Key Points
Mogamulizumab was well-tolerated in 41 patients with previously treated mycosis fungoides or Sézary syndrome.
Durable responses observed with a global overall response rate of 36.8%; patients with Sézary syndrome had a response rate of 47.1%.
Abstract
This phase 1/2 study evaluated the efficacy of mogamulizumab, a defucosylated, humanized, anti-CC chemokine receptor 4 monoclonal antibody, in 41 pretreated patients with cutaneous T-cell lymphoma. No dose-limiting toxicity was observed and the maximum tolerated dose was not reached in phase 1 after IV infusion of mogamulizumab (0.1, 0.3, and 1.0 mg/kg) once weekly for 4 weeks followed by a 2-week observation. In phase 2, patients were dosed with 1.0 mg/kg mogamulizumab according to the same schedule for the first course followed by infusion every 2 weeks during subsequent courses until disease progression. The most frequent treatment-emergent adverse events were nausea (31.0%), chills (23.8%), headache (21.4%), and infusion-related reaction (21.4%); the majority of events were grade 1/2. There were no significant hematologic effects. Among 38 evaluable patients, the overall response rate was 36.8%: 47.1% in Sézary syndrome (n = 17) and 28.6% in mycosis fungoides (n = 21). Eighteen of 19 (94.7%) patients with ≥B1 blood involvement had a response in blood, including 11 complete responses. Given the safety and efficacy of mogamulizumab, phase 3 investigation of mogamulizumab is warranted in cutaneous T-cell lymphoma patients. This trial was registered at www.clinicaltrials.gov as #NCT00888927.
Introduction
Cutaneous T-cell lymphomas (CTCL), a diverse group of non-Hodgkin lymphomas characterized by primary cutaneous infiltration of malignant T cells, include a growing number of subtypes characterized by clonal expansion of T cells that produce clinically heterogeneous skin lesions.1 Mycosis fungoides (MF), the most common type of CTCL, arises from accumulation of aberrant effector memory CD4+ T cells in skin lesions. Sézary syndrome (SS), the erythrodermic and leukemic form of CTCL, may arise de novo as an expansion of central memory T cells.2 Although very early-stage MF patients have an indolent course, those with ≥stage IIB and SS patients have a compromised survival.3-6
The pathogenesis of CTCLs is not fully understood, but an alteration in the skin-homing and/or skin-resident T cells, lack of normal cellular differentiation, and apoptosis of T cells are common. Other than allogeneic hematopoietic stem cell transplantation,7 no treatment has been shown to be curative and advanced disease can become refractory, leading to serious clinical complications. Thus, newer therapies for CTCL are needed, especially targeted therapies focused on malignant T cells.
CC chemokine receptor 4 (CCR4), the receptor for macrophage-derived chemokine and thymus- and activation-regulated chemokine (TARC), is present on T cells expressing the T-helper type 2 phenotype,8 as well as on certain functional regulatory T cells, particularly on CD4+CD25+ FoxP3+ cells.9,10 Interaction between CCR4 and TARC was first suggested in patients with MF.11 CCR4-expressing neoplastic T cells have been demonstrated in approximately 40% of patients with CTCL12 and peripheral T-cell lymphoma (PTCL)10,13 by immunohistochemistry or multicolor flow cytometry (MFC), and the interplay between CCR4 and its ligands may be involved in malignant T-cell trafficking and distant organ involvement. In certain T-cell neoplasms (eg, adult T-cell leukemia/lymphoma), the extent of expression of CCR4 by malignant T cells is related to the degree of skin involvement.14 CCR4 therefore represents a potentially attractive target for the treatment of CTCL and other T-cell neoplasms.9,10,14-17
Mogamulizumab (KW-0761) is a defucosylated, humanized anti-CCR4 monoclonal antibody.16 Removal of fucose results in the antibody eliciting more potent antibody-dependent cellular cytotoxicity than conventionally produced antibodies.18,19 Mogamulizumab binds with high affinity to the N-terminal domain of CCR4, but is not internalized and does not exhibit complement-dependent cytotoxic activity or directly induce apoptosis. Early in vivo and clinical experiences suggest encouraging response rates with limited short- or long-term disruption of homeostasis of the immune system or development of autoimmunity.1,18 Because of the capacity of mogamulizumab to mediate tumor cell killing via antibody-dependent cellular cytotoxicity, the tolerability and preliminary activity of mogamulizumab were determined in this phase 1/2 study.
Patients and methods
Study design
This was an open-label, multicenter (5 US centers), two-part study. Phase 1 employed a standard “3 plus 3” dose-escalation scheme to assess safety, pharmacokinetics, maximum tolerated dose (MTD), and dose-limiting toxicity (DLT). At the maximum dose level tested, a Simon 2-stage design was employed to test that the response rate was significantly greater than 10% at the 0.05 significance level with 90% power assuming a true rate of 30%. The primary objective of the phase 2 component was to determine the overall response rate (ORR) with response duration and time to progression as the secondary objectives. The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and relevant federal regulations after approval by each institutional review board. All patients gave written informed consent to participate.
The study protocol permitted inclusion of patients with CTCL or PTCL. However, only 1 patient with PTCL was recruited (during phase 2). This patient was excluded from efficacy analyses to maintain a homogeneous study population of patients with CTCL but was included in safety analyses. Reasons for poor recruitment of PTCL patients were that the study centers were already recruiting PTCL patients in a clinical trial for another drug and/or did not see PTCL patients in their practice.
Patients
Eligible patients were ≥18 years old with histologically/cytologically confirmed CTCL (limited to MF or SS), who had failed ≥1 prior systemic therapy (completed at least 4 weeks before study entry), and had an Eastern Cooperative Oncology Group performance status of ≤2 with adequate hematological, hepatic, and renal function. Patients were not eligible if pregnant or lactating; had a significant intercurrent illness including, but not limited, to uncontrolled infection requiring antibiotics; class III or IV cardiac disease and other specified cardiac conditions; or uncontrolled diabetes. Patients were also not included with known autoimmune disease or positive viral tests (ie, for HIV, human T-cell leukemia virus-1, hepatitis B, or hepatitis C); evidence of central nervous system metastasis; had received monoclonal antibodies ≤6 weeks of study entry; had prior allergic reaction to monoclonal antibodies or other therapeutic proteins; or who required immunomodulatory drugs (other than low-dose corticosteroids). Patients were allowed blood products, hematopoietic growth factors, and prophylactic treatment of the infectious complications of T-cell lymphoma; low- or medium-strength topical steroids; and to continue chronic steroid therapy (≤10 mg/day prednisone equivalent; 2 mg/day dexamethasone).
Treatment
Mogamulizumab was administered weekly as a 1-hour IV infusion for 4 weeks followed by a 2-week observation period for the first treatment course. Patients received the mogamulizumab starting dose (0.1 mg/kg) and succeeding doses (0.3 and 1.0 mg/kg) based on a standard “3 plus 3” dose-escalation scheme. In the phase 2 portion, patients received the MTD or the maximum dose level judged to be tolerable from the dose-escalation phase. After the first course of treatment, patients with partial response or stable disease continued subsequent treatment doses every 2 weeks until the development of progressive disease (PD) or withdrawal. After a patient achieved a complete response (CR), up to 2 additional doses were permitted.
DLT was defined as ≥grade 3 hematologic or nonhematologic toxicity and ≥grade 3 allergic or acute infusion reactions. Patients with grade 2 hepatic enzyme increases resulting from hepatic involvement had to have an increase to grade 3 that persisted for ≥7 days to represent a DLT. Lymphopenia, a pharmacologic effect of the antibody treatment, was not considered a DLT. For neutropenia and thrombocytopenia, only grade 3 febrile neutropenia, grade 4 nonfebrile neutropenia lasting >7 days, grade 4 thrombocytopenia, or grade 3 thrombocytopenia with bleeding were considered DLTs.
Safety
Safety evaluations included physical examinations, 12-lead electrocardiograms, weight, vital signs, monitoring of adverse events (AEs) from initial study drug infusion until 30 days postdose, and repeated blood chemistry and hematologic measurements (on day 1, then every 7 days for the first treatment course, and then every 2 weeks). Toxicities were reported according to the Common Terminology Criteria for Adverse Events, version 3.0. All dosed patients were included in the safety analysis.
Disease assessment and response criteria
Patients who received at least 4 mogamulizumab doses and had baseline plus at least 1 on-study response assessment comprised the efficacy population. Disease assessment was performed by the investigators on day 29 of the first treatment course and every 8 weeks thereafter to provide confirmation of response. The method of assessment was dependent on organ involvement with the disease. The modified Severity Weighted Assessment Tool was used for quantitative assessment of disease burden in the skin of all patients.20,21 Positron emission tomography/computed tomography scans were used to determine activity of mogamulizumab treatment in lymph nodes and viscera of all patients. Finally, MFC was used to monitor the number of circulating CTCL/Sézary cells in peripheral blood based on aberrancies in expression levels of CD3, CD4, CD7, and/or CD26.22 The overall global response score was for all components (skin, lymph nodes, and viscera) as well as response in blood (eg, patients with ≥B1 blood involvement).20 A global score of stable disease was defined as the failure to attain CR or partial response and no evidence of PD in any component.20
Pharmacokinetic analyses
Blood samples were drawn before and after infusion and at the specified time points postinfusion of the first and fourth weekly dose in the first cycle during phase 1. Plasma concentrations for mogamulizumab were measured using a validated enzyme-linked immunosorbent assay with a lower limit of quantitation of 10 ng/mL. Assay accuracy was up to –6.3% and precision was up to 10.7%. Pharmacokinetic parameters including area under the plasma concentration-time curve from time 0 to 7 days (AUC0-7days), maximum (Cmax) and trough (Ctrough) plasma concentration, and elimination half-life (t1/2) were calculated using noncompartmental methods with WinNonlin (version 6.3, Pharsight Corporation, CA).
CCR4 expression
Exploratory assessments of CCR4 expression at baseline were performed on blood samples by MFC using a FACSCanto II cytometer (BD Biosciences, San Jose, CA). Circulating CTCL cells were identified using aberrancies in expression levels of CD3, CD4, CD7, and/or CD26. CD194 (CCR4) expression was assessed using fluorescein isothiocyanate-conjugated KM2160 for pretreatment peripheral blood specimens; clone 1G1-biotin (BD Biosciences) and streptavidin-phycoerythrin (BD Biosciences) were used for pretreatment and follow-up specimens.
Statistical analyses
Safety and efficacy were analyzed in a descriptive manner. Efficacy end points included best response, time to progression, progression-free survival, and duration of response. The Kaplan-Meier method was used to determine survival plots. The 95% Clopper-Pearson exact confidence intervals (CI) were calculated for ORR. All statistical analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC).
Role of the funding source
The data were collected by the investigators and analyzed by the sponsor (Dr. Rocco Ballerini of Kyowa Hakko Kirin Pharma, Inc., and Dr. Li Liu of ReSearch Pharmaceutical Service, Inc., performed the statistical analyses). All authors had access to the final data, participated in data analysis and interpretation, and vouch for the completeness and accuracy of the data. The first draft was written by K.M.D., X.Z., and M.R.K. of Kyowa Hakko Kirin Pharma, Inc., with input from all coauthors and assistance from medical writers funded by the sponsor. All of the authors participated in revising subsequent drafts of the manuscript and made the final decision to submit it for publication.
Results
Patients
Forty-one patients with histologically confirmed CTCL were enrolled. Their demographic and baseline characteristics are shown in Table 1. The primary diagnosis was MF (n = 22) or SS (n = 19). Twenty-six (63.4%) patients had stage IV disease. Median patient age was 66 years (range, 35-85). The median number of prior systemic treatments for lymphoma was 3 (range, 1-17). The majority of patients (59.5%) had also received prior radiation therapy, which included psoralen plus ultraviolet A (n = 13), electron beam (n = 13), ultraviolet B (n = 8), and external beam (n = 3) therapy.
Demographic and baseline clinical characteristics of CTCL patients
| . | Mogamulizumab dose . | . | ||
|---|---|---|---|---|
| . | 0.1 mg/kg (n = 3) . | 0.3 mg/kg (n = 3) . | 1.0 mg/kg (n = 35)* . | Total (n = 41) . |
| Age, y, median (min, max) | 68 (62, 68) | 74 (64, 79) | 66 (35, 85) | 66 (35, 85) |
| Gender | ||||
| Male | 3 (100) | 1 (33.3) | 20 (57.1) | 24 (58.5) |
| Female | 0 (0) | 2 (67.7) | 15 (42.9) | 17 (41.5) |
| Race | ||||
| White/Caucasian | 1 (33.3) | 3 (100) | 32 (91.4) | 36 (87.8) |
| Black/African American | 1 (33.3) | 0 (0) | 2 (5.7) | 3 (7.3) |
| Other | 1 (33.3) | 0 (0) | 0 (0) | 1 (2.4) |
| Not reported | 0 | 0 (0) | 1 (2.9) | 1 (2.4) |
| Primary diagnosis | ||||
| Mycosis fungoides | 2 (67.7) | 2 (67.7) | 18 (51.4) | 22 (53.7) |
| Sézary syndrome | 1 (33.3) | 1 (33.3) | 17 (48.6) | 19 (46.3) |
| Clinical stage | ||||
| IB | 0 (0) | 0 (0) | 3 (8.6) | 3 (7.3) |
| II | 0 (0) | 0 (0) | 1 (2.9) | 1 (2.4) |
| IIB | 0 (0) | 1 (33.3) | 8 (22.9) | 9 (22.0) |
| III | 0 (0) | 0 (0) | 1 (2.9) | 1 (2.4) |
| IIIA | 0 (0) | 0 (0) | 1 (2.9) | 1 (2.4) |
| IVA | 3 (33.3) | 2 (66.7) | 17 (48.6) | 22 (53.7) |
| IVB | 0 (0) | 0 (0) | 4 (11.4) | 4 (9.8) |
| ECOG performance status | ||||
| 0 | 2 (67.7) | 3 (100) | 26 (74.3) | 31 (75.6) |
| 1 | 0 (0) | 0 (0) | 9 (25.7) | 9 (21.9) |
| 2 | 1 (33.3) | 0 (0) | 0 (0) | 1 (2.4) |
| No. of prior systemic therapies, median (min, max) | 3 (1, 17) | |||
| Previous systemic therapies† | ||||
| Retinoids | 28 (68.3) | |||
| Interferon | 19 (46.3) | |||
| Chemotherapy | 24 (58.5) | |||
| Denileukin diftitox | 12 (29.3) | |||
| HDAC inhibitor | 13 (31.7) | |||
| Extracorporeal photopheresis | 19 (46.3) | |||
| Systemic steroids | 5 (12.2) | |||
| Investigational therapies | 18 (43.9) | |||
| Other (Revlimid, alemtuzumab, Velcade, etc.) | 8 (19.5) | |||
| . | Mogamulizumab dose . | . | ||
|---|---|---|---|---|
| . | 0.1 mg/kg (n = 3) . | 0.3 mg/kg (n = 3) . | 1.0 mg/kg (n = 35)* . | Total (n = 41) . |
| Age, y, median (min, max) | 68 (62, 68) | 74 (64, 79) | 66 (35, 85) | 66 (35, 85) |
| Gender | ||||
| Male | 3 (100) | 1 (33.3) | 20 (57.1) | 24 (58.5) |
| Female | 0 (0) | 2 (67.7) | 15 (42.9) | 17 (41.5) |
| Race | ||||
| White/Caucasian | 1 (33.3) | 3 (100) | 32 (91.4) | 36 (87.8) |
| Black/African American | 1 (33.3) | 0 (0) | 2 (5.7) | 3 (7.3) |
| Other | 1 (33.3) | 0 (0) | 0 (0) | 1 (2.4) |
| Not reported | 0 | 0 (0) | 1 (2.9) | 1 (2.4) |
| Primary diagnosis | ||||
| Mycosis fungoides | 2 (67.7) | 2 (67.7) | 18 (51.4) | 22 (53.7) |
| Sézary syndrome | 1 (33.3) | 1 (33.3) | 17 (48.6) | 19 (46.3) |
| Clinical stage | ||||
| IB | 0 (0) | 0 (0) | 3 (8.6) | 3 (7.3) |
| II | 0 (0) | 0 (0) | 1 (2.9) | 1 (2.4) |
| IIB | 0 (0) | 1 (33.3) | 8 (22.9) | 9 (22.0) |
| III | 0 (0) | 0 (0) | 1 (2.9) | 1 (2.4) |
| IIIA | 0 (0) | 0 (0) | 1 (2.9) | 1 (2.4) |
| IVA | 3 (33.3) | 2 (66.7) | 17 (48.6) | 22 (53.7) |
| IVB | 0 (0) | 0 (0) | 4 (11.4) | 4 (9.8) |
| ECOG performance status | ||||
| 0 | 2 (67.7) | 3 (100) | 26 (74.3) | 31 (75.6) |
| 1 | 0 (0) | 0 (0) | 9 (25.7) | 9 (21.9) |
| 2 | 1 (33.3) | 0 (0) | 0 (0) | 1 (2.4) |
| No. of prior systemic therapies, median (min, max) | 3 (1, 17) | |||
| Previous systemic therapies† | ||||
| Retinoids | 28 (68.3) | |||
| Interferon | 19 (46.3) | |||
| Chemotherapy | 24 (58.5) | |||
| Denileukin diftitox | 12 (29.3) | |||
| HDAC inhibitor | 13 (31.7) | |||
| Extracorporeal photopheresis | 19 (46.3) | |||
| Systemic steroids | 5 (12.2) | |||
| Investigational therapies | 18 (43.9) | |||
| Other (Revlimid, alemtuzumab, Velcade, etc.) | 8 (19.5) | |||
Values are n (%); summation of percentages does not always equal 100% because of rounding.
ECOG, Eastern Cooperative Oncology Group; HDAC, histone deacetylase; max, maximum; min, minimum.
Includes patients from phase 1 (n = 3) and phase 2 (n = 32).
If a subject received the same category of therapy multiple times (eg, if subject received methotrexate; cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisone; and gemcitabine, 3 different chemotherapy regimens), the subject was only counted as having prior chemotherapy once (counts are by subject, not by treatment).
The mean duration of treatment was 23.3 weeks (range, 2-156). Two (4.8%) patients discontinued treatment (both receiving 1.0 mg/kg) before completing the first course: 1 withdrew consent and 1 died (not related to study drug).
DLT and safety
Forty-two patients received at least 1 dose of mogamulizumab and were included in the safety population. In phase 1, 9 patients with CTCL (3 per cohort) were dosed with mogamulizumab. No DLTs were observed in any cohort during phase 1 for the first treatment course, so the MTD was not reached. In phase 2, 33 patients (CTCL, n = 32; PTCL, n = 1) were enrolled and were treated at the maximum dose level judged to be tolerable in phase 1 (1.0 mg/kg mogamulizumab).
The most frequently reported nonhematological AEs (>10%) regardless of relationship to treatment are reported in Table 2. The majority of AEs were grade 1/2; there were no grade 4 AEs. The most frequent AEs were nausea (31.0%), chills (23.8%), infusion-related reaction (21.4%), headache (21.4%), pyrexia (19.0%), fatigue (16.7%), and cutaneous drug eruption (16.7%). Thirteen patients (30.9%) had at least 1 skin infection, only 1 of which was considered related to treatment. All of the infections seen were those frequently encountered in patients with CTCL.23,24 No patient experienced an AE consistent with an autoimmune phenomenon.
Nonhematologic adverse events regardless of relationship to treatment reported by >10% of patients in the safety population (N = 42)
| . | Patients, n (%) . | |||
|---|---|---|---|---|
| Preferred term* . | Grade 1-2 . | Grade 3 . | Grade 4-5 . | Total . |
| Nausea | 11 (26.2) | 2 (4.8) | 0 (0) | 13 (31.0) |
| Chills | 10 (23.8) | 0 (0) | 0 (0) | 10 (23.8) |
| Infusion-related reaction | 9 (21.4) | 0 (0) | 0 (0) | 9 (21.4) |
| Headache | 9 (21.4) | 0 (0) | 0 (0) | 9 (21.4) |
| Pyrexia | 8 (19.0) | 0 (0) | 0 (0) | 8 (19.0) |
| Fatigue | 7 (16.7) | 0 (0) | 0 (0) | 7 (16.7) |
| Cutaneous drug eruption | 6 (14.3) | 1 (2.4) | 0 (0) | 7 (16.7) |
| Diarrhea | 5 (11.9) | 1 (2.4) | 0 (0) | 6 (14.3) |
| Pruritus | 5 (11.9) | 0 (0) | 0 (0) | 5 (11.9) |
| Upper respiratory tract infection | 5 (11.9) | 0 (0) | 0 (0) | 5 (11.9) |
| Vomiting | 3 (7.1) | 2 (4.8) | 0 (0) | 5 (11.9) |
| . | Patients, n (%) . | |||
|---|---|---|---|---|
| Preferred term* . | Grade 1-2 . | Grade 3 . | Grade 4-5 . | Total . |
| Nausea | 11 (26.2) | 2 (4.8) | 0 (0) | 13 (31.0) |
| Chills | 10 (23.8) | 0 (0) | 0 (0) | 10 (23.8) |
| Infusion-related reaction | 9 (21.4) | 0 (0) | 0 (0) | 9 (21.4) |
| Headache | 9 (21.4) | 0 (0) | 0 (0) | 9 (21.4) |
| Pyrexia | 8 (19.0) | 0 (0) | 0 (0) | 8 (19.0) |
| Fatigue | 7 (16.7) | 0 (0) | 0 (0) | 7 (16.7) |
| Cutaneous drug eruption | 6 (14.3) | 1 (2.4) | 0 (0) | 7 (16.7) |
| Diarrhea | 5 (11.9) | 1 (2.4) | 0 (0) | 6 (14.3) |
| Pruritus | 5 (11.9) | 0 (0) | 0 (0) | 5 (11.9) |
| Upper respiratory tract infection | 5 (11.9) | 0 (0) | 0 (0) | 5 (11.9) |
| Vomiting | 3 (7.1) | 2 (4.8) | 0 (0) | 5 (11.9) |
Using MedDRA Version 12.0.
Twenty-four serious AEs were reported by 10 patients (23.8%), including 1 patient receiving 0.3 mg/kg and 9 patients receiving 1.0 mg/kg. Two patients (4.8%) experienced a total of 4 serious AEs that were considered at least possibly or probably related to the study drug: this included 1 patient (1.0 mg/kg) with hypotension, abdominal pain, and a secondary T-cell malignancy, and 1 patient with a secondary T-cell malignancy represented by clonal rearrangement. All other serious AEs were considered not related to the study drug. There were no reported deaths related to the study drug. There was no significant treatment-related hematologic toxicity. Lymphopenia (40.5% with grade 3 and 26.2% with grade 4) was expected given the pharmacologic effect of mogamulizumab.
A total of 7 patients (16.7%) experienced rash (drug eruption), and all were withdrawn from the study. These rashes first appeared a median of 66 days (range, 22-309) from the first dose of mogamulizumab. Six patients experienced grade 2 (n = 1, 0.3 mg/kg; n = 5, 1.0 mg/kg) and 1 patient experienced grade 3 (1.0 mg/kg) rashes. Biopsies of most cases showed a perivascular lymphocytic infiltrate with eosinophils, with or without epidermal spongiosis. One patient had psoriasiform dermatitis and another had a lymphomatoid drug eruption.25,26
Clinical efficacy
Tumor response was evaluable in 38 of 41 CTCL patients in both phases of the trial. Three patients were excluded from the efficacy analysis because they had no on-study assessment for response, 2 of whom received only 3 doses. One excluded patient withdrew consent, a second discontinued because of PD, and the third discontinued because of treatment-unrelated dehydration.
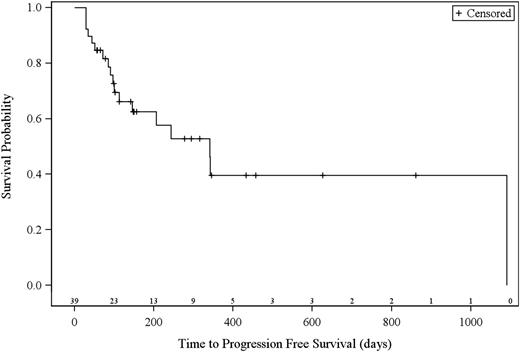
The 38 efficacy evaluable patients (21 patients with MF, 17 patients with SS) had a best global response rate of 36.8% (95% CI 21.8-54.0), with a higher rate in patients with SS (47.1%; 95% CI 23.0-72.2) compared with patients with MF (28.6%; 95% CI 11.3-52.2) (Table 3). Three patients (n = 1, 0.1 mg/kg; n = 2, 1.0 mg/kg) had a global CR. Eleven patients (n = 1, 0.1 mg/kg; n = 2, 0.3 mg/kg; n = 8, 1.0 mg/kg) had a global PR. Overall global clinical responses were observed in patients with stage IIB (n = 1), stage IIIA (n = 1), stage IVB (n = 1), and stage IVA (n = 11) disease. Median time to response (TTR) was 31.5 days (range, 26-154). Median progression-free survival of evaluable patients was 11.4 months (range, 1.1 to not estimable; 95% CI, 16.1-not estimable; Figure 1). Median duration of response was 10.4 months (interquartile range, 6.9-33.2).
Best response to mogamulizumab (0.1, 0.3, or 1.0 mg/kg) by compartment for all evaluable CTCL patients and subgroups with Sézary syndrome and mycosis fungoides
| All evaluable CTCL patients (n = 38) . | Blood (n = 19) . | Skin (n = 38) . | Lymph nodes (n = 28) . | Global response (n = 38) . |
|---|---|---|---|---|
| ORR, n (%) [95% CI] | 18 (94.7) [74.0-99.9] | 16 (42.1) [26.4-59.2] | 7 (25.0) [10.7-44.9] | 14 (36.8) [21.8-54.0] |
| CR | 11 (57.9) | 4 (10.5) | 4 (14.3) | 3 (7.9) |
| PR | 7 (36.8) | 12 (31.6) | 3 (10.7) | 11 (28.9) |
| SD | 1 (5.3) | 20 (52.6) | 17 (60.7) | 19 (50.0) |
| PD | 0 (0) | 2 (5.3) | 4 (14.3) | 5 (13.2) |
| All evaluable CTCL patients (n = 38) . | Blood (n = 19) . | Skin (n = 38) . | Lymph nodes (n = 28) . | Global response (n = 38) . |
|---|---|---|---|---|
| ORR, n (%) [95% CI] | 18 (94.7) [74.0-99.9] | 16 (42.1) [26.4-59.2] | 7 (25.0) [10.7-44.9] | 14 (36.8) [21.8-54.0] |
| CR | 11 (57.9) | 4 (10.5) | 4 (14.3) | 3 (7.9) |
| PR | 7 (36.8) | 12 (31.6) | 3 (10.7) | 11 (28.9) |
| SD | 1 (5.3) | 20 (52.6) | 17 (60.7) | 19 (50.0) |
| PD | 0 (0) | 2 (5.3) | 4 (14.3) | 5 (13.2) |
| Subgroup with Sézary syndrome (n = 17) . | Blood (n = 17) . | Skin (n = 17) . | Lymph nodes (n = 15) . | Global response (n = 17) . |
|---|---|---|---|---|
| ORR, n (%) [95% CI] | 16 (94.1) [71.3-99.9] | 9 (52.9) [27.8-77.0] | 5 (33.3) [11.8-61.6] | 8 (47.1) [23.0-72.2] |
| CR | 9 (52.9) | 2 (11.8) | 2 (13.3) | 2 (11.8) |
| PR | 7 (41.2) | 7 (41.2) | 3 (20.0) | 6 (35.3) |
| SD | 1 (5.9) | 8 (47.1) | 7 (46.7) | 7(41.2) |
| PD | 0 (0) | 0 (0) | 3 (20.0) | 2 (11.8) |
| Subgroup with Sézary syndrome (n = 17) . | Blood (n = 17) . | Skin (n = 17) . | Lymph nodes (n = 15) . | Global response (n = 17) . |
|---|---|---|---|---|
| ORR, n (%) [95% CI] | 16 (94.1) [71.3-99.9] | 9 (52.9) [27.8-77.0] | 5 (33.3) [11.8-61.6] | 8 (47.1) [23.0-72.2] |
| CR | 9 (52.9) | 2 (11.8) | 2 (13.3) | 2 (11.8) |
| PR | 7 (41.2) | 7 (41.2) | 3 (20.0) | 6 (35.3) |
| SD | 1 (5.9) | 8 (47.1) | 7 (46.7) | 7(41.2) |
| PD | 0 (0) | 0 (0) | 3 (20.0) | 2 (11.8) |
| Subgroup with mycosis fungoides (n = 21) . | Blood (n = 2) . | Skin (n = 21) . | Lymph nodes (n = 13) . | Global response (n = 21) . |
|---|---|---|---|---|
| ORR, n (%) [95% CI] | 2 (100) [15.8-100] | 7 (33.3) [14.6-57.0] | 2 (15.4) [1.9-45.4] | 6 (28.6) [11.3-52.2] |
| CR | 2 (100) | 2 (9.5) | 2 (15.4) | 1 (4.8) |
| PR | 0 (0) | 5 (23.8) | 0 (0) | 5 (23.8) |
| SD | 0 (0) | 12 (57.1) | 10 (76.9) | 12 (57.1) |
| PD | 0 (0) | 2 (9.5) | 1 (7.7) | 3 (14.3) |
| Subgroup with mycosis fungoides (n = 21) . | Blood (n = 2) . | Skin (n = 21) . | Lymph nodes (n = 13) . | Global response (n = 21) . |
|---|---|---|---|---|
| ORR, n (%) [95% CI] | 2 (100) [15.8-100] | 7 (33.3) [14.6-57.0] | 2 (15.4) [1.9-45.4] | 6 (28.6) [11.3-52.2] |
| CR | 2 (100) | 2 (9.5) | 2 (15.4) | 1 (4.8) |
| PR | 0 (0) | 5 (23.8) | 0 (0) | 5 (23.8) |
| SD | 0 (0) | 12 (57.1) | 10 (76.9) | 12 (57.1) |
| PD | 0 (0) | 2 (9.5) | 1 (7.7) | 3 (14.3) |
CTCL, cutaneous T-cell lymphoma; PR, partial response; SD, stable disease.
Kaplan-Meier curves of estimated progression-free survival. Number of patients at risk are indicated above the x-axis.
Kaplan-Meier curves of estimated progression-free survival. Number of patients at risk are indicated above the x-axis.
Response by disease compartment was assessed in a subgroup analysis. Eighteen of 19 (94.7%) patients had a response in the blood with CR in 11 patients (Table 3). Median TTR was 30 days (range, 27-95). Figure 2 shows representative MFC results for a patient with SS (stage IVA) who had failed 6 prior systemic therapies and who achieved a CR in blood that had lasted for at least 309 days at last assessment. Responses in skin were assessed in all 38 patients; 16 (42.1%) patients had responses, including 4 who had a complete clearing of skin involvement with median TTR of 32 days (range, 26-154). Twenty-eight patients had disease in the lymph nodes, with 7 (25.0%) demonstrating a response, although the median TTR of 92 days (range, 30-155) was greater than for the other disease compartments. One patient who relapsed after CR was retreated with mogamulizumab and subsequently achieved a PR.
Response in blood to mogamulizumab in Sézary syndrome. Patient had received 6 prior systemic therapies. Total CD3+ lymphocytes from peripheral blood are shown. Red, circulating cutaneous T-cell lymphoma cells; green, normal CD3+CD4+ cells; blue, normal CD3+CD4− cells.
Response in blood to mogamulizumab in Sézary syndrome. Patient had received 6 prior systemic therapies. Total CD3+ lymphocytes from peripheral blood are shown. Red, circulating cutaneous T-cell lymphoma cells; green, normal CD3+CD4+ cells; blue, normal CD3+CD4− cells.
Clinical response and CCR4 expression
The median percentage of CCR4-positive aberrant T cells detected in blood by MFC was 63.8% (range, 21.3-99.4). Of 19 patients with disease in the blood (≥B1) who were positive for CCR4 expression by flow cytometry, all but 1 had a response in blood and there were 11 CRs.
Pharmacokinetics
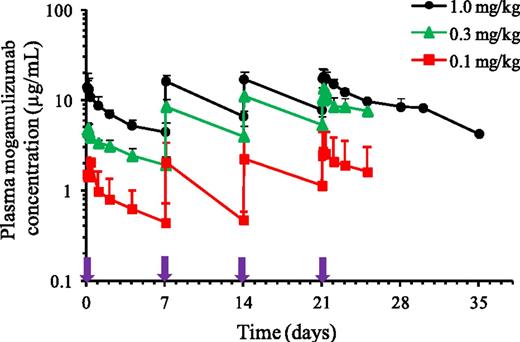
Plasma mogamulizumab concentrations (AUC0-7day) showed 1.88- to 3.05-fold accumulation following repeated once-weekly administration for 4 weeks. The pharmacokinetic exposure to mogamulizumab (Cmax and AUC0-7days) increased in a dose-proportional manner from 0.1 to 1.0 mg/kg following the first and fourth administrations. Plasma Cmax, Ctrough,, and AUC0-7days increased as dose increased from 0.1 to 1.0 mg/kg, as shown in Figure 3 and Table 4. At 1.0 mg/kg, mean (± standard deviation) Cmax, Ctrough, and AUC0-7days were 14.7 ± 4.78 µg/mL, 4.42 ± 1.18 µg/mL, and 1050 ± 221 µg/h per mL, respectively, after the first infusion, whereas the corresponding values after the fourth infusion were 18.8 ± 4.14 µg/mL, 8.33 ± 2.18 µg/mL, and 1970 ± 342 µg/h per mL. Mean (± standard deviation) values for t1/2 varied across the 3 dose levels and were 334 ± 84.3 hours (13.9 ± 3.5 days) after the fourth infusion of 1.0 mg/kg.
Mean plasma mogamulizumab concentration-time profiles for the dose cohorts in phase 1. Bar indicates upper limit of the standard deviation. Purple arrows indicate timing of weekly mogamulizumab administration.
Mean plasma mogamulizumab concentration-time profiles for the dose cohorts in phase 1. Bar indicates upper limit of the standard deviation. Purple arrows indicate timing of weekly mogamulizumab administration.
Pharmacokinetics of mogamulizumab following the first and fourth weekly doses in phase 1
| Dose . | Week . | No. of patients . | Mean ± standard deviation . | |||||
|---|---|---|---|---|---|---|---|---|
| Tmax (h) . | Cmax (ng/mL) . | Ctrough (µg/mL) . | AUC0-7days (µg/h per mL) . | t1/2 (h) . | R . | |||
| 0.1 mg/kg | 1 | 3 | 2.11 ± 2.76 | 1.52 ± 0.769 | 0.436 ± 0.288 | 122 ± 758 | 130 ± 16.4 | – |
| 4 | 3 | 3.08 ± 1.73 | 2.72 ± 2.02 | 0.116* | 301 ± 254 | 350 ± 270 | 2.17 ± 1.01 | |
| 0.3 mg/kg | 1 | 3 | 2.41 ± 1.15 | 4.90 ± 0.595 | 1.91 ± 0.45 | 468 ± 97.8 | 184 ± 51.9 | – |
| 4 | 3 | 2.51 ± 1.16 | 13.6 ± 2.76 | 7.07† | 1440† | 430† | 3.05† | |
| 1.0 mg/kg | 1 | 3 | 1.74 ± 0.583 | 14.7 ± 4.78 | 4.42 ± 1.18 | 1050 ± 221 | 184 ± 64.4 | – |
| 4 | 3 | 2.54 ± 0.50 | 18.8 ± 4.14 | 8.33 ± 2.18 | 1970 ± 342 | 334 ± 84.3 | 1.88 ± 0.075 | |
| Dose . | Week . | No. of patients . | Mean ± standard deviation . | |||||
|---|---|---|---|---|---|---|---|---|
| Tmax (h) . | Cmax (ng/mL) . | Ctrough (µg/mL) . | AUC0-7days (µg/h per mL) . | t1/2 (h) . | R . | |||
| 0.1 mg/kg | 1 | 3 | 2.11 ± 2.76 | 1.52 ± 0.769 | 0.436 ± 0.288 | 122 ± 758 | 130 ± 16.4 | – |
| 4 | 3 | 3.08 ± 1.73 | 2.72 ± 2.02 | 0.116* | 301 ± 254 | 350 ± 270 | 2.17 ± 1.01 | |
| 0.3 mg/kg | 1 | 3 | 2.41 ± 1.15 | 4.90 ± 0.595 | 1.91 ± 0.45 | 468 ± 97.8 | 184 ± 51.9 | – |
| 4 | 3 | 2.51 ± 1.16 | 13.6 ± 2.76 | 7.07† | 1440† | 430† | 3.05† | |
| 1.0 mg/kg | 1 | 3 | 1.74 ± 0.583 | 14.7 ± 4.78 | 4.42 ± 1.18 | 1050 ± 221 | 184 ± 64.4 | – |
| 4 | 3 | 2.54 ± 0.50 | 18.8 ± 4.14 | 8.33 ± 2.18 | 1970 ± 342 | 334 ± 84.3 | 1.88 ± 0.075 | |
R, accumulation ratio (fourth dose/first dose for AUC0-7days); Tmax, time to Cmax.
n = 1.
n = 2.
Discussion
The prognosis of patients with advanced and refractory CTCL is poor, and patients are often treated with single-agent and combination therapies that are associated with treatment-related toxicities as well as immunosuppression. Because of the relatively long clinical course of CTCL, controlled clinical trials have typically used response rates, rather than time-based end points, to evaluate the potential benefits of novel therapies. The histone deacetylase inhibitors27-29 and folate analogs30 have shown activity in this heavily pretreated population with advanced-stage disease. However, responses in this population are often not durable and there remains an unmet medical need to develop additional novel approaches with targeted, tumor-selective agents.31 Other monoclonal antibodies that have demonstrated clinical activity for the treatment of CTCL include brentuximab vedotin, an anti-CD30 antibody–drug conjugate, and alemtuzumab, directed against CD52.
In the current study, mogamulizumab, a humanized monoclonal antibody targeting the chemokine receptor CCR4, has demonstrated activity and an acceptable safety profile in patients with advanced and refractory CTCL. Consistent with the pharmacologic effect of lymphopenia, 75% of patients had a reduction of lymphocyte counts from pretreatment levels; however, no increased risk of clinically threatening infections was observed in a patient population predisposed to skin infections.
Encouraging clinical responses to mogamulizumab were seen across all disease stages and disease compartments in both MF and SS patients. A global ORR of 36.8% (95% CI 21.8-54.0) was observed for all evaluable patients: 47.1% (95% CI 23.0-72.2) for SS patients and 28.6% (95% CI 11.3-52.2) for MF patients. Median progression-free survival was 11.4 months and the median duration of response was 10.4 months. Mogamulizumab therapy resulted in a dramatic clearance of malignant cells as measured by flow cytometry in 18 of 19 patients with blood involvement. Response in the blood was noted during the first 4 weeks of treatment and persisted for up to 3 years in several patients.
The MTD for mogamulizumab was not reached during dose escalation to 1.0 mg/kg during phase 1 of this study. This is in agreement with a previous phase 1 study of mogamulizumab in Japanese patients with adult T-cell leukemia-lymphoma and PTCL in which 1.0 mg/kg was also the highest dose investigated.16 The maximum dose level tested was considered the recommended phase 2 dose in both studies. A phase 2 study of mogamulizumab (1.0 mg/kg) in Japanese patients with CCR4-positive PTCL and CTCL has been recently reported.32 This study enrolled a small number of CTCL patients (n = 8) with only relapsed disease. However, the ORR of 35% was similar to the rate of 36.8% among the 38 patients in our study. Treatment-related nonhematological toxicity ≥grade 3 was infrequent in both studies.
A reversible, drug-related skin eruption was reported in 7 patients (16.7%) after a median of 66 days on study. In some patients, the rash was treated successfully with topical steroids alone. Two patients with SS, both at the same study site, were reported to have secondary T-cell malignancies that were immunophenotypically distinct T-cell lymphomas (ie, gene rearrangements different from their initial diagnoses and in 1 case, with a CD8 immunophenotype when the initial disease was CD4+). It is well known, however, that in patients with CTCL, the presence of multiple T-cell clones, although not common, does occur. These different clones may be represented by differing T-cell clones found in different areas of involved skin or in different clones found among involved skin, lymph node, and blood compartments.33,34 It is not therefore possible to definitively say whether these patients developed de novo secondary T-cell malignancies or that these events represented clonal evolution of the underlying disease.
In summary, the MTD of mogamulizumab was not reached in the phase 1 part of this study so the highest tested dose of 1.0 mg/kg became the recommended phase 2 dose. This dose produced durable global clinical responses in heavily pretreated CTCL patients. Of significance, rapid reduction in peripheral blood Sézary cells was observed in 16 of 17 SS patients. In this SS population, the overall global response rate was 47.1%. Mogamulizumab was well-tolerated without drug-related increased risk of infections. Based on these findings, a phase 3 randomized trial comparing mogamulizumab vs vorinostat in patients with relapsed or refractory MF or SS with respect to efficacy, safety, and quality of life is ongoing (#NCT01728805).
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Editorial and medical writing assistance was provided by Fiona M. Herr (Aranmore Medical Communications LLC) and Peter A. Todd (Tajut Ltd). The authors acknowledge Xiao Ni, Nehal Mohammed, and George Spitalny for important intellectual content; Colleen Sullivan for administrative support; and representatives of Kyowa Hakko Kirin Pharma for assistance with study design.
This study was sponsored by Kyowa Hakko Kirin Pharma Inc. The study drug was provided free of charge by Kyowa Hakko Kirin Pharma, Inc. All participating institutions received support from Kyowa Hakko Kirin Pharma for the conduct of the study.
Authorship
Contribution: M.D. and F.M.F. designed the research, performed research, collected data, and analyzed data; L.C.P.-B. and L.S. performed research, collected data, and analyzed data; J.L.J., P.C., K.M.D., and X.Z. collected data and analyzed data; M.R.K. designed the research, collected data, and analyzed data; Y.H.K. designed the research, performed research, collected data, and analyzed data; M.D., Y.H.K., and F.M.F. designed the study; R.B. and L.L. collected and analyzed data in conjunction with the other investigators; and all authors had access to the primary data and participated in writing and editing the article.
Conflict-of-interest disclosure: M.D. received research funding from Kyowa Hakko Kirin Pharma. L.C.P. received research funding and is a consultant for Kyowa Hakko Kirin Pharma. F.M.F. received research funding from Kyowa Hakko Kirin Pharma, Celgene, and Biogen; honoraria from Celgene; and is a consultant for Celgene, Seattle Genetics, and Millennium. L.S. received research funding from Kyowa Hakko Kirin Pharma. J.L.J. received research funding from Kyowa Hakko Kirin Pharma. P.C. received research funding from Kyowa Hakko Kirin Pharma. K.M.D. and X.Z. are employed by Kyowa Hakko Kirin Pharma. M.R.K. is a consultant for Kyowa Hakko Kirin Pharma. Y.H.K. received research funding, honoraria and is a consultant for Kyowa Hakko Kirin Pharma.
Correspondence: Madeleine Duvic, Department of Dermatology, University of Texas, MD Anderson Cancer Center, 1400 Pressler, Unit 1452, Houston, TX 77030; e-mail: mduvic@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal