Key Points
EBV LMP2A alters B-cell gene expression; E47 and PU.1 are repressed by LMP2A, resulting in downregulation of MHC class II expression.
Abstract
Oncogenic Epstein-Barr virus (EBV) uses various approaches to escape host immune responses and persist in B cells. Such persistent infections may provide the opportunity for this virus to initiate tumor formation. Using EBV-immortalized lymphoblastoid cell lines (LCLs) as a model, we found that the expression of major histocompatibility complex (MHC) class II and CD74 in B cells is repressed after EBV infection. Class II transactivator (CIITA) is the master regulator of MHC class II–related genes. As expected, CIITA was downregulated in LCLs. We showed that downregulation of CIITA is caused by EBV latent membrane protein 2A (LMP2A) and driven by the CIITA-PIII promoter. Furthermore, we demonstrated that LMP2A-mediated E47 and PU.1 reduction resulted in CIITA suppression. Mechanistically, the LMP2A immunoreceptor tyrosine-based activation motif was critical for the repression of E47 and PU.1 promoter activity via Syk, Src, and the phosphatidylinositol 3-kinase/Akt pathway. Elimination of LMP2A in LCLs using a shLMP2A approach showed that the expression levels of E47, PU.1, CIITA, MHC class II, and CD74 are reversed. These data indicated that the LMP2A may reduce MHC class II expression through interference with the E47/PU.1-CIITA pathway. Finally, we demonstrated that MHC class II may be detected in tonsils and EBV-negative Hodgkin disease but not in EBV-associated posttransplant lymphoproliferative disease and Hodgkin disease.
Introduction
During infection, viruses face the various challenges of the host immune response and have evolved a number of immune evasion strategies to enable successful infection of the host cells. Epstein-Barr virus (EBV) is a ubiquitous, human γ-herpesvirus that persistently infects more than 90% of the human population.1 EBV has evolved several mechanisms to escape immune surveillance: EBV limits the expression of its proteins and persists in immune B cells. EBV uses various strategies to attenuate the first line of innate immunity; for example, latent membrane protein 1 (LMP1) negatively regulates the expression of the important sensor Toll-like receptor 9,2 B-cell receptor F1 (BCRF1)-encoded viral interleukin-10 inhibits interferon production,3 and the tegument protein BPLF1 blocks Toll-like receptor signaling.4 In counteracting the adaptive immune response, EBV interferes with the major histocompatibility complex (MHC) class I and II antigen presentations, which are the key factors for adaptive immunity. MHC antigens are critical in the cellular immune response, which is important for viral clearance. Several studies have addressed the question how EBV downregulates MHC class I antigen expression. EBNA1 has a glycine-alanine repeat motif that protects it from degradation and also inhibits its own synthesis.5,6 The EBV lytic protein BNLF2a interferes with both the peptide and adenosine triphosphate binding to the transporter associated with antigen processing.7 As with the HSV1 virion host shut-off protein, EBV DNase BGLF5 inhibits MHC class I protein production.8 The viral G-coupled receptor protein BILF1 also downregulates MHC class I expression by promoting the degradation of its messenger RNA (mRNA), increasing endocytosis and degradation.9 Regarding EBV and MHC class II antigen, the transactivator Zta can inhibit class II antigen expression via direct downregulation of the class II transactivator (CIITA) by binding to its promoter or, indirectly, by inhibition of interferon-γ (IFN-γ) production.10,11 In addition, the viral host protein shut-off master BGLF5 directly blocks class II antigen synthesis.8 Furthermore, the EBV viral interleukin-10 also inhibits the production of transporter associated with antigen processing 1 and the proteasome subunit bi-LMP2 at the transcriptional level to prevent MHC I antigen presentation and interferes with IFN-γ to block MHC class II induction.12,13 In addition, the BZLF2-encoded gp42 can associate with MHC class II molecules in the endoplasmic reticulum and accompany the class II complexes to the cell surface. Then, gp42 in the class II complex blocks the interaction with the receptors on CD4+ T cells.14 Taken together, EBV uses many strategies to prevent MHC antigen expression; however, most involve lytic gene products. According to the evidence provided by Thorley-Lawson, LMP2A may be the critical viral protein expressed in the healthy EBV carrier.15 So, we wonder how EBV escapes immune surveillance in this situation.
It is interesting to consider the protein structure of LMP2A.16 This latent protein contains 12 transmembrane domains and has a short C terminus and long, 119-aa N terminus containing 8 tyrosine residues. More impressively, the residues tyrosine 74 and 85 can form a motif that mimics the immunoreceptor tyrosine-based activation motif (ITAM) of the BCR. So, the biological functions of LMP2A are similar to the BCR in some ways.17,18 A series of studies from Longnecker’s laboratory revealed that LMP2A may provide survival signaling to B cells in LMP2A-transgenic mice and also facilitate the oncogenicity of c-Myc.19,20 Of interest, they demonstrated that LMP2A requires Notch1 to alter B-cell gene expression in a very similar manner to the gene expression in Reed-Sternberg cells of EBV-associated Hodgkin disease (HD).21 In addition, they speculated that these cells can survive with an intact p5322 because of cell growth being directly promoted through the c-Myc pathway.20 In our previous study, we showed that LMP2A can regulate c-Jun activity and so promote cell mobility.23 Furthermore, we found that Syk can be activated by the ITAM of LMP2A and facilitate nasopharyngeal carcinoma metastasis.24 Thus, we address the question whether LMP2A plays any role in immune evasion because it may be the only EBV protein expressed in normal carriers.
In our preliminary complementary DNA (cDNA) array screening, we noted the downregulation of MHC class II antigen expression (data not shown). These results encouraged us to further investigate the molecular mechanism of how EBV gene products repress the expression of MHC class II antigen. In this study, we found that LMP2A uses an indirect strategy to downregulate the expression of MHC class II proteins via suppression of the B-cell transcriptional factors PU.1 and E47. Moreover, MHC class II expression is reduced in biopsies from EBV-associated posttransplant lymphoproliferative disease (PTLD) and HD. The mechanism and significance of this downregulation will be discussed.
Materials and methods
B-cell purification and EBV infection
Peripheral blood mononuclear cells were isolated from the whole blood of anonymous donors and CD19-positive B cells were purified using Dynabeads (Invitrogen). Production of EBV virions (B95-8 strain) and infection of B cells by EBV have been described previously.25 Experiments involving human samples were approved by the institutional review boards of National Taiwan University Hospital (NTUH, Taipei, Taiwan).
Cell culture and inhibitors
Akata and BJAB are EBV-negative Burkitt lymphoma–derived cell lines. Raji cells are an EBV-positive Burkitt lymphoma–derived cell line. Lymphoblastoid cell lines (LCLs) were established from EBV-infected peripheral blood mononuclear cells or purified B cells. All B-cell lines were cultured in complete RPMI medium (containing 10% fetal calf serum, 1 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). Syk inhibitor (Piceatannol), Src inhibitor (PP2), mitogen-activated protein kinase kinase inhibitor (U0126), and phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002) were purchased from Merck Millipore. SP1 inhibitor (mithramycin) was purchased from CalBiochem.
Construction of plasmids
The EBNA1 plasmid pCEP4 (Invitrogen) carries the EBNA1 gene. BARF0 was amplified from the genomic DNA of Akata cells by polymerase chain reaction (PCR) and then inserted into pcDNA3. EBER1 and EBER2 were constructed into pLKO vector at the 5′ AgeI site and the 3′ EcoRI site. The pSIN-LMP1 was constructed by inserting LMP1 cDNA into the pSIN.26 The pSIN-LMP2A was constructed by insertion of full-length LMP2A cDNA into the pSIN at 5′ BamHI site and 3′ NotI site. The LMP2A-deleted 74-85 and Y112F were constructed by site-directed mutagenesis. The LMP2A PY motif mutant constructs were kindly provided by Dr Yao Chang (National Health Research Institutes, Tainan, Taiwan).27 A series of luciferase reporter plasmids driven by the E47 or PU.1 promoter were constructed in the pCDH-GL3-basic vector. The pcDNA3-E47fD was kindly provided by Dr Rudolf Grosschedl (Max-Planck Institute of Immunobiology, Freiburg, Germany). The pLKO-forced-dimered-E47-puro was constructed by insertion of the E47fd fragment from pcDNA3-E47fd into the pLKO-AS2-puro at the NheI site. The pLKO-PU.1-neo was constructed by the insertion of PU.1 cDNA into the pLKO-AS2-neo at the 5′ EcoRI site and the 3′ AscI site.
Preparation and infection of lentiviruses
RNA interference fragments were purchased from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan) and their sequences are shown in supplemental Table 1. Briefly, plasmids p8.91, pMD2.G, and pLKO.1-shLuc, pLKO.1-shPIK3CA, pLKO.1-shPAX5, pLKO.1-shSP1, pLKO.1-shLMP2A, pSIN-LMP1, pSIN-LMP2A, pSIN-Zta, pLKO-PU.1, and pLKO-E47fd were cotransfected into HEK293T cells using lipofectamine 2000 (Invitrogen). Infectious expressing lentiviruses were collected at day 3 posttransfection and stored at −80°C. The method of production and infection with lentiviruses has been described previously.25 For lentivirus infection, LCLs or BJAB cells were infected with lentiviruses at a multiplicity of infection of 1 to 2.
Electroporation
The method of electroporation was described previously.26 Cells were electroporated using a Neon kit (Invitrogen).
Analysis of RT-PCR and Q-PCR
Total RNA was isolated from cells using TRIzol (Invitrogen). Synthesis of cDNA has been described in our previous article.26 The cDNA was used as a template for PCR in the presence of specific primers shown in supplemental Table 1. Analysis of quantitative PCR (Q-PCR) was performed using the TaqMan primer/probe set (Pre-Developed Assay Reagents; Applied Biosystems) in some experiments. Primers and probes for other transcripts are shown as supplemental Table 1. The relative intensity fold of reverse transcriptase PCR (RT-PCR) was normalized with internal control and then standardized to the vector control.
Western blotting and antibodies
Cells were lysed by buffer, and western blotting was performed according to our previous study.25 Anti-E47, -PU.1, -CIITA, -HLA-DR, -CD74, -phospho-extracellular signal-regulated kinase (ERK) Thr202/Tyr204, -ERK, and -Akt antibodies were purchased from Santa Cruz Biotechnology. Anti-phospho-Akt Ser473 antibody was purchased from Cell Signaling Technology and anti-β-actin antibody was purchased from Sigma-Aldrich. Anti-EBNA1 (NPC47),28 -LMP1 (S12)29 and -LMP2A23 antibodies were used according to previous studies. The relative folds of the protein of interest were determined by normalizing the level of each group to the corresponding β-actin intensity and then standardized with the vector control.
Reporter assay
Cells were infected with the pCDH-GL3 promoter luciferase reporter lentiviruses at a multiplicity of infection of 1. On day 4 postinfection, the luciferase activities and green fluorescence protein (GFP) intensity were detected using the Bright-Glo Luciferase Assay System kit (Promega). The relative fold induction of luciferase activity from each transfectant was normalized to its GFP intensity and standardized with the vector control.
IHC and in situ hybridization of EBER assays
Tonsil, PTLD, and HD biopsies were obtained from the NTUH. Experiments involving human samples were approved by the institutional review boards of NTUH. IHC assays were performed using the Super Sensitive Link-Label IHC Detection System (BioGenex), and in situ hybridization of EBER assays were described and performed according to our previous article.26
Results
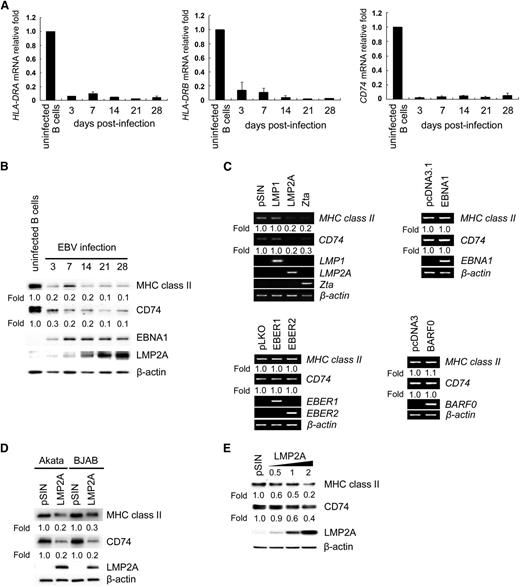
The expression of MHC class II and CD74 mRNAs decreases following EBV infection
Regulation of MHC class II and its associated chaperone, CD74, has been reported during virus infection.30,31 To determine whether the expression of MHC class II and CD74 is influenced by EBV infection, expression of HLA-DRA and HLA-DRB was detected in primary B cells with or without EBV infection by RT-Q-PCR and western blotting. As shown in Figure 1B, we demonstrated that downregulation of MHC class II and CD74 was observed when infected B cells expressed LMP2A at day 3 postinfection. After that, LMP2A was constitutively expressed in infected cells and low-level expression of MHC class II and CD74 was detected at the same time (Figure 1B). In the EBV-infected primary B-cell system, we concluded that EBV may block viral antigen presentation by downregulation of MHC class II and CD74.
Downregulation of the expression of MHC class II and CD74 in LCLs. CD19-positive B cells were seeded in a 12-well plate at a density of 1 × 106 cells per well and infected with EBV. RNA and proteins were harvested at the time points indicated. (A) The expression of HLA-DRA, HLA-DRB1, and CD74 transcripts was measured by RT-Q-PCR. The relative fold of the transcripts was normalized to uninfected B cells with the corresponding β-actin mRNA. This is a representative result from 6 independent experiments from anonymous donors. (B) Protein expression of MHC class II, CD74, EBNA1, LMP2A, and β-actin was detected by western blotting. Detection of β-actin served as an internal control. (C) BJAB cells were transfected with EBNA1 or BARF0 expression plasmids or infected with EBER1, EBER2, LMP1, LMP2A, or Zta lentiviruses. Expression of MHC class II, CD74, EBNA1, EBER1, EBER2, BARF0, LMP1, LMP2A, and Zta transcripts in the transfectants and lentiviruses-infected cells were analyzed by RT-PCR. β-actin was detected as an internal control. (D) Akata and BJAB cells were infected with LMP2A-expressing lentiviruses. Cell lysates were harvested and the expression of MHC class II, CD74, and LMP2A was detected by western blotting. β-actin was detected as an internal control. (E) BJAB cells were infected with various doses of LMP2A-expressing lentiviruses. At day 5 postinfection, cell lysates were harvested for the detection of MHC class II, CD74, and LMP2A by western blot analysis. β-actin was detected as an internal control. The experiment was performed 3 times, and 1 representative is shown.
Downregulation of the expression of MHC class II and CD74 in LCLs. CD19-positive B cells were seeded in a 12-well plate at a density of 1 × 106 cells per well and infected with EBV. RNA and proteins were harvested at the time points indicated. (A) The expression of HLA-DRA, HLA-DRB1, and CD74 transcripts was measured by RT-Q-PCR. The relative fold of the transcripts was normalized to uninfected B cells with the corresponding β-actin mRNA. This is a representative result from 6 independent experiments from anonymous donors. (B) Protein expression of MHC class II, CD74, EBNA1, LMP2A, and β-actin was detected by western blotting. Detection of β-actin served as an internal control. (C) BJAB cells were transfected with EBNA1 or BARF0 expression plasmids or infected with EBER1, EBER2, LMP1, LMP2A, or Zta lentiviruses. Expression of MHC class II, CD74, EBNA1, EBER1, EBER2, BARF0, LMP1, LMP2A, and Zta transcripts in the transfectants and lentiviruses-infected cells were analyzed by RT-PCR. β-actin was detected as an internal control. (D) Akata and BJAB cells were infected with LMP2A-expressing lentiviruses. Cell lysates were harvested and the expression of MHC class II, CD74, and LMP2A was detected by western blotting. β-actin was detected as an internal control. (E) BJAB cells were infected with various doses of LMP2A-expressing lentiviruses. At day 5 postinfection, cell lysates were harvested for the detection of MHC class II, CD74, and LMP2A by western blot analysis. β-actin was detected as an internal control. The experiment was performed 3 times, and 1 representative is shown.
EBV LMP2A is a critical factor in the downregulation of MHC class II and CD74
To identify which EBV gene product is responsible for the reduction of MHC class II and CD74 expression, individual EBV genes were ectopically expressed in EBV-negative BJAB cells. In Figure 1C, we observed that only LMP2A or Zta downregulates expression of MHCclass II and CD74 transcripts, but not the other viral gene products tested, including EBNA1, LMP1, EBER1, EBER2, or BARF0. The EBV lytic cycle transactivator Zta functions as a repressor of MHC class II and CD74 expression, which is consistent with a previous report.31 In particular, we were interested in LMP2A because it is expressed predominately during the viral latent stage. We found that the protein levels of MHC class II and CD74 were downregulated in Akata and BJAB cells expressing ectopic LMP2A (Figure 1D). As shown in Figure 1E, MHC class II and CD74 proteins decreased in a dose-dependent manner following transduction of the cells with LMP2A-expressing lentiviruses.
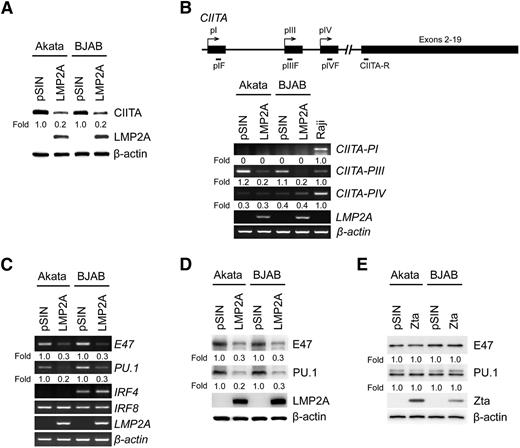
LMP2A triggers the downregulation of CIITA, E47, and PU.1
We speculated that downregulation of MHC class II and CD74 possibly resulted from LMP2A-triggered downstream signaling. Based on previous studies, CIITA is the master regulator of MHC class II expression,32 so the expression of CIITA was measured in LMP2A-transduced Akata and BJAB cells. In Figure 2A, LMP2A suppressed CIITA expression. In general, expression of CIITA is driven by 3 independent promoter units—PI, PIII, and PIV—which are cell type–specific.10 To determine which promoter unit is regulated by LMP2A, the expression of CIITA-PI, CIITA-PIII, and CIITA-PIV transcripts was detected in Akata and BJAB cells transduced with LMP2A-lentivirus. LMP2A suppressed the expression of CIITA-PIII in this system, consistent with a previous study10 that showed CIITA-PIII mRNA is specifically expressed in B cells (Figure 2B). According to previous studies, several B-cell transcriptional factors are involved in the regulation of CIITA-PIII, including E47, PU.1, IRF4, and IRF8.33 In Figure 2C, a significant reduction of E47 and PU.1 was seen in Akata and BJAB cells expressing LMP2A, but the expression of IRF4 and IRF8 did not change. Protein expression levels of E47 and PU.1 were also reduced in Akata and BJAB cells expressing ectopic LMP2A (Figure 2D). In Li’s study,31 Zta-mediated suppression of MHC class II was via direct binding to the CIITA promoter; our results indicated that Zta does not alter the expression of E47 and PU.1 expression (Figure 2E). Taken together, it seems that EBV uses different regulatory mechanisms to downregulate MHC class II and CD74 during latency and the lytic replication cycle.
LMP2A downregulates the expression of CIITA, E47, and PU.1. (A) Akata and BJAB cells were infected with LMP2A-expressing lentiviruses. Expression of CIITA and LMP2A was detected by western blotting. (B) The expression of the CIITA-PI, CIITA-PIII, CIITA-PIV, and LMP2A transcripts was measured by RT-PCR analysis. Raji cells were used as a positive control for the CIITA promoters. (C) Expression of E47, PU.1, IRF4, IRF8, and LMP2A was measured by RT-PCR analysis. (D) Expression of E47, PU.1, and LMP2A was detected by western blotting. (E) Akata and BJAB cells were infected with Zta-expressing lentiviruses. Expression of E47, PU.1, and Zta was detected by western blotting. β-actin was detected as an internal control. Each experiment was performed 3 times, and 1 representative is shown.
LMP2A downregulates the expression of CIITA, E47, and PU.1. (A) Akata and BJAB cells were infected with LMP2A-expressing lentiviruses. Expression of CIITA and LMP2A was detected by western blotting. (B) The expression of the CIITA-PI, CIITA-PIII, CIITA-PIV, and LMP2A transcripts was measured by RT-PCR analysis. Raji cells were used as a positive control for the CIITA promoters. (C) Expression of E47, PU.1, IRF4, IRF8, and LMP2A was measured by RT-PCR analysis. (D) Expression of E47, PU.1, and LMP2A was detected by western blotting. (E) Akata and BJAB cells were infected with Zta-expressing lentiviruses. Expression of E47, PU.1, and Zta was detected by western blotting. β-actin was detected as an internal control. Each experiment was performed 3 times, and 1 representative is shown.
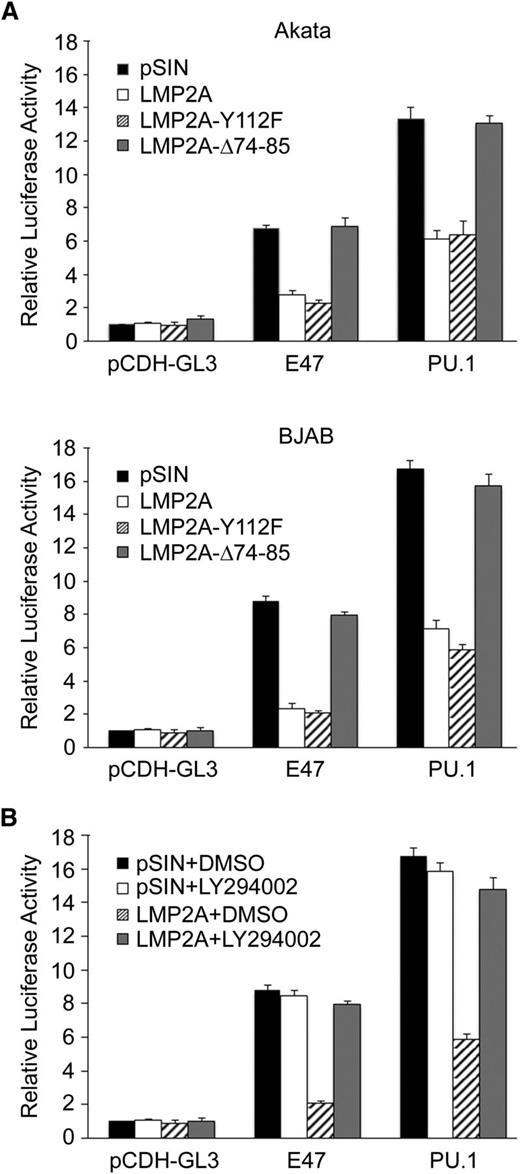
LMP2A-mediated suppression of E47 and PU.1 is through the PI3K/Akt pathway
LMP2A contains 8 tyrosine residues that are important for LMP2A function. LMP2A is phosphorylated on tyrosine 112 through its interaction with Lyn34 and interacts constitutively with Syk through the ITAM (tyrosine 74 and 85).35 In addition, LMP2A PY motifs play an important role in protein stability and phosphorylation of LMP2A-associated proteins.36 To evaluate the potencies of the phosphorylated tyrosine 112 and ITAM domain of LMP2A involved in the expression of E47, PU.1, MHC class II, and CD74, lentiviruses carrying Y112F-mutated or Δ74-85 deleted LMP2A were prepared. In Figure 3A, compared with the vector control, LMP2A wild-type and the Y112F mutant reduced the expression of E47, PU.1, MHC class II, and CD74. However, the LMP2A-Δ74-85 mutant had significantly lost the ability to suppress the expression of E47 and PU.1. In addition, we addressed the involvement of PY domains in LMP2A-mediated suppression of PU.1 and E47. In Figure 3B, expression of PU.1 and E47 was not restored when cells express LMP2A with mutated PY domains. Thus, downstream signaling molecules of the ITAM in LMP2A were required for E47 and PU.1 suppression.
LMP2A downregulates the expression of E47 and PU.1 through the PI3K/Akt pathway. (A) BJAB cells were infected with LMP2A-, LMP2A-Δ74-85–, or LMP2A-Y112F–expressing lentiviruses. Expression of LMP2A, E47, PU.1, CD74, and MHC class II was analyzed by western blotting. β-actin was detected as an internal control. (B) BJAB cells were transfected with wild-type LMP2A or LMP2A with mutated PY motif expression plasmids, and the transfectants were subjected to western analysis of LMP2A, E47, and PU.1. β-actin was detected as an internal control. (C) LCLs were cultured in the presence of 25 μM Piceatannol or 10 μM PP2 at the indicated time. Expression of phospho-Akt (pAkt), total Akt, E47, and PU.1 was analyzed by western blotting. β-actin was detected as an internal control. (B-C) Experiments were performed twice, and 1 representative is shown. (D-E) BJAB cells were infected with LMP2A-expressing lentiviruses or vector control. After 5 days, the cells were cultured in the presence of 20 μM LY294002 (D) or 20 μM U0126 (E) paired to dimethylsulfoxide (DMSO) control for 48 hours. Cell lysates were subjected to western analysis of E47, PU.1 CD74, MHC class II, and LMP2A. β-actin was detected as an internal control. (D) Special detection of pAkt and total Akt and (E) detection of phosphoERK1/2 (pERK1/2) and total ERK. (F) LCLs were infected with sh-Luc or sh-PIK3CA expressing lentivirus and the cell lysates were subjected to western analysis of E47, PU.1, CD74, and MHC class II. β-actin was detected as an internal control. (G) BJAB cells were infected with LMP2A, PU.1, or forced-dimered-E47 (FD-E47) lentiviruses and transfectants were subjected to western analysis of FD-E47, endogenous E47 (endo-E47), PU.1, CD74, MHC class II, and LMP2A. β-actin was detected as an internal control. Each experiment was performed 3 times, and 1 representative is shown.
LMP2A downregulates the expression of E47 and PU.1 through the PI3K/Akt pathway. (A) BJAB cells were infected with LMP2A-, LMP2A-Δ74-85–, or LMP2A-Y112F–expressing lentiviruses. Expression of LMP2A, E47, PU.1, CD74, and MHC class II was analyzed by western blotting. β-actin was detected as an internal control. (B) BJAB cells were transfected with wild-type LMP2A or LMP2A with mutated PY motif expression plasmids, and the transfectants were subjected to western analysis of LMP2A, E47, and PU.1. β-actin was detected as an internal control. (C) LCLs were cultured in the presence of 25 μM Piceatannol or 10 μM PP2 at the indicated time. Expression of phospho-Akt (pAkt), total Akt, E47, and PU.1 was analyzed by western blotting. β-actin was detected as an internal control. (B-C) Experiments were performed twice, and 1 representative is shown. (D-E) BJAB cells were infected with LMP2A-expressing lentiviruses or vector control. After 5 days, the cells were cultured in the presence of 20 μM LY294002 (D) or 20 μM U0126 (E) paired to dimethylsulfoxide (DMSO) control for 48 hours. Cell lysates were subjected to western analysis of E47, PU.1 CD74, MHC class II, and LMP2A. β-actin was detected as an internal control. (D) Special detection of pAkt and total Akt and (E) detection of phosphoERK1/2 (pERK1/2) and total ERK. (F) LCLs were infected with sh-Luc or sh-PIK3CA expressing lentivirus and the cell lysates were subjected to western analysis of E47, PU.1, CD74, and MHC class II. β-actin was detected as an internal control. (G) BJAB cells were infected with LMP2A, PU.1, or forced-dimered-E47 (FD-E47) lentiviruses and transfectants were subjected to western analysis of FD-E47, endogenous E47 (endo-E47), PU.1, CD74, MHC class II, and LMP2A. β-actin was detected as an internal control. Each experiment was performed 3 times, and 1 representative is shown.
It is known that the ITAM of LMP2A mediates activation of Syk and Src, which then activate the PI3K/Akt signaling pathway.37,38 An inhibitor assay was used to determine whether Syk and Src signaling pathways are involved in LMP2A-mediated downregulation. As shown in Figure 3C, blockage of activated Syk and Src by their inhibitors, Piceatannol and PP2, respectively, resulted in decreased amounts of phospho-Akt and the expression of E47 and PU.1 was upregulated in LCL cells. Furthermore, LMP2A-mediated suppression of E47, PU.1, MHC class II, and CD74 was abolished in the presence of an inhibitor of PI3K (LY294002). In contrast, LMP2A-mediated suppression of these molecules was not altered in cells treated with the mitogen-activated protein kinase kinase inhibitor, U0126 (Figure 3D-E). Furthermore, the reduction of E47, PU.1, MHC class II, and CD74 was recovered when the p110 subunit of PI3K was knocked down by short hairpin RNA (shRNA) (Figure 3F). Taken together, these data suggest that LMP2A downregulates the expression of E47 and PU.1 through the PI3K/Akt pathway. We then sought to confirm whether the LMP2A-mediated downregulation of MHC class II also was through this pathway. Expression of MHC class II, CD74, and CIITA was measured in cells expressing LMP2A, with or without overexpression of E47 and PU.1. In Figure 3G, coexpression of E47 and PU.1 significantly reversed the LMP2A-mediated suppression of MHC class II and CD74.
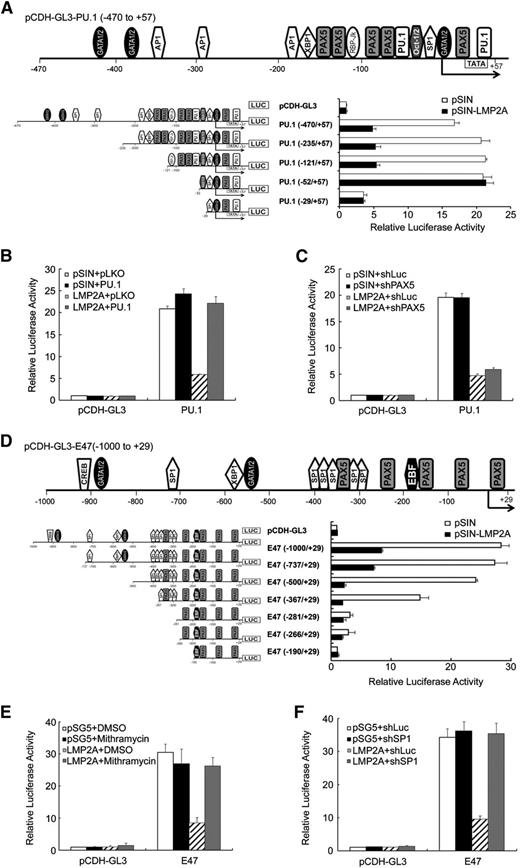
LMP2A inhibits the promoter activities of E47 and PU.1
Luciferase promoter reporter assays were performed to understand more of the mechanism of LMP2A-mediated repression of E47 and PU.1. The promoter activities of PU.1 and E47 in Akata and BJAB cells were repressed by LMP2A and the LMP2A-Y112F mutant, but not by the LMP2A-Δ74-85 mutant (Figure 4A). In addition, the promoter activities were restored by the PI3K inhibitor, LY294002 (Figure 4B). To further dissect which regions of the promoters are crucial for this inhibition, Figure 5A,D illustrate the transcriptional factor binding sites in schematic maps of the promoter regions of PU.1 (−470 to +57) and E47 (−1000 to +29). According to the results of serial deletions of the E47 and PU.1 promoters, the region −121 to −52 bp in the PU.1 promoter might be critical for LMP2A-mediated inhibition (Figure 5A). There are 5 PAX5 and 2 PU.1 putative binding sites in this region. Furthermore, the promoter activity of PU.1 was restored in cells overexpressing PU.1 (Figure 5B). However, the promoter activity of PU.1 was not affected in PAX5 knockdown cells (Figure 5C). These results demonstrated that PU.1 itself plays a key role in regulating of LMP2A-mediated suppression of PU.1 promoter activity. However, we could not find a specific region on the E47 promoter that is involved in LMP2A-mediated downregulation (Figure 5D). There are many putative SP1 sites on E47 promoter, so we tried to block the SP1-DNA binding activity. Mithramycin, an SP1 inhibitor that blocks the binding between SP1 and GC-rich DNA, was used to test the involvement of SP1 in regulating E47 promoter activity. According to the reporter assay results in Figure 5E-F, SP1 is involved in LMP2A-mediated downregulation of E47. These results reveal that LMP2A downregulates E47 and PU.1 promoter activity through ITAM-dependent activated PI3K/Akt signaling. Meanwhile, PU.1 and SP1 were crucial for this regulation.
LMP2A inhibited the E47 and PU.1 promoter activity through the ITAM motif. (A) Akata and BJAB cells were infected with pSIN, LMP2A, LMP2A-Y112F, and LMP2A-Δ74-85 expressing lentiviruses. After 3 days, the cells were infected with pCDH-GL3–, E47-, and PU.1 reporter–expressing lentiviruses. Luciferase activities were normalized with the GFP intensities of each transfectant. The activated fold was calculated by normalizing luciferase activities for the transfectant vs that for the pSIN with pCDH-GL3 vector control. (B) BJAB cells were infected with pSIN- and LMP2A-expressing lentiviruses. After 3 days, the cells were infected with pCDH-GL3–, E47-, and PU.1-expressing lentiviruses and incubated with dimethylsulfoxide (DMSO) or 20 μM LY294002 for another 48 hours. Luciferase activities from each transfectant were normalized with the GFP intensities. The activated fold for each reporter was calculated by normalizing luciferase activities for the transfectant vs that for the pSIN with pCDH-GL3 vector control. These data are a composite of 3 independent experiments (mean ± standard deviation).
LMP2A inhibited the E47 and PU.1 promoter activity through the ITAM motif. (A) Akata and BJAB cells were infected with pSIN, LMP2A, LMP2A-Y112F, and LMP2A-Δ74-85 expressing lentiviruses. After 3 days, the cells were infected with pCDH-GL3–, E47-, and PU.1 reporter–expressing lentiviruses. Luciferase activities were normalized with the GFP intensities of each transfectant. The activated fold was calculated by normalizing luciferase activities for the transfectant vs that for the pSIN with pCDH-GL3 vector control. (B) BJAB cells were infected with pSIN- and LMP2A-expressing lentiviruses. After 3 days, the cells were infected with pCDH-GL3–, E47-, and PU.1-expressing lentiviruses and incubated with dimethylsulfoxide (DMSO) or 20 μM LY294002 for another 48 hours. Luciferase activities from each transfectant were normalized with the GFP intensities. The activated fold for each reporter was calculated by normalizing luciferase activities for the transfectant vs that for the pSIN with pCDH-GL3 vector control. These data are a composite of 3 independent experiments (mean ± standard deviation).
LMP2A inhibited the E47 and PU.1 promoter activity. (A) Schematic map of the promoter region of PU.1 (−470 to +57). Deletion constructs derived from the PU.1 construct (−470 to +57) were subcloned into the pCDH-GL3 luciferase reporter vector. BJAB cells were infected with pSIN- and LMP2A-expressing lentiviruses. After 3 days, the cells were infected with the reporter lentiviruses indicated. After 4 days, luciferase activities from each transfectant were normalized with the GFP intensities. (B) BJAB cells were transduced with pLKO or PU.1 lentiviruses. After 5 days, infected BJAB cells were transduced with PU.1- (−470 to +57), pSIN-, or LMP2A-expressing lentiviruses and then the luciferase activities were measured and normalized to GFP activity at day 5 postinfection. (C) BJAB cells were transduced with shLuc or shPAX5 lentiviruses. After 5 days, infected BJAB cells were further transduced with PU.1- (−470 to +57), pSIN-, or LMP2A-expressing lentiviruses. At day 5 postinfection, the luciferase activities were measured and normalized to GFP activity. (D) Schematic map of the promoter region of E47 (−1000 to +29). Deletion constructs derived from the E47 construct (−1000 to +29) were subcloned into the pCDH-GL3 luciferase reporter vector. The 293T cells were transfected with pSG5- and LMP2A-expressing plasmids. After 3 days, the luciferase activities were measured and normalized to GFP activity. (E) The 293T cells were transfected with pCDH-GL3-E47– (−1000 to +29), pSG5-, or LMP2A-expressing plasmids and treated with 500 nM Mithramycin. At day 3 postinfection, the luciferase activities were measured and normalized to GFP activity. (F) The 293T cells were infected with shLuc or shSP1 lentiviruses. After 5 days, infected 293T cells were transfected with pCDH-GL3-E47– (−1000/+29), pSG5-, or LMP2A-expressing plasmids. At day 3 postinfection, the luciferase activities were measured and normalized to GFP activity. Each experiment was performed 3 times, and 1 representative is shown.
LMP2A inhibited the E47 and PU.1 promoter activity. (A) Schematic map of the promoter region of PU.1 (−470 to +57). Deletion constructs derived from the PU.1 construct (−470 to +57) were subcloned into the pCDH-GL3 luciferase reporter vector. BJAB cells were infected with pSIN- and LMP2A-expressing lentiviruses. After 3 days, the cells were infected with the reporter lentiviruses indicated. After 4 days, luciferase activities from each transfectant were normalized with the GFP intensities. (B) BJAB cells were transduced with pLKO or PU.1 lentiviruses. After 5 days, infected BJAB cells were transduced with PU.1- (−470 to +57), pSIN-, or LMP2A-expressing lentiviruses and then the luciferase activities were measured and normalized to GFP activity at day 5 postinfection. (C) BJAB cells were transduced with shLuc or shPAX5 lentiviruses. After 5 days, infected BJAB cells were further transduced with PU.1- (−470 to +57), pSIN-, or LMP2A-expressing lentiviruses. At day 5 postinfection, the luciferase activities were measured and normalized to GFP activity. (D) Schematic map of the promoter region of E47 (−1000 to +29). Deletion constructs derived from the E47 construct (−1000 to +29) were subcloned into the pCDH-GL3 luciferase reporter vector. The 293T cells were transfected with pSG5- and LMP2A-expressing plasmids. After 3 days, the luciferase activities were measured and normalized to GFP activity. (E) The 293T cells were transfected with pCDH-GL3-E47– (−1000 to +29), pSG5-, or LMP2A-expressing plasmids and treated with 500 nM Mithramycin. At day 3 postinfection, the luciferase activities were measured and normalized to GFP activity. (F) The 293T cells were infected with shLuc or shSP1 lentiviruses. After 5 days, infected 293T cells were transfected with pCDH-GL3-E47– (−1000/+29), pSG5-, or LMP2A-expressing plasmids. At day 3 postinfection, the luciferase activities were measured and normalized to GFP activity. Each experiment was performed 3 times, and 1 representative is shown.
LMP2A mediation of MHC class II suppression via E47 and PU.1 was verified in LCLs using an shRNA approach
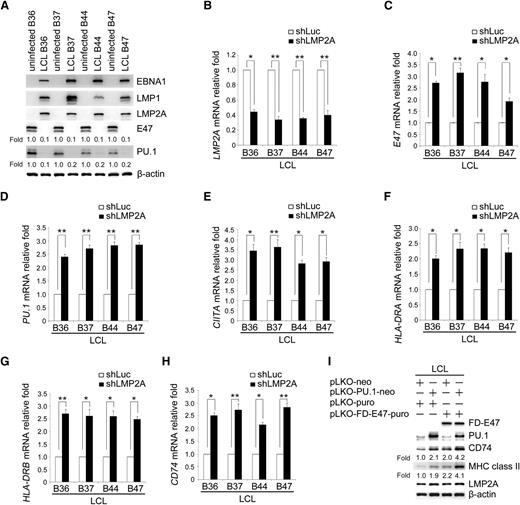
We then investigated whether downregulation of E47 and PU.1 in EBV-infected primary B cells is a general phenomenon. Expression of the E47 and PU.1 proteins was detected in 4 pairs of uninfected B cells and LCLs. EBV infection strongly suppressed expression of the E47 and PU.1 proteins (Figure 6A). To validate the importance of LMP2A-mediated reduction of the various molecules, endogenous LMP2A was knocked down in LCLs (Figure 6B). As shown in Figure 6C-H, the expression of E47, PU.1, CIITA, HLA-DRA, HLA-DRB, and CD74 transcripts was restored in LMP2A knocked-down LCLs. In addition, compared with uninfected B cells, knockdown of LMP2A restored approximately 60% of E47 and PU.1 mRNA levels (data not shown). Furthermore, ectopic coexpression of E47 and PU.1 restored the expression of MHC class II and CD74 in LCLs (Figure 6I). Collectively, LMP2A potentially is the primary EBV-encoded product that contributes to the downregulation of MHC II molecules, which may be critical for EBV persistence in healthy carriers.
LMP2A is the key factor of EBV inhibiting the expression of E47 and PU.1 and their downstream genes. (A) Expression of EBNA1, LMP1, LMP2A, E47, PU.1, and β-actin in various pairs of uninfected primary B cells and EBV-immortalized LCLs was detected by western blotting. β-actin served as an internal control. (B-H) The LCL lines B36, B37, B44, and B47 were infected with shLuc or shLMP2A lentiviruses. After 5 days, the RNA and protein were harvested. Expression levels of the LMP2A (B), E47 (C), PU.1 (D), CIITA (E), HLA-DRA (F), HLA-DRB (G), and CD74 (H) transcripts were measured by RT-Q-PCR. (I) LCL B47 cells were infected with PU.1 or FD-E47 expression lentiviruses. Expression of E47, PU.1, CD74, MHC class II, and LMP2A was analyzed by western blotting. β-actin was detected as an internal control. (Paired t test, *P < .05; **P < .01). Each experiment was performed 3 times, and 1 representative is shown.
LMP2A is the key factor of EBV inhibiting the expression of E47 and PU.1 and their downstream genes. (A) Expression of EBNA1, LMP1, LMP2A, E47, PU.1, and β-actin in various pairs of uninfected primary B cells and EBV-immortalized LCLs was detected by western blotting. β-actin served as an internal control. (B-H) The LCL lines B36, B37, B44, and B47 were infected with shLuc or shLMP2A lentiviruses. After 5 days, the RNA and protein were harvested. Expression levels of the LMP2A (B), E47 (C), PU.1 (D), CIITA (E), HLA-DRA (F), HLA-DRB (G), and CD74 (H) transcripts were measured by RT-Q-PCR. (I) LCL B47 cells were infected with PU.1 or FD-E47 expression lentiviruses. Expression of E47, PU.1, CD74, MHC class II, and LMP2A was analyzed by western blotting. β-actin was detected as an internal control. (Paired t test, *P < .05; **P < .01). Each experiment was performed 3 times, and 1 representative is shown.
HLA-DR is expressed in tonsil biopsies but not in PTLD or EBV-positive HD biopsies
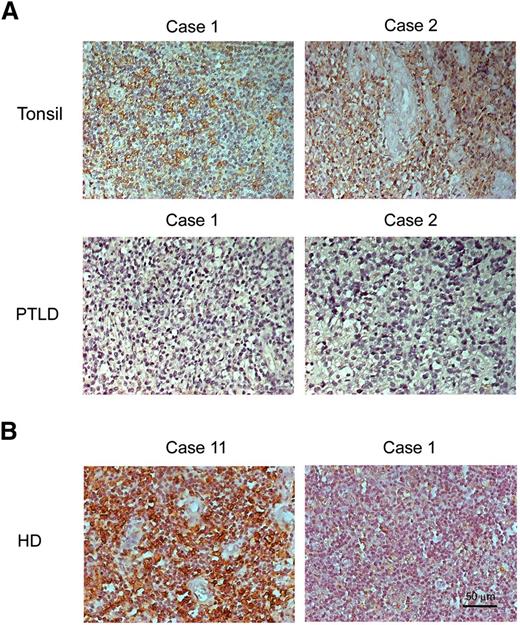
The expression of HLA-DR was detected by IHC assay in 14 PTLD and 4 tonsil biopsies (supplemental Table 2-1). All PTLD samples were EBER-positive and HLA-DR–negative (supplemental Table 2-2). The IHC assay showed that HLA-DR is expressed in 4 EBER-negative tonsil biopsies and HLA-DR is predominantly expressed in the cytoplasm and at the cell membrane (Figure 7A). In addition, we investigated 16 cases of HD. According to our results, 10 cases of EBER-positive HD were all negative for HLA-DR expression. However, 4 of 6 cases of EBER-negative HD were positive for HLA-DR expression (Figure 7B; supplemental Tables 2 and 3). Based on these results, it seems that EBV-positive PTLD and HD specimens are similar in terms of suppression of HLA-DR expression in vivo.
HLA-DR is not expressed in PTLD biopsies compared with tonsil biopsies. PTLD, tonsils, and HD sections were used for IHC assays and the nuclei counterstained with hematoxylin. (A) Positive signals of HLA-DR were observed as a brown color in tonsil biopsies but not in PTLD biopsies. (B) Positive signals of HLA-DR were observed as a brown color in EBER− biopsy (case 11) but not in EBER+ biopsy (case 1). The nuclei are stained blue. Original magnification, ×200. Scale bar indicates 50 μm.
HLA-DR is not expressed in PTLD biopsies compared with tonsil biopsies. PTLD, tonsils, and HD sections were used for IHC assays and the nuclei counterstained with hematoxylin. (A) Positive signals of HLA-DR were observed as a brown color in tonsil biopsies but not in PTLD biopsies. (B) Positive signals of HLA-DR were observed as a brown color in EBER− biopsy (case 11) but not in EBER+ biopsy (case 1). The nuclei are stained blue. Original magnification, ×200. Scale bar indicates 50 μm.
Discussion
Recognition of antigens by CD4 T cells is through complexes of MHC class II and viral peptides, which are endocytosed and digested by lysosomes.39 However, EBV is a pathogen that may persist for decades. In general, EBV is maintained in a latent form in healthy individuals and escapes immune surveillance by expressing a restricted set of viral proteins. As the only protein expressed in latency I, EBNA1 is essential for maintaining the viral genome in an episomal form. Although it may evade antigen presentation,5 EBNA1 can be recognized as an endogenous antigen that is digested by autophagy for presentation by MHC class II molecules,40 so that CD4 T cells are generated against EBNA1 in infected healthy individuals. In healthy carriers, the CD4 T cells consistently respond to EBV latent antigens, including EBNA1 and EBNA2. In the latent stage of EBV infection, EBV-specific CD4 T cells play a protective role, preventing reactivation of EBV. However, the numbers of cytotoxic T lymphocytes against EBNA1 are very low in individuals with EBV-associated malignancy.41,42 To avoid targeting by EBV-specific CD4 T cells, EBV has evolved several strategies to hide itself in infected cells, including in B cells. In this study, we found that the EBV latent protein, LMP2A, plays a critical role in downregulating the expression of MHC class II molecules in infected B cells.
Viruses evade antiviral CD4 T cells responses via interference with MHC class II molecule presentation. For example, human cytomegalovirus, human parainfluenza virus type 3, and varicella zoster virus suppress IFN-γ–induced MHC class II expression.43-45 Mechanistically, downregulation of IFN-γ–induced MHC class II expression is through inhibition of activation of the Janus kinase–signal transducer and activator of transcription pathway, which results in reduction of CIITA expression.30 In EBV infection, most studies of strategies of viral escape from the immune system have focused on lytic products. For example, BZLF1 downregulates expression of MHC class I and II by binding directly to the ZRE element on the CIITA promoters.31 In addition, BGLF5 protein induces the global degradation of mRNA, resulting in reduction of expression of MHC class I and II molecules.8
It is well-documented that BCR-triggered signaling facilitates the formation of complexes of MHC class II and viral peptides on the cell surface.46 Functionally, LMP2A mimics constitutively activated BCR signaling; however, the LMP2A-activated PI3K pathway mediates the suppression of MHC class II and CD74 in EBV-infected B cells. Previous studies have revealed that CIITA is a master regulator of the expression of MHC class II molecules and CD74.10 Here, we showed that knockdown of LMP2A in LCLs rescues the expression of CIITA, MHC class II, and CD74 (Figure 6E-H).
Expression of CIITA transcripts is controlled by 3 defined promoters, depending on the cell type (promoters I and III) and IFN-γ–inducible expression (promoter IV) of CIITA. In B cells, expression of CIITA is regulated by promoter III, producing type III transcripts that encode a 124-kDa CIITA protein.47 The transcription factors PU.1, E47, and IRF4 have been reported to bind to promoter III of CIITA.33 In this study, we demonstrated that LMP2A-mediated the reduction of CIITA levels by downregulation of PU.1 and E47 expression. In contrast, in Kaposi sarcoma-associated herpesvirus infection, LANA protein-mediated inhibition of MHC class II presentation is through blocking the DNA binding activity of IRF4 on the CIITA promoter.48
E47, the bHLH transcription factor, has been shown to regulate many of the processes involved in the development of B lymphocytes.49 The transcription factor PU.1 is required for the differentiation of both lymphoid and myeloid cells.50,51 Knockout of the PU.1 gene in B cells impairs cell differentiation and causes pre–B-cell acute lymphoblastic leukemia.52 Of interest, the expression of E47 and PU.1 is reduced in Hodgkin lymphoma–derived cell lines and EBV-positive Reed-Sternberg cells.53,54 In LMP2A transgenic mice, both the E47 and PU.1 genes are specifically downregulated in the B cells.55 In this study, we demonstrated that LMP2A reduces the expression of E47 and PU.1 in EBV-infected B cells.
Regulation of PU.1 and E47 levels is commonly controlled by epigenetic modification, such as promoter activity and phosphorylation.56-60 For example, PU.1 is downregulated in classical Hodgkin lymphoma cells through methylation of the PU.1 promoter.61 Phosphorylation of E47 through the mitogen-activated protein kinase pathway induces degradation of the E47 protein.58 In this study, we found that LMP2A inhibits the E47 and PU.1 promoter activities through its ITAM motif and the associated kinases, Syk and Src. Thus, we speculate that the ITAM motif is critical for LMP2A-mediated repression of cellular genes.
SP1 is a constitutively expressed transcription factor and can be a transactivator or repressor of the promoter activities of viral and cellular genes, depending on its mode of interaction.62 In our previous study, we found that SP1 is repressed by the Zta promoter when SP1 is associated with histone deacetylase 2. However, SP1 is phosphorylated by protein kinase C-δ and releases histone deacetylase 2 in the presence of a histone deacetylase inhibitor, which is an EBV reactivation factor.63 In this study, SP1 acted as a repressor of the promoter activity of E47. So far, phosphorylation of SP1 by the PI3K/Akt, protein kinase Cδ, and mitogen-activated protein kinase pathways may affect the associated protein partners in regulating promoter activity.
In summary, LMP2A-mediated reduction of E47 and PU.1 may downregulate the expression of MHC class II and CD74 in B lymphocytes. These data provide novel insights into the roles of EBV and LMP2A in EBV-associated malignancies, in particular, PTLD and HD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Tim J. Harrison of University College London Medical School (London, United Kingdom) for reviewing the manuscript critically. The authors also thank the Taipei Blood Center of Taiwan Blood Service Foundation for providing whole blood.
This work was supported by the Ministry of Science and Technology (MOST 103-2320-B-002-038-MY3), the National Health Research Institute (NHRI-EX102-10031BI and NHRI-EX103-10306BI), Excellent Translational Medicine Research Projects of National Taiwan University College of Medicine and National Taiwan University Hospital (103C-101-A1) (C.-H.T.), and by the Ministry of Science and Technology and Chang Gung Memorial Hospital (MOST-103-2320-B-182-028-MY3 and CMRPD1D0011) (S.-J.L.).
Authorship
Contribution: J.-H.L. designed experiments, performed experiments, analyzed the data, and cowrote the manuscript; J.-Y.L. designed experiments and performed experiments; Y.-C.C. performed experiments and analyzed the data; M.-R.C. provided materials; T.-H.Y. provided materials; C.-W.L. provided materials; S.-J.L. designed experiments and cowrote the manuscript; and C.-H.T. designed experiments and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sue-Jane Lin, No. 259, Wen-Hwa 1st Rd, Research Center for Emerging Viral Infections, College of Medicine, Chang Gung University, Kwei-Shan, Tao-Yuan 333, Taiwan ; e-mail:suejane.lin@mail.cgu.edu.tw; and Ching-Hwa Tsai, Room 719, No. 1, 1st Section, Jen-Ai Rd, Graduate Institute of Microbiology, College of Medicine, National Taiwan University, Taipei 10051, Taiwan; e-mail: chtsai@ntu.edu.tw.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal