Glutamax medium together with 0.05 μL of Dharmafect1 reagent (Dharmacon) to the siRNA-containing 384-well plates. After 30-minute incubation at ambient temperature, HeLa cells (2500 per well) were added to the siRNA transfection mix in a 30-μL volume of high-glucose Dulbecco modified Eagle medium (Life Technologies) supplemented with 10% heat-inactivated low-endotoxin fetal bovine serum (Gibco). Forty-eight hours after siRNA transfection, the medium was replaced with 30 μL of fresh medium containing 0.5 μg/mL doxycycline (Sigma-Aldrich). Three hours later, doxycycline was removed by extensive washing with Dulbecco phosphate-buffered saline (Sigma-Aldrich) and fresh culture medium was added to cells. The cells were lysed after 18-hour incubation with 20 μL of passive lysis buffer 1× (Promega) and frozen. All dispensing steps were performed with the use of MultidropCombi dispensing systems (Thermo Scientific). Renilla luciferase activity was measured by adding Renilla luciferase assay substrate (Promega) and quantified using a Centro LB 960 luminometer (Berthold Technologies).
Bone marrow–derived macrophage isolation
Bone marrow cells were flushed from tibiae and femurs using ice-cold Hank balanced salt solution and filtered through a 70-μm cell strainer. Cells were seeded at a density of 350 000 cells/cm2 in RPMI 1640–Glutamax medium (Life Technologies) supplemented with 10% of heat-inactivated fetal bovine serum (Hyclone; Thermo Scientific), 1% penicillin/streptomycin (Sigma-Aldrich), and 10 ng/mL macrophage colony-stimulating factor 1 (Sigma-Aldrich). After 4 days, nonadherent cells were removed by washing with Hank balanced salt solution and the medium was replaced daily. Stimulation was performed by adding FSL1, PAM3CSK4 (InvivoGEN), PamOct2C-(VPG)4VPGKG (EMC Microcollection), and LPS (Escherichia coli serotype O111:B4) to the medium at a concentration of 100 ng/mL for the indicated time points.
Mice
C57BL/6 wild-type (WT) male mice aged between 10 and 11 weeks were purchased from Charles River Laboratories. C326S ferroportin mutant mice on a N8F2 C57BL/6 background aged 4 weeks were generated as reported.15 Mice were housed in the European Molecular Biology Laboratory (EMBL) Animal Facility under constant light-dark cycle and maintained on a standard diet containing 200 parts per million iron (Teklad 2018S; Harlan) with ad libitum access to water and food. Mice were treated by intraperitoneal injection of FSL1 (InvivoGEN) and LPS (E coli serotype O111:B4) and PAM3CSK4 (InvivoGEN) 25 ng/g body weight, unless otherwise indicated. Control mice were injected with an equivalent volume of sterile saline solution. Heparinized blood was collected by cardiac puncture from mice killed by CO2 inhalation. All mouse breeding and animal experiments were approved by and conducted in observation of the guidelines of the EMBL Institutional Animal Care and Use Committee. Bone marrow–derived macrophages (BMDMs) were obtained from TLR6- or TLR2-deficient mice housed at Forschungszentrum (Borstel, Germany) or at the Universitatsklinikum (Essen, Germany), respectively.
Preparation of total RNA, reverse transcription, and quantitative real-time PCR analysis
Total RNA was isolated from BMDMs using the RNeasyPlus Mini kit (Qiagen) and from tissues using TRIzol (Life Technologies) according to the manufacturer’s instructions. The conditions for reverse transcription and real-time quantitative polymerase chain reaction (qRT-PCR) were reported previously.15 The mRNA/complementary DNA abundance of each gene was calculated relative to the expression of the housekeeping gene 36B4 encoding for an acidic ribosomal phosphoprotein P0 (RPLP0) and data were analyzed by applying the ΔΔ cycle threshold method.16 The primers used are listed in supplemental Table 1 (see supplemental Data, available on the Blood Web site).
Western blotting
Protein lysate and western blot analysis were performed as previously described.15 All western blot signals were acquired using the Vilbert Lourmat Fusion FX7 system. Anti-ferroportin antibody was purchased from Alpha Diagnostics and anti-β-actin antibody from Sigma-Aldrich.
Plasma biochemistry and tissue iron quantification
Plasma iron concentrations, transferrin saturation, and tissue non-heme iron content were assessed as previously described.15
Splenic macrophage isolation
Mouse macrophages were magnetically separated from splenic cell suspensions by using CD11b MicroBeads (magnetic-activated cell sorting [MACS]) according to the manufacturer’s instruction.
DAB-enhanced Perls’ staining
Tissues were fixed in 10% formalin overnight at room temperature and embedded in paraffin. Microtome sections, 5-μm thick, were stained with potassium ferrocyanide solution (Sigma-Aldrich) followed by 3,3-diaminobenzidinetetrahydrochloride (DAB) (Sigma-Aldrich) development.
Statistical analyses
For analysis of the screening data, the cellHTS2 package (Bioconductor) was used to calculate z-scores as a measure of the generated phenotype. High z-scores were indicative of reduced ferroportin-Rluc activity; low z-scores were indicative of increased ferroportin-Rluc activity. The threshold value was computed as the mean signal of the distribution plus 2 times the standard deviation. Mean z-scores for control siRNAs were first calculated within each replicate and then between replicates. For the screening data, the mean z-score of 2 replicates was calculated.
All other results from this study are expressed as a mean of at least 3 independent experiments plus or minus standard error of the mean (SEM). The 2-tailed, Student t test was used for estimation of statistical significance.
Results
TLR6 is a novel ferroportin regulator
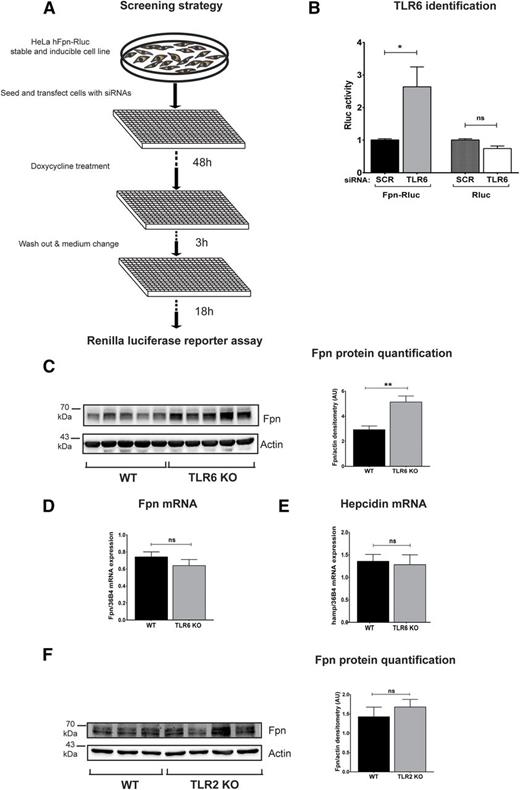
To identify new regulators of ferroportin-mediated iron export, we generated an HeLa cell line that stably expresses an inducible human ferroportin-Renilla luciferase (Fpn-Rluc) fusion protein for an RNA interference (RNAi) screen targeting 779 genes encoding kinases and other signaling molecules (Figure 1A). In this screen, we measured Renilla luciferase activity as readout of ferroportin expression and we identified TLR6 as a ferroportin suppressor.
Identification of TLR6 as a novel regulator of ferroportin protein. (A) A stable and doxycycline-inducible HeLa cell line expressing a human ferroportin-Renilla luciferase fusion protein (Fpn-RLuc) was used for RNAi screening. Renilla luciferase activity (Rluc), used as a reporter of ferroportin expression, was measured 70 hours after reverse transfection of siRNA pools. The screen was performed in duplicates and the cellHTS2 software was used for data analysis. (B) Rluc activity was measured upon scramble (scr) or TLR6 interference with pooled siRNAs in the HeLa cell line expressing Fpn-Rluc and in a HeLa cell line expressing only the reporter protein. Data are presented as means ± SEM from at least 4 independent experiments. *P < .05; Student t test. (C,F) Western blot analysis of endogenous ferroportin expression in BMDMs isolated from WT or TLR6-deficient (TLR6 KO) mice or TLR2-deficient (TLR2 KO) mice; β-actin was used as loading control. Western blot images were acquired and quantified with the Vilber Lourmat Fusion-FX Chemiluminescence system. (D-E) Ferroportin and hepcidin mRNA levels were determined by qRT-PCR and calibrated to 36B4 mRNA levels. Data are means ± SEM; BMDMs were derived from at least 4 different mice per group. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. **P < .01; Student t test.
Identification of TLR6 as a novel regulator of ferroportin protein. (A) A stable and doxycycline-inducible HeLa cell line expressing a human ferroportin-Renilla luciferase fusion protein (Fpn-RLuc) was used for RNAi screening. Renilla luciferase activity (Rluc), used as a reporter of ferroportin expression, was measured 70 hours after reverse transfection of siRNA pools. The screen was performed in duplicates and the cellHTS2 software was used for data analysis. (B) Rluc activity was measured upon scramble (scr) or TLR6 interference with pooled siRNAs in the HeLa cell line expressing Fpn-Rluc and in a HeLa cell line expressing only the reporter protein. Data are presented as means ± SEM from at least 4 independent experiments. *P < .05; Student t test. (C,F) Western blot analysis of endogenous ferroportin expression in BMDMs isolated from WT or TLR6-deficient (TLR6 KO) mice or TLR2-deficient (TLR2 KO) mice; β-actin was used as loading control. Western blot images were acquired and quantified with the Vilber Lourmat Fusion-FX Chemiluminescence system. (D-E) Ferroportin and hepcidin mRNA levels were determined by qRT-PCR and calibrated to 36B4 mRNA levels. Data are means ± SEM; BMDMs were derived from at least 4 different mice per group. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. **P < .01; Student t test.
The knockdown of TLR6 specifically increases ferroportin-Renilla activity, indicating an increase in ferroportin protein levels, although it does not affect the expression of the Renilla protein by itself as assessed in an independent HeLa control cell line (Figure 1B). BMDMs obtained from TLR6-deficient mice display increased ferroportin protein levels (Figure 1C), validating the result of the RNAi screen in a genetic ex vivo model. TLR6 deficiency caused an increase of ferroportin protein levels independent of alterations in ferroportin and hepcidin mRNA levels (Figure 1D-E), suggesting that the lack of TLR6 affects ferroportin posttranscriptionally in a hepcidin-independent manner. TLR6 is an inflammatory sensor, which recognizes specific ligands via heterodimerization with TLR2 on the cell surface.17 However, BMDMs from TLR2-deficient mice do not show changes in ferroportin protein levels (Figure 1F). TLRs mostly play a role under inflammatory conditions which are known to modulate iron homeostasis. Thus, we next chose to explore ferroportin regulation following TLR6 ligation mimicking a pathogen-induced inflammatory response.
TLR2 and TLR6 control the hepcidin-independent ferroportin response to FSL1
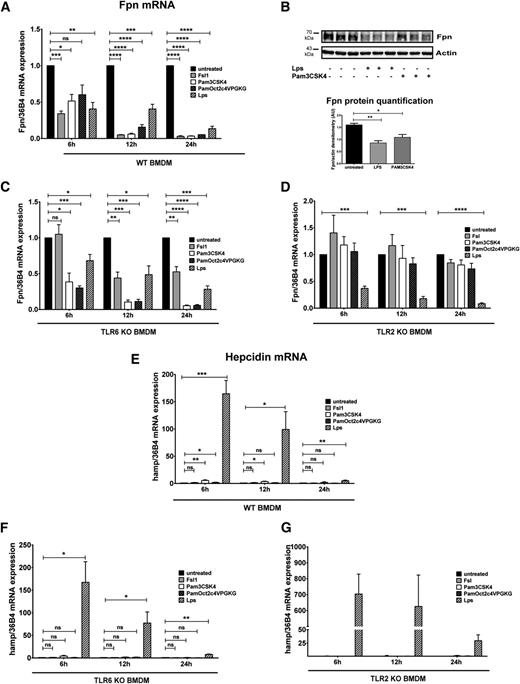
We next stimulated TLR2/6 by FSL1, a lipoprotein ligand from Mycoplasma salivarium known to activate an inflammatory response.18-20 Treatment of BMDMs with different FSL1 concentrations causes a profound decrease in ferroportin mRNA and protein levels (Figure 2A-B). FSL1-mediated ferroportin regulation seems to occur predominantly at the transcriptional level because the rate of mRNA decay remains unaffected by application of the transcription inhibitor actinomycin D (supplemental Figure 1).
FSL1-mediated TLR2/6 ligation reduces ferroportin expression in BMDMs without activating hepcidin mRNA expression. (A,C) qRT-PCR analysis of ferroportin mRNA in BMDMs from WT and TLR6-deficient mice (A), and from WT and TLR2-deficient mice (C) stimulated with FSL1 (20 ng/mL or 100 ng/mL) for the indicated time. (B,D) Western blot analysis and quantification of ferroportin expression in BMDMs from WT and TLR6-deficient mice (B) and from WT and TLR2-deficient mice (D) treated with 100 ng/mL FSL1 for 24 hours. β-actin detection ascertains equal sample loading. (E-F) Ferroportin and hepcidin mRNA expression in BMDMs after FSL1 and LPS (100 ng/mL) stimulation. mRNA levels were normalized to 36B4 mRNA levels. All data are reported as means ± SEM; BMDMs were derived from at least 4 different mice per group. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. *P < .05; **P < .01; ***P < .001; Student t test.
FSL1-mediated TLR2/6 ligation reduces ferroportin expression in BMDMs without activating hepcidin mRNA expression. (A,C) qRT-PCR analysis of ferroportin mRNA in BMDMs from WT and TLR6-deficient mice (A), and from WT and TLR2-deficient mice (C) stimulated with FSL1 (20 ng/mL or 100 ng/mL) for the indicated time. (B,D) Western blot analysis and quantification of ferroportin expression in BMDMs from WT and TLR6-deficient mice (B) and from WT and TLR2-deficient mice (D) treated with 100 ng/mL FSL1 for 24 hours. β-actin detection ascertains equal sample loading. (E-F) Ferroportin and hepcidin mRNA expression in BMDMs after FSL1 and LPS (100 ng/mL) stimulation. mRNA levels were normalized to 36B4 mRNA levels. All data are reported as means ± SEM; BMDMs were derived from at least 4 different mice per group. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. *P < .05; **P < .01; ***P < .001; Student t test.
The mechanism underlying the ferroportin response to FSL1 is distinct from the one mediated by TLR6 deficiency, which affects ferroportin protein levels in the absence of mRNA alterations. Transcriptional repression in response to FSL1 is mediated by TLR6 because it is absent or significantly blunted in TLR6-deficient BMDMs (Figure 2A-B). However, we observed that the response to FSL1 was partially retained even in the absence of TLR6 at the 12-hour and 24-hour time points, demonstrating that TLR2 homodimers may also contribute to the ferroportin response.
Interestingly, the FSL1-mediated ferroportin response is completely absent in TLR2-deficient BMDMs at all time points analyzed and even at high doses of FSL1 (Figure 2C-D), suggesting a central role of TLR2. In addition to TLR6, TLR2 also heterodimerizes with TLR1. However, FSL1 treatment of TLR1-deficient BMDMs results in reduced ferroportin expression similar to BMDM from WT mice (data not shown). Thus, our data reveal that the regulation of ferroportin is mediated by TLR2/6 heterodimers and TLR2 homodimers.
The suppression of ferroportin in macrophages during inflammation is a well-studied response to TLR4 stimulation by LPS involving hepcidin induction.10,11,21 We next compared FSL1 (TLR2/6) and LPS (TLR4) stimulation of BMDMs. Both ligands trigger the expected reduction of ferroportin mRNA levels (Figure 2E),10,11 whereas only LPS treatment increases hepcidin mRNA expression (Figure 2F). Nevertheless, both stimuli activate inflammatory cytokine IL1β, IL6, and tumor necrosis factor α (TNFα) mRNA expression, albeit quantitatively more moderately in FSL1-treated BMDMs (supplemental Figure 2). This finding suggests that increased expression of IL1β, IL6, and TNFα mRNAs per se is not sufficient for hepcidin activation in macrophages. We next tested other bacterial lipopeptides that activate TLR2-dependent signaling (PAM3CSK4 and PamOct2C-(VPG)4VPGKG).19,22 We show that ferroportin mRNA (Figure 3A) and protein (Figure 3B) expression responds with a quantitatively similar decrease in BMDMs of WT mice, indicating that ferroportin transcriptional regulation is a conserved response in the inflammatory context. Conversely, persistent and considerable hepcidin induction is mediated only by LPS (Figure 3E), suggesting a negligible contribution of TLR2 signaling to hepcidin production in macrophages.
Ferroportin downregulation is mediated by TLR2 and TLR4 ligands whereas hepcidin activation is limited to the TLR4 ligand LPS in BMDMs. (A,C-D) Ferroportin mRNA expression was determined by qRT-PCR in WT (A), TLR6-deficient (C), and TLR2-deficient (D) BMDMs stimulated with 100 ng/mL TLR2 ligands (FSL1, PAM3CSK4, PamOct2C-(VPG)4VPGKG) or TLR4 ligand (LPS) for the indicated time. (B) Western blot analysis and quantification of ferroportin expression in BMDMs from WT mice treated with 100 ng/mL LPS and PAM3CSK4 for 24 hours. β-actin was used as loading control. (E-G) Hepcidin mRNA expression was analyzed in the same samples. The mRNA quantification was calibrated to 36B4 mRNA levels. All data are reported as means ± SEM; BMDMs were derived from at least 4 different mice per group. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. *P < .05; **P < .01; ***P < .001; ****P < .0001; Student t test.
Ferroportin downregulation is mediated by TLR2 and TLR4 ligands whereas hepcidin activation is limited to the TLR4 ligand LPS in BMDMs. (A,C-D) Ferroportin mRNA expression was determined by qRT-PCR in WT (A), TLR6-deficient (C), and TLR2-deficient (D) BMDMs stimulated with 100 ng/mL TLR2 ligands (FSL1, PAM3CSK4, PamOct2C-(VPG)4VPGKG) or TLR4 ligand (LPS) for the indicated time. (B) Western blot analysis and quantification of ferroportin expression in BMDMs from WT mice treated with 100 ng/mL LPS and PAM3CSK4 for 24 hours. β-actin was used as loading control. (E-G) Hepcidin mRNA expression was analyzed in the same samples. The mRNA quantification was calibrated to 36B4 mRNA levels. All data are reported as means ± SEM; BMDMs were derived from at least 4 different mice per group. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. *P < .05; **P < .01; ***P < .001; ****P < .0001; Student t test.
Although FSL1-triggered ferroportin suppression depends on TLR2 and TLR6, the ferroportin response mediated by PAM3CSK4 and PamOct2C-(VPG)4VPGKG only depends on TLR2-dependent signaling (Figure 3A,C,D). As expected, LPS stimulation reduced ferroportin mRNA expression throughout the time course by TLR2- and TLR6-independent mechanisms (Figure 3E-G).
Hepcidin-independent ferroportin downregulation is sufficient to induce acute hypoferremia in mice
We next investigated whether hepcidin-independent ferroportin regulation can cause hypoferremia in an animal model. WT mice were injected with either LPS or FSL1 (25 ng/g bodyweight, corresponding to <1 µg per mouse) and iron-related parameters were compared. Low-dose injection of LPS or FSL1 elicits hypoferremia already after 3 hours (Figure 4A-B), associated with a robust reduction of ferroportin mRNA levels in the liver (Figure 4C). By contrast, only LPS, but not FSL1, stimulates hepcidin, TNFα, and IL6 mRNA expression in the liver (Figure 4D-F). Despite the lack of hepcidin induction in FSL1-treated mice, ferroportin protein levels are reduced (Figure 4H) and the hepatic non-heme iron content is elevated to similar levels as in LPS-injected mice (Figure 4G).
Hepcidin-independent acute hypoferremia in mice. WT mice were injected with saline (ctrl), FSL1, or LPS (25 ng of ligand per g body weight) and analyzed 3 hours after injection. (A-B) Plasma iron levels and transferrin saturation were assessed. (C-F) Hepatic ferroportin, hepcidin, TNFα, and IL6 mRNA levels were determined by qRT-PCR and normalized to 36B4 mRNA levels. (G) The hepatic non-heme iron content was quantified as indicated. (H) Western blot analysis and quantification of ferroportin expression in the liver of injected mice. β-actin was used as loading control. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. Data are means ± SEM. Results are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001; Student t test; n = 6 mice per group.
Hepcidin-independent acute hypoferremia in mice. WT mice were injected with saline (ctrl), FSL1, or LPS (25 ng of ligand per g body weight) and analyzed 3 hours after injection. (A-B) Plasma iron levels and transferrin saturation were assessed. (C-F) Hepatic ferroportin, hepcidin, TNFα, and IL6 mRNA levels were determined by qRT-PCR and normalized to 36B4 mRNA levels. (G) The hepatic non-heme iron content was quantified as indicated. (H) Western blot analysis and quantification of ferroportin expression in the liver of injected mice. β-actin was used as loading control. Each lane in the Western blot analysis represents the protein lysate obtained from a single mouse. Data are means ± SEM. Results are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001; Student t test; n = 6 mice per group.
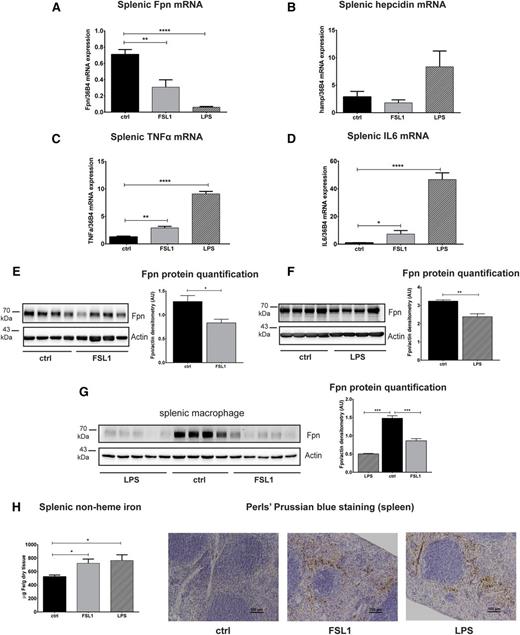
In parallel, ferroportin mRNA (Figure 5A) and protein (Figure 5E-F) levels are decreased in the spleen upon LPS or FSL1 injection, a finding even more pronounced in magnitude in isolated splenic macrophages (Figure 5G). The less pronounced ferroportin decrease observed in tissue samples of LPS- or FSL1-injected mice may be explained by enhanced macrophage recruitment into inflamed tissues. Because macrophages express high levels of ferroportin, the overall decrease in ferroportin levels may not be as evident. Consistent with previous reports, ferroportin mRNA decreased whereas hepcidin mRNA expression increased11,21 in the spleen of LPS-injected mice (Figure 5B), although hepcidin expression is generally low in this tissue. As predicted from the experiments in BMDMs, splenic hepcidin mRNA expression is unchanged following FSL1 injection (Figure 5B). In addition, TNFα and IL6 mRNA levels are stimulated to higher levels in LPS-injected compared with FSL1-injected mice (Figure 5C-D). Consistent with the findings in the liver, splenic iron retention occurs without hepcidin induction in FSL1-injected mice (Figure 5H).
The decrease in splenic ferroportin levels is independent of hepcidin activation. WT mice were injected with saline (ctrl), FSL1, or LPS (25 ng of ligand per gram of body weight) and the spleens were analyzed 3 hours after injection. (A-D) Ferroportin, hepcidin, TNFα, and IL6 mRNA expression was assessed by qRT-PCR. (E-G) Western blot analysis and quantification of ferroportin expression in the whole spleen (E-F) and in splenic macrophages isolated from the injected mice (G). β-actin was used as loading control. (H) Splenic non-heme iron content and DAB-enhanced Perls’ iron staining show iron retention in red pulp macrophages of the spleen of FSL1- and LPS-injected mice. Each lane in the western blot analysis represents the protein lysate obtained from a single mouse. Data are means ± SEM. *P < .05; **P < .01; ***P < .001; Student t test; n = 6 mice per group.
The decrease in splenic ferroportin levels is independent of hepcidin activation. WT mice were injected with saline (ctrl), FSL1, or LPS (25 ng of ligand per gram of body weight) and the spleens were analyzed 3 hours after injection. (A-D) Ferroportin, hepcidin, TNFα, and IL6 mRNA expression was assessed by qRT-PCR. (E-G) Western blot analysis and quantification of ferroportin expression in the whole spleen (E-F) and in splenic macrophages isolated from the injected mice (G). β-actin was used as loading control. (H) Splenic non-heme iron content and DAB-enhanced Perls’ iron staining show iron retention in red pulp macrophages of the spleen of FSL1- and LPS-injected mice. Each lane in the western blot analysis represents the protein lysate obtained from a single mouse. Data are means ± SEM. *P < .05; **P < .01; ***P < .001; Student t test; n = 6 mice per group.
Recent findings in hepcidin knockout mice suggested that hypoferremia upon LPS injection is not caused by altered ferroportin protein levels in the spleen but is attributed to diminished dietary iron absorption due to decreased duodenal ferroportin and divalent metal transporter 1 mRNA expression.23 We find that the duodenal mRNA expression of ferroportin and divalent metal transporter 1 is unchanged in FSL1-injected mice and observe a trend toward decreased ferroportin and divalent metal transporter 1 mRNA levels upon LPS injection (supplemental Figure 3A-B). Likewise, ferroportin protein levels appear unaltered by immunohistochemical analysis (supplemental Figure 3D). Furthermore, Perls’ Prussian blue staining of duodenal sections does not reveal pronounced differences in iron distribution in FSL1- and LPS-injected mice (supplemental Figure 3C), suggesting that at least in FSL1-injected mice iron absorption is not significantly altered.
FSL1-mediated ferroportin downregulation is preserved in a mouse model with a disrupted hepcidin/ferroportin regulatory circuitry
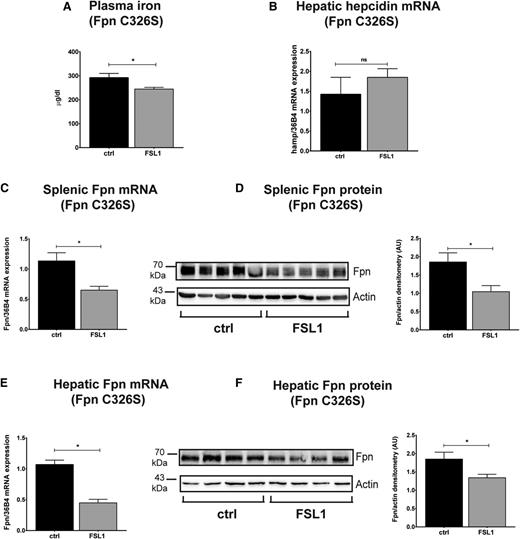
To directly confirm that FSL1-induced hypoferremia and ferroportin regulation is independent of the hepcidin-ferroportin interaction in vivo, we tested a C326S knock-in mouse strain in which ferroportin is rendered hepcidin-resistant.15 It is of note that BMDMs derived from these mice respond to FSL1 and LPS treatment in a highly similar manner like BMDMs from WT mice (eg, by decreasing ferroportin mRNA and protein levels, elevated cytokine expression, and a strong increase in hepcidin mRNA expression exclusively in response to LPS stimulation [supplemental Figure 4]). In vivo data support the observation in BMDMs. Despite the systemic iron overload developed by these mice, FSL1 injection significantly reduces serum iron levels within 3 hours (Figure 6A), accompanied by a reduction in ferroportin mRNA and protein levels in the spleen (Figure 6C-D) and liver (Figure 6E-F) despite unaltered hepcidin levels (Figure 6B). Importantly, injection of a different TLR2 ligand, Pam3CSK4, recapitulates the in vivo findings both in WT mice (supplemental Figure 5) as well as in C326S knock-in mice (supplemental Figure 6), demonstrating that TLR2/6-mediated ferroportin regulation is conserved for different ligands.
Inflammatory hypoferremia and ferroportin protein downregulation are preserved in C326S ferroportin knock-in mice with a disrupted hepcidin/ferroportin regulatory circuitry. (A) Plasma iron level in C326S ferroportin mutant mice injected with FSL1 (100 ng/g body weight) for 3 hours. (B) Hepatic hepcidin mRNA levels were determined by qRT-PCR and normalized to 36B4 mRNA levels. Splenic (C-D) and hepatic (E-F) ferroportin mRNA and protein levels were analyzed by qRT-PCR and western blot in the same groups of mice. β-actin was used as loading control. Each lane in the western blot analysis represents the protein lysate obtained from a single mouse. Data are means ± SEM. *P < .05; Student t test; n = 6 mice per group.
Inflammatory hypoferremia and ferroportin protein downregulation are preserved in C326S ferroportin knock-in mice with a disrupted hepcidin/ferroportin regulatory circuitry. (A) Plasma iron level in C326S ferroportin mutant mice injected with FSL1 (100 ng/g body weight) for 3 hours. (B) Hepatic hepcidin mRNA levels were determined by qRT-PCR and normalized to 36B4 mRNA levels. Splenic (C-D) and hepatic (E-F) ferroportin mRNA and protein levels were analyzed by qRT-PCR and western blot in the same groups of mice. β-actin was used as loading control. Each lane in the western blot analysis represents the protein lysate obtained from a single mouse. Data are means ± SEM. *P < .05; Student t test; n = 6 mice per group.
Discussion
Iron holds a central position at the host-pathogen interface because it is an essential micronutrient for hosts and pathogens alike. Pathogens developed multiple strategies to acquire iron and an adequate supply of iron is linked to pathogen proliferation, virulence, and persistence. Consequently, preclusion of iron from invading microbes represents a key pathway in host defense, for which the term nutritional immunity was coined. The inflammatory response of the innate immune system alters the expression of genes involved in iron metabolism causing sequestration of iron in macrophages of the reticuloendothelial system and hypoferremia. A critical player among these genes is hepcidin, which is induced by inflammatory stimuli in the liver and myeloid cells.5-7,11,21,24 It binds to the iron exporter ferroportin to trigger its degradation, thus reducing iron export from tissue macrophages and a drop in serum iron levels. This is thought to be a critical step in causing hypoferremia in inflammatory and infectious conditions. Infectious agents, such as fungi and viruses, increase hepcidin levels but additionally reduce ferroportin mRNA expression,10,11,25,26 suggesting that different modes of regulation contribute to ferroportin expression during infection.
Toll-like receptors are critical sensors of the innate immune system. Previous studies focused on TLR4 ligation by LPS and demonstrated that reduction of serum iron levels is mediated by increased hepcidin and decreased ferroportin protein levels.10,11 Here, we show that bacterial lipopeptides that mainly target TLR2 and TLR6 exclusively reduce ferroportin expression (Figure 3). TLR2 and TLR6 are dispensable for hepcidin activation in macrophages, which relies on TLR4 stimulation by LPS (Figures 2-3). These differences are qualitatively independent of mRNA expression of IL1β, IL6, or TNFα, which is induced by both TLR2 and TLR4 ligation in macrophages and the spleen (supplemental Figure 2, Figure 5C-D). The amount of hepcidin produced by myeloid cells is minor compared with hepatocytes and a physiological effect of macrophage-expressed hepcidin could not be demonstrated.27 However, it was postulated that hepcidin released from macrophages into an infectious or inflammatory microenvironment contributes to reduce ferroportin expression in an autocrine manner to restrict iron for invading pathogens.11,21,28,29 In this study, we show for the first time that under specific inflammatory conditions the control of ferroportin and hepcidin expression can be uncoupled and that a decrease of ferroportin protein levels downstream of TLR2/6 signaling is independent of hepcidin activation (Figure 2).
We analyzed the Mycoplasma-derived ligand FSL1 as a specific TLR2/TLR6 stimulator. It is widely accepted that diacylated lipopeptides, such as FSL1, signal through TLR2/TLR6 heteromers, whereas triacylated lipopeptides, such as PAM3CSK4 signal through TLR2/TLR1 heteromers.30 However, recent studies applying new synthetic lipopeptide derivates showed that this distinction is not exclusive and that some lipopeptide can be recognized by TLR2 in a TLR1- and TLR6-independent manner, suggesting that TLR2 may signal as homomer.22,31 Our data show that FSL1-mediated ferroportin mRNA downregulation does not depend on TLR1 (data not shown) and only partially depends on TLR6 (Figure 2A-B). By contrast, TLR2 plays the predominant role because the FSL1-mediated control of ferroportin is completely abolished in TLR2-deficient BMDMs (Figure 2C-D). Taken together, our results demonstrate that FSL1-mediated ferroportin regulation is mediated by TLR2/6 heterodimers and/or TLR2 homodimers. Such redundancy may enable the immune system to trigger a more immediate and robust response to rapidly reduce iron supply to pathogens through ferroportin reduction.
A current model suggests that hypoferremia is predominantly induced by a decrease of hepcidin-mediated ferroportin protein expression during acute and chronic inflammation.4 This notion is supported by several studies that analyze LPS-challenged mice10,11,32 and humans.6 However, this model was challenged by the analysis of LPS-injected hepcidin knockout mice that showed hypoferremia.23 The authors concluded that compromised iron absorption due to a decrease of duodenal ferroportin protein levels, and of the membrane iron transporter divalent metal transporter 1 and the oxidoreductase Dcytb, may explain this observation. Here, we demonstrate that hypoferremia can be induced in a hepcidin-independent manner not only in conditions of iron overload but also under steady-state conditions. We show that the rapid decrease of ferroportin mRNA and protein expression at major sites of iron recycling is responsible for this. Specifically, we show that mice injected with FSL1 or LPS both reduce ferroportin mRNA and protein levels in the liver (Figure 4) and in the spleen (Figure 5), whereas only LPS-treated mice induce hepcidin mRNA expression (Figure 4D). Hypoferremia appeared to be a rapid response triggered by both stimuli, as plasma iron and transferrin saturation decreased 3 hours postinjection (Figure 4A-B). Likewise, the hepatic (Figure 4G) and splenic (Figure 5H) iron content increased, indicating iron retention in both tissues. The ferroportin protein reduction observed in the spleen was even more pronounced in magnitude in isolated splenic macrophages (Figure 5G). Contrasting the analyses in hepcidin knockout mice, expression of the duodenal iron transporters ferroportin and divalent metal transporter 1 remained unaffected, suggesting that decreased iron absorption does not significantly contribute to hypoferremia in FSL1-injected mice (supplemental Figure 3).
The analysis of C326S ferroportin mutant mice challenged by FSL1 or Pam3CSK4 injection convincingly demonstrates that the decrease of ferroportin protein levels in the spleen and liver is sufficient to decrease circulating iron levels in the absence of hepcidin-mediated ferroportin control. Despite the severe systemic iron overload, which hallmarks this mouse model, FSL1 and PAM3CSK4 decreased ferroportin mRNA and protein levels and reduced plasma iron levels within 3 hours following injection (Figure 6, supplemental Figure 6).
It is of note that the concentrations of TLR ligands applied in our study are much lower than those reported previously in mouse models to induce acute inflammation in response to LPS,10,32 FSL1,8,33,34 or PAM3CSK48,35-37 stimulation. Clinical measurements of endotoxins in patients remain unclear38-40 and it is thus difficult to estimate the concentrations of TLR ligands that can be achieved in the macrophage microenvironment during bacterial infections. Further analyses are required to clarify the physiological relevance of our findings in models studying infections with whole pathogens.
This report challenges the prevailing notion of how inflammatory hypoferremia is caused. We show that TLRs 2/6 and their stimulation by lipopeptides can mediate hepcidin-independent ferroportin downregulation in BMDMs, in liver and in spleen, probably as a first-line response to restrict iron access to pathogens.
During an infection complex, PAMPs may activate more than one TLR, and the relative contribution of individual TLRs to the immune response is difficult to analyze.41 For example, TLRs can be activated in a time-dependent manner during Salmonella infection, where the initial macrophage response is mediated by TLR4, whereas the latter response is controlled by TLR2.42 Individual TLRs play specific roles in the defense against pathogens. Thus, TLR2 recognizes gram-positive cell wall components, such as peptidoglycan, and plays a critical role in the defense against Mycobacterium leprae43 or Staphylococcus aureus.44,45 In addition, a polymorphism in the human TLR2 gene (R753G) is associated with a decreased response to septic shock after infection with gram-positive bacteria, especially S aureus.46
The inflammatory response triggered by individual TLRs is partially overlapping; that is, TLR2 and TLR4 both activate signaling cascades that induce transcriptional responses mediated by the transcription factor NFκB, but additional regulatory mechanisms are operational specific to each TLR.47,48 Here, we show that TLR4 ligation increases hepcidin and represses ferroportin expression, while TLR2 ligation exclusively decreases ferroportin levels. To address the role of individual TLRs during the immune response most mechanistic studies apply synthetic ligands. Although such an experimental approach cannot fully recapitulate a pathogen infection, it allows deciphering innate immune pathways operational during the immune defense. Our data highlight the importance of ferroportin transcriptional downregulation as a front-line response to inflammation and/or infection and suggest that the pathways described here complement the hepcidin response in generating hypoferremia. This finding may explain previous studies that have questioned the critical role of hepcidin in the anemia of inflammation.49,50 Future investigation of the regulators involved in the ferroportin decrease may enable the identification of novel targets for pharmacologic treatment of anemia of inflammation that control systemic iron levels in a more direct manner compared with drugs that affect the pathways regulating hepcidin levels.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors particularly thank Prof Dr Carsten Kirschning who provided TLR2 KO mice from which they derived BMDMs and Dr Christina Falschlehner for the help in performing the high-throughput screening.
This work was supported by a grant from the University of Heidelberg (Excellenzinitiative; M.U.M.), from the BMBF (Virtual Liver; M.U.M. and M.W.H.), financial support from the Dietmar Hopp Stiftung, and a grant from the University of Heidelberg (Excellenzinitiative II) as well as by a postdoctoral fellowship of the Med. Faculty Heidelberg (S.A.). M.U.M. acknowledges funding from SFB 1036.
Authorship
Contribution: C.G. designed research, performed experiments, and wrote the manuscript; S.A. designed research and performed experiments; F.A.K. analyzed screening data; B.G. and A.J.U. provided reagents and mice; M.B. supervised screening experiments and edited the manuscript; and M.W.H. and M.U.M. designed the project, supervised research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martina U. Muckenthaler, Department of Pediatric Oncology, Hematology and Immunology, University of Heidelberg, Im Neuenheimer Feld 350, 69120 Heidelberg, Germany; e-mail: martina.muckenthaler@med.uni-heidelberg.de; or Matthias W. Hentze, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany; e-mail: hentze@embl.de.
References
Author notes
C.G. and S.A. are co-first authors.
M.W.H. and M.U.M. are co-senior authors.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal