In this issue of Blood, Guida et al reveal a novel role for the Toll-like receptors (TLR)2 and TLR6 in mediating hypoferremia in response to inflammatory stimuli.1
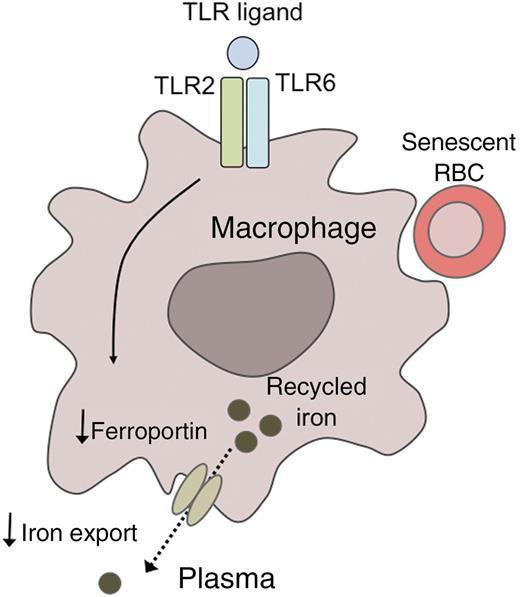
A model for TLR2/6-mediated hypoferremia. Binding of specific TLR ligands to the Toll-like receptors TLR2 and TLR6 downregulates the expression of the cellular iron exporter ferroportin in reticuloendothelial macrophages. As a result, iron that has been recycled from senescent red blood cells fails to be exported into the plasma, promoting the development of hypoferremia.
A model for TLR2/6-mediated hypoferremia. Binding of specific TLR ligands to the Toll-like receptors TLR2 and TLR6 downregulates the expression of the cellular iron exporter ferroportin in reticuloendothelial macrophages. As a result, iron that has been recycled from senescent red blood cells fails to be exported into the plasma, promoting the development of hypoferremia.
The development of hypoferremia, ie, a decrease in circulating iron levels, is a common response to systemic infections and inflammatory conditions. Hypoferremia in these disorders results from the sequestration of iron within macrophages, a response that may have evolved as a host defense mechanism to reduce iron availability to pathogens.2 However, hypoferremia also restricts iron availability to erythroid precursors, and if prolonged, may contribute to the development of the anemia of inflammation.
Previous studies have implicated hepcidin, a circulating peptide hormone produced primarily by hepatocytes, as a central mediator of the hypoferremic response to inflammatory stimuli. Hepcidin synthesis is induced by several cytokines, including interleukin (IL)-6, IL-1, and IL-22, as well as bacterial lipopolysaccharide (LPSs).2 Hepcidin binds to ferroportin, an iron exporter primarily expressed in cell types that process large amounts of iron, including hepatic and splenic macrophages (which recycle iron from senescent erythrocytes), duodenal enterocytes (which absorb dietary iron), and hepatocytes (which serve as the major site of iron storage). Because hepcidin binding triggers the internalization and degradation of ferroportin, hepcidin limits the export of iron into the circulation. Although hepatocytes serve as the major source of circulating hepcidin, hepcidin is also produced by other cell types, including macrophages, where it may contribute to the local regulation of iron fluxes.3
In the present study, Guida et al sought to identify novel genes that regulate ferroportin-mediated iron export from cells. Using gene silencing by small interfering RNA-based knockdown technology in HeLa cells, the authors screened a large collection of protein kinases and related signaling molecules for the ability to modulate ferroportin expression levels. This screen identified TLR6 as a suppressor of ferroportin protein levels.1
TLRs serve as key regulators of specific innate immune responses by recognizing microbe-specific molecular signatures. TLRs are single transmembrane spanning proteins expressed in both immune and nonimmune cells and are known to function as dimers. TLR6, the TLR identified in the present study, is known to heterodimerize with TLR2.4 To test whether TLR6 signaling could alter ferroportin expression in macrophages, Guida et al treated mouse bone marrow-derived macrophages (BMDMs) with FSL1,1 a synthetic diacylated lipoprotein derived from Mycoplasma salivarium that is recognized by TLR2 and TLR6.5 FSL1 treatment caused a profound reduction in ferroportin mRNA and protein levels in BMDMs from wild-type mice. However, the ability of FSL1 to lower ferroportin expression was blunted in BMDMs from TLR6-knockout mice and was completely lost in BMDMs from TLR2-knockout mice, supporting roles for both TLR6 and TLR2 in mediating the ferroportin response.
Suppression of ferroportin mRNA and protein levels in macrophages has been shown previously to occur in response to stimulation of another TLR family member, TLR4, with LPS; notably, this process has been shown to be accompanied by hepcidin induction in macrophages.6,7 Guida et al1 found that, like LPS, FSL1 stimulation of wild-type BMDMs increased expression of inflammatory cytokines and decreased ferroportin mRNA levels. However, in contrast to LPS treatment, treatment of wild-type BMDMs with FSL1 did not induce hepcidin expression.
Next, the authors examined whether administration of TLR ligands to mice could induce acute changes in systemic iron homeostasis. Similar to injection with LPS, injection of wild-type mice with FSL1 reduced ferroportin expression in the liver and spleen, promoted iron sequestration in these organs, and induced hypoferremia within 3 hours. However, in contrast to LPS, injection of FSL1 failed to increase hepcidin mRNA in either liver or spleen of wild-type mice. Importantly, FSL1 injection was also effective in acutely lowering hepatic and splenic ferroportin expression and plasma iron levels in mice harboring a mutant form of ferroportin that is rendered insensitive to hepcidin due to an amino acid substitution (C326S) in the hepcidin-binding domain. Administration of Pam3CSK4, a synthetic ligand recognized by TLR2, to wild-type mice as well as to the hepcidin-insensitive ferroportin mutant mice, produced changes in ferroportin expression and plasma iron levels similar to those induced by FSL1, demonstrating conservation of the response across different TLR ligands.
Based on their novel findings, Guida et al1 propose a model whereby FSL1 binds to TLR2/6, which in turn downregulates ferroportin, resulting in hypoferremia by a mechanism that is hepcidin independent (see figure). Interestingly, a recent study of hepcidin-knockout mice also suggested that the hypoferremic response to inflammatory stimuli may not be entirely hepcidin dependent, as these mice remained capable of responding to LPS injection with a moderate, but significant, reduction in plasma iron levels.8
Guida et al1 have revealed a novel role for TLR2/6 in mediating an acute hypoferremic response to inflammatory stimuli, which may have important implications for the pathophysiological understanding of anemia of inflammation. Further studies are needed to clarify whether this hepcidin-independent pathway is important for the hypoferremic response to intact pathogens. It will be important to understand whether the concentrations of TLR ligands achieved by murine injection in the present study have relevance to the TLR ligand concentrations present in the macrophage microenvironment during microbial infections in humans. It will also be interesting to clarify the specific downstream signaling pathway by which TLR2/6 stimulation results in altered ferroportin expression. Given that common polymorphisms in genes encoding several TLRs have been associated with altered susceptibility to infectious, inflammatory, and allergic diseases,9 the present results raise the intriguing possibility that polymorphisms in TLR2 and/or TLR6 might influence the hypoferremic response to inflammatory stimuli.
Several approaches that antagonize the hepcidin-ferroportin interaction are under investigation as potential therapies for iron-restricted anemias, including reducing hepcidin production, neutralizing the hepcidin peptide, blocking hepcidin binding to ferroportin, and enhancing ferroportin membrane expression.10 Future study of the hepcidin-independent role of TLR2/6 to the development of hypoferremia may be informative in elucidating additional strategies that might be used for treatment of iron-restricted anemias.
KEF is a recipient of a Burroughs Wellcome Career Award for Medical Scientists.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal