Case presentation
Case 1. Anne is a 23-year-old nursing student who presents with bilateral cervical, left supraclavicular, and left axillary adenopathy. Computed tomography (CT) scan of the thorax reveals a 6-cm anterior mediastinal mass. Biopsy of the left supraclavicular lymph node reveals classical nodular sclerosis Hodgkin lymphoma. A complete blood count is normal and the erythrocyte sedimentation rate (ESR) is 25 mm/h. CT scan of the abdomen/pelvis show normal liver and spleen and no evidence of abdominal lymphadenopathy. You are asked to advise her on the role of radiation therapy in her treatment plan.
Case 2. John is a 48-year-old teacher who presents with chest pain following a hockey game. A chest radiograph reveals mediastinal widening, and a contrast-enhanced CT scan shows a 7.5-cm anterior mediastinal mass, with enlarged paratracheal and right hilar lymph nodes. Image-guided core biopsy of the mediastinal mass is diagnostic for classical Hodgkin lymphoma. The hemoglobin is 130 g/L, white blood cell countand platelets are normal, and the ESR is 35 mm/h. John asks you about the role of radiotherapy in the management of his lymphoma.
Introduction
Patients with limited- or early-stage Hodgkin lymphoma (HL; Ann Arbor stage I and II) can expect an excellent outcome with current management approaches. These approaches have been evaluated in patient populations predicted to have different risks of disease recurrence based on the absence (early favorable HL) or presence (early unfavorable HL) of clinical risk factors at diagnosis. These risk factors have differed among study populations and include elevated erythrocyte sedimentation rate (ESR), B symptoms, extranodal extension, older age, multiple sites of nodal involvement, and large mediastinal mass (Table 1).1-3 Treatment approaches for favorable and unfavorable limited-stage HL differ in number of cycles of chemotherapy, treatment intensity, and radiation dose; regardless, a freedom-from-treatment-failure rate of ∼85% and an overall survival rate of 95% at 8 to 10 years can be achieved in this patient population.
Clinical risk factors used by cooperative groups to predict risk of disease recurrence in patients with early-stage HL
| EORTC/LYSA/FIL . | NCIC CTG . | GHSG . |
|---|---|---|
| Age >50 y | Age >40 y | E lesions |
| >3 Nodal areas | LD/MC | LMM |
| Bulky mediastinal >0.35 MTD | >3 Nodal areas | >2 Regions |
| ESR <50 mm/h (B Sx <30) | ESR >50 mm/h | ESR >50mm/h |
| EORTC/LYSA/FIL . | NCIC CTG . | GHSG . |
|---|---|---|
| Age >50 y | Age >40 y | E lesions |
| >3 Nodal areas | LD/MC | LMM |
| Bulky mediastinal >0.35 MTD | >3 Nodal areas | >2 Regions |
| ESR <50 mm/h (B Sx <30) | ESR >50 mm/h | ESR >50mm/h |
Presence of any of the risk factors indicates early unfavorable HL.
B Sx, B symptoms; E, extranodal extension; EORTC/LYSA/FIL, European Organization for Research and Treatment of Cancer/Lymphoma Study Association/Italian Lymphoma Foundation; ESR, erythrocyte sedimentation rate; GHSG, German Hodgkin Study Group; LD, lymphocyte depleted; LMM, large mediastinal mass; MC, mixed cellularity; MTD, maximum thoracic diameter on an anteroposterior chest radiograph; NCIC CTG, National Cancer Institute of Canada Clinical Trials Group; RCT, randomized controlled trial.
For more than 50 years, radiation therapy has been an integral component of the treatment of early-stage HL.4,5 Despite excellent results in terms of freedom from lymphoma recurrence, the young median age at diagnosis (35-38 years in North America) and the long life expectancy of HL survivors has uncovered an increased mortality from second cancers and cardiovascular disease.6,7 Most of this excess morbidity and mortality is attributable to extended-field radiation therapy (EFRT; mantle and upperabdominal or subtotal nodal irradiation). Although the majority of treatment failure is observed within the first few years after completion of combination chemotherapy and radiation, cardiac and other late effects and second cancers are observed after 10 to 30 years of follow-up. In some studies, mortality from late effects exceeds the risk of death from HL itself.8 Careful radiation dosimetry studies, randomized trials of radiation dose and extent, and observational cohort studies suggest that the risk of late effects may be reduced by the use of smaller radiation fields, such as involved-field radiotherapy (IFRT) and involved-nodal radiotherapy (INRT), and lower radiation doses.9-11 There are early indications from clinical studies that smaller radiation fields will be associated with a lower risk of breast cancer, but longer follow-up from more recent studies of combined-modality therapy (CMT) are needed.
The choice of treatment of limited-stage HL, therefore, frequently represents a trade-off between optimum disease control, most often assessed as progression-free survival (PFS), and the risk of later morbidity and mortality from the effects of treatment. The objective of this focused review is to systematically review the literature on the use of radiotherapy in the treatment of limited-stage HL. We have concentrated on data from randomized trials; because the follow-up of these studies is more limited than that from observational cohort studies, the focus will be on disease control measured by PFS, and, where possible, overall survival.
Methods
We performed a systematic review that followed a process developed by the Cancer Care Ontario Program in Evidence-Based Care, using validated methodology of the Practice Guideline Development Cycle.12 A systematic search of MEDLINE (Ovid, 2003 to June 7, 2013), EMBASE (Ovid, 2003 to 2013 Week 25), and the Cochrane Library (Central Register of Controlled Trials, Database of Systematic Reviews, and Database of Abstracts of Effects, 2003 to June 18, 2013) was completed (see supplemental Appendix 1, available on the Blood Web site). In addition, abstracts from the American Society of Hematology (2003-2013), the American Society of Clinical Oncology (2003-2013), the International Conference on Malignant Lymphoma and the Cologne Hodgkin Lymphoma Meeting (2003-2012), and reference lists of included articles were also searched. Ongoing trials were obtained from the www.clinicaltrials.gov database.
Study selection criteria
English-language articles were selected for inclusion if they met the following criteria: inclusion of patients >15 years old with early-stage (I and II) HL; evaluation of radiation therapy (including extent of field and dose); RCTs published from 2003 onward or systematic reviews published from 2011 onward; and evaluation of overall survival, disease control (eg, PFS), response rate, quality of life, or adverse events (early or late). Citations and full-text review were independently reviewed by a methodologist (F.B.) and a clinician (J.H., M.C.C., or M.C.). Discrepancies were resolved by consensus. Studies that included stage III patients were excluded when the results of the stage I and II patients were not reported separately because the natural history of such patients and the extent of radiation used may differ. The quality of the included studies was evaluated according to the Cochrane Risk of Bias tool13 and the Grading of Recommendations Assessment, Development, and Evaluation approach14 independently by a methodologist (F.B.) and a clinician (M.C.C.).
Data were extracted to address the following three questions: (1) What are the results of radiation in the treatment of early-stage HL? (2) What is the role of fluorodeoxyglucose positron emission tomography (FDG-PET) scanning in guiding the use of radiation therapy in early-stage HL? and (3) Are there subgroups of patients that benefit (or do not benefit) from radiation therapy?
Initially, it was planned to pool data in a meta-analysis if clinically homogeneous results were found. If data were not considered clinically and statistically homogeneous according to the consensus of the working group members, a narrative synthesis would be performed.
Results
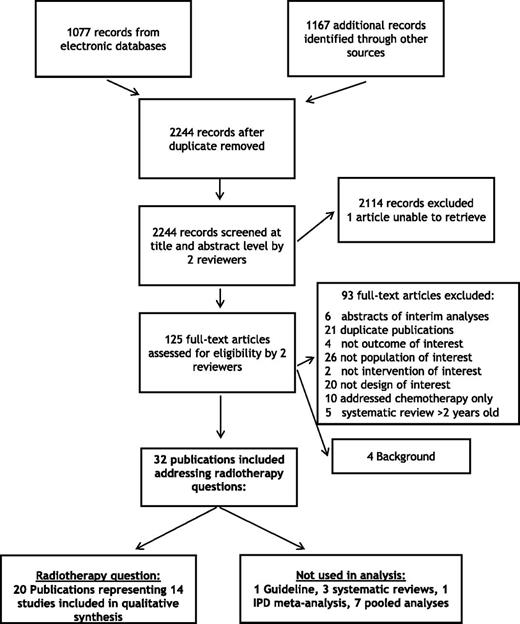
The search of electronic databases, conference abstracts, and the reference lists of included articles resulted in 2244 potentially eligible citations; from these, 125 full-text articles were assessed for eligibility (Figure 1). Twenty publications representing 14 unique studies examined questions regarding the use of radiotherapy for patients with early-stage HL. The general characteristics of the relevant RCTs are shown in Tables 2-6. The studies were grouped according to 4 comparisons: (1) radiotherapy in combination with chemotherapy vs chemotherapy alone; (2) low-dose radiotherapy vs high-dose radiotherapy; (3) smaller-field vs large-field radiation; and (4) standard therapy vs tailored therapy using FDG-PET scanning.
Diagram of literature search. Twenty publications were identified reporting results of randomized trials examining questions regarding use of radiation in early HL.
Diagram of literature search. Twenty publications were identified reporting results of randomized trials examining questions regarding use of radiation in early HL.
Results of RCTs comparing radiotherapy plus chemotherapy to chemotherapy alone
| Trial . | Intervention control participants . | OS, % . | EFS, % . | PFS, % . | Late AE, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|
| HD.6 | Arm A: ABVD only (n = 196) | *94 vs 87 at 12 y | 85 vs 80 at 12 y | 87 vs 92 at 12 y | Second cancers: 5.1 vs 11.33 | 11.3 y | 14 |
| Arm B: STNI with or without ABVD (n = 203) | HR for death: 0.50; 95% CI: 0.25-0.99 (P = .04) | HR: 0.88; 95% CI: 0.54-1.43 (P = .60) | HR: 1.91; 95% CI: 0.99-3.69 (P = .05) | Death: 6.1 vs 11.8 |
| Trial . | Intervention control participants . | OS, % . | EFS, % . | PFS, % . | Late AE, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|
| HD.6 | Arm A: ABVD only (n = 196) | *94 vs 87 at 12 y | 85 vs 80 at 12 y | 87 vs 92 at 12 y | Second cancers: 5.1 vs 11.33 | 11.3 y | 14 |
| Arm B: STNI with or without ABVD (n = 203) | HR for death: 0.50; 95% CI: 0.25-0.99 (P = .04) | HR: 0.88; 95% CI: 0.54-1.43 (P = .60) | HR: 1.91; 95% CI: 0.99-3.69 (P = .05) | Death: 6.1 vs 11.8 |
ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AE, adverse events; CI, confidence interval; EFS, event-free survival; HR, hazard ratio; OS, overall survival; STNI, subtotal nodal irradiation.
Primary outcome.
Results of RCTs that examined the efficacy of a lower compared to a higher radiotherapy dose
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| GOELAMS H 97E | Arm A (reduced-dose arm): 3 × ABVD + irradiation at 36 Gy to initially involved sites and 24 Gy to adjacent sites, the upper infra-diaphragmatic area, and the spleen (n = 89) | 97.8 ± 3.1 vs 95 ± 4.9 at 10 y (P = NS) | *88.6 ± 11.4 vs 92.6 ± 5.9 at 10 y (P = NS) | NR | NR | NR | NR | 10 y | 20 |
| GHSG HD11 | Arm A: 4 × ABVD + IFRT 30 Gy (n = 386) vs standard treatment | Arm A: 94.3 (95% CI: 91-96) at 5 y | 4 × ABVD arm at 5 y: inferiority of 20 Gy vs 30 Gy could not be excluded (n = 682), difference −4.7% (95% CI: −10.3 to 0.8) | †Arm B vs arm A: HR: 1.49; 95% CI: 1.04-2.15 (P = .03) | NR | SC: 30 Gy vs 20 Gy: 3.4 vs 4.0 (P = NS) Death: 30 Gy vs 20 Gy: 6.5 vs 6.2 | Complete response: 30 Gy vs 20 Gy: 94.5 vs 93.7 (P = NS) | 105 mo | 21 |
| Arm B: 4 × ABVD + IFRT 20 Gy (n = 395) vs Arm C | Arm B: 93.8 (95% CI: 91-96) at 5 y | ||||||||
| Arm C: 4 × BEACOPP + 30 Gy IFRT (n = 394) vs Arm D | Arm C: 94.6 (95% CI: 92-97) at 5 y | *4 × BEACOPP arm at 5 y: 20 Gy was not inferior to 30 Gy (n = 669), difference −0.8% (95% CI: −5.8 to 4.2) | |||||||
| Arm D: 4 × BEACOPP + 20 Gy IFRT (n = 395) | Arm D: 95.1 (95% CI: 92-97) at 5 y (P = NS) | ||||||||
| GHSG HD10 | Arm A: 4 × ABVD + IFRT 30 Gy (n = 346) vs Arm B | Arms A & C: 94.9 (95% CI: 92.2-96.6) at 8 y Arms B & D: 95.6 (95% CI: 93.2-97.1) at 8 y HR for death: 0.86; 95% CI: 0.49-1.53 (P = .61) | Arms B & D at 8 y: 88.6 (95% CI: 85.1-91.3) Arms A & C at 8 y: 87.8 (95% CI: 83.8-90.9) HR: 1.00 (95% CI: 0.68-1.47) Group difference at 5 y of Arms B & D vs arms A & C: −0.5 (95% CI: −3.6 to 2.6); the 7% inferiority of 20 Gy can be excluded | Arms A & C at 8 y: 88.1 (95% CI: 84.1-91.2) Arms B & D at 8 y: 88.9 (95% CI: 85.4-91.6) | NR | SC at 7.5 y: Arms A & C: 5.4 Arms B & D: 4.1 Death at 7.5 y: Arms A & C: 4.3 Arms B & D: 3.7 (P = .34) | Complete response: Arms A & C: 99.0 Arms B & D: 97.4 (P = NS) | 90 mo | 22 |
| Arm B: 4 × ABVD + IFRT 20 Gy (n = 340) vs Arm C | |||||||||
| Arm C: 2 × ABVD + IFRT 30 Gy (n = 341) vs Arm D | |||||||||
| Arm D: 2 × ABVD + IFRT 20 Gy (n = 343) | |||||||||
| EORTC-GELA H9F (favorable) | Arm A: IFRT 36 Gy + 6 × EBVP (n = 239) | 98 vs 98 vs 98 | NR | NR | 87 vs 84 vs 70 (P < .001) | NR | NR | 4 y | 15 |
| Arm B: IFRT 20 Gy + 6 × EBVP (n = 239) | |||||||||
| Arm C: no RT + 6 × EBVP (arm stopped early) (n = 130) |
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| GOELAMS H 97E | Arm A (reduced-dose arm): 3 × ABVD + irradiation at 36 Gy to initially involved sites and 24 Gy to adjacent sites, the upper infra-diaphragmatic area, and the spleen (n = 89) | 97.8 ± 3.1 vs 95 ± 4.9 at 10 y (P = NS) | *88.6 ± 11.4 vs 92.6 ± 5.9 at 10 y (P = NS) | NR | NR | NR | NR | 10 y | 20 |
| GHSG HD11 | Arm A: 4 × ABVD + IFRT 30 Gy (n = 386) vs standard treatment | Arm A: 94.3 (95% CI: 91-96) at 5 y | 4 × ABVD arm at 5 y: inferiority of 20 Gy vs 30 Gy could not be excluded (n = 682), difference −4.7% (95% CI: −10.3 to 0.8) | †Arm B vs arm A: HR: 1.49; 95% CI: 1.04-2.15 (P = .03) | NR | SC: 30 Gy vs 20 Gy: 3.4 vs 4.0 (P = NS) Death: 30 Gy vs 20 Gy: 6.5 vs 6.2 | Complete response: 30 Gy vs 20 Gy: 94.5 vs 93.7 (P = NS) | 105 mo | 21 |
| Arm B: 4 × ABVD + IFRT 20 Gy (n = 395) vs Arm C | Arm B: 93.8 (95% CI: 91-96) at 5 y | ||||||||
| Arm C: 4 × BEACOPP + 30 Gy IFRT (n = 394) vs Arm D | Arm C: 94.6 (95% CI: 92-97) at 5 y | *4 × BEACOPP arm at 5 y: 20 Gy was not inferior to 30 Gy (n = 669), difference −0.8% (95% CI: −5.8 to 4.2) | |||||||
| Arm D: 4 × BEACOPP + 20 Gy IFRT (n = 395) | Arm D: 95.1 (95% CI: 92-97) at 5 y (P = NS) | ||||||||
| GHSG HD10 | Arm A: 4 × ABVD + IFRT 30 Gy (n = 346) vs Arm B | Arms A & C: 94.9 (95% CI: 92.2-96.6) at 8 y Arms B & D: 95.6 (95% CI: 93.2-97.1) at 8 y HR for death: 0.86; 95% CI: 0.49-1.53 (P = .61) | Arms B & D at 8 y: 88.6 (95% CI: 85.1-91.3) Arms A & C at 8 y: 87.8 (95% CI: 83.8-90.9) HR: 1.00 (95% CI: 0.68-1.47) Group difference at 5 y of Arms B & D vs arms A & C: −0.5 (95% CI: −3.6 to 2.6); the 7% inferiority of 20 Gy can be excluded | Arms A & C at 8 y: 88.1 (95% CI: 84.1-91.2) Arms B & D at 8 y: 88.9 (95% CI: 85.4-91.6) | NR | SC at 7.5 y: Arms A & C: 5.4 Arms B & D: 4.1 Death at 7.5 y: Arms A & C: 4.3 Arms B & D: 3.7 (P = .34) | Complete response: Arms A & C: 99.0 Arms B & D: 97.4 (P = NS) | 90 mo | 22 |
| Arm B: 4 × ABVD + IFRT 20 Gy (n = 340) vs Arm C | |||||||||
| Arm C: 2 × ABVD + IFRT 30 Gy (n = 341) vs Arm D | |||||||||
| Arm D: 2 × ABVD + IFRT 20 Gy (n = 343) | |||||||||
| EORTC-GELA H9F (favorable) | Arm A: IFRT 36 Gy + 6 × EBVP (n = 239) | 98 vs 98 vs 98 | NR | NR | 87 vs 84 vs 70 (P < .001) | NR | NR | 4 y | 15 |
| Arm B: IFRT 20 Gy + 6 × EBVP (n = 239) | |||||||||
| Arm C: no RT + 6 × EBVP (arm stopped early) (n = 130) |
BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; EBVP, epirubicin, bleomycin, vinblastine, prednisone; FFTF, freedom from treatment failure; GELA, French Adult Lymphoma Study Group; NR, not reported; NS, not significant; GOELAMS, East West Study Group of Leukemia and Other Diseases of the Blood; SC, second cancers.
Primary outcome
The single experimental arms (arms B, C, and D) were compared to the standard arm (arm A) in a Cox regression model, together with all candidate prognostic factors.
Results of RCTs comparing the efficacy of smaller vs larger radiotherapy field
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| GHSG HD8 | Arm A: COPP-ABVD + 30 Gy EF + 10 Gy to bulk (n = 565) | 90.8 vs 92.4 at 5 y (difference: EF − IF = −1.6% (95% CI: −5.6 to 2.5); P = NS | *85.8 vs 84.2 at 5 y (difference of 1.6%, upper boundary, 5.9%); IF was not inferior to EF; margin at 6% | 79.8 vs 80.0 at 10 y (95% CI: −5.2 to 5.6); P = NS | NR | SC: 4.6 vs 2.8 (P = .191) Deaths: 8.1 vs 6.4 (P = .344) | CR: 98.5 vs 97.2 | 55 mo | 8†, 23 |
| Arm B: COPP-ABVD + 30 Gy IF + 10 Gy to bulk (n = 571) | |||||||||
| EORTC GELA H8U (unfavorable) | Arm A: 6 × MOPP-ABV + IFRT (n = 663) | 88 (95% CI: 84-91) vs 85 (95% CI: 78-90) vs 84 (95% CI: 74-90) at 10 y (P = .93) | NR | NR | *82 (95% CI: 77-86) vs 80 (95% CI: 75-85) vs 80 (95% CI: 71-86) at 10 y (P = .80) | SC at 10 y: Arm A: 4.5 (95% CI: 2.5-7.9) Arm B: 7.1 (95% CI: 4.3-11.6) Arm C: 8.8 (95% CI: 4.3-17.3), P = .63 Death: 11 vs 11 vs 10 (P values NR) | CR/Cru at the end of chemotherapy: 69 vs 64 vs 64 CR/Cru at the end of treatment: 83 vs 85 vs 86 | 89 mo | 25 |
| Arm B: 4 × MOPP-ABV + IFRT (n = 333) | |||||||||
| Arm C: 4 × MOPP-ABV + STNI (n = 327) | |||||||||
| INCI | Arm A: ABVD + STNI (n = 66) | 96 (95% CI: 91-100) vs 94 (95% CI: 89-100) at 12 y | NR | 93 (95% CI: 83-100) vs 94 (95% CI: 88-100) at 12 y | 87 (95% CI: 85-98) vs 91 (95% CI: 85-98) at 12 y | NR | CR: 100 vs 97 | 116 mo | 26 |
| Arm B: ABVD + IFRT (n = 70) | |||||||||
| DNHSG | Arm A: MFRT + 6 × MOPP (n = 163) | For patients surviving >15 y, OS was better for arm A than for arm B (P < .02); estimate: 62 vs 50 at 30 y | NR | NR | NR | SC at 20 y: NS | NR | 25 y | 16 |
| Arm B: (S)TNI (n = 164) |
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| GHSG HD8 | Arm A: COPP-ABVD + 30 Gy EF + 10 Gy to bulk (n = 565) | 90.8 vs 92.4 at 5 y (difference: EF − IF = −1.6% (95% CI: −5.6 to 2.5); P = NS | *85.8 vs 84.2 at 5 y (difference of 1.6%, upper boundary, 5.9%); IF was not inferior to EF; margin at 6% | 79.8 vs 80.0 at 10 y (95% CI: −5.2 to 5.6); P = NS | NR | SC: 4.6 vs 2.8 (P = .191) Deaths: 8.1 vs 6.4 (P = .344) | CR: 98.5 vs 97.2 | 55 mo | 8†, 23 |
| Arm B: COPP-ABVD + 30 Gy IF + 10 Gy to bulk (n = 571) | |||||||||
| EORTC GELA H8U (unfavorable) | Arm A: 6 × MOPP-ABV + IFRT (n = 663) | 88 (95% CI: 84-91) vs 85 (95% CI: 78-90) vs 84 (95% CI: 74-90) at 10 y (P = .93) | NR | NR | *82 (95% CI: 77-86) vs 80 (95% CI: 75-85) vs 80 (95% CI: 71-86) at 10 y (P = .80) | SC at 10 y: Arm A: 4.5 (95% CI: 2.5-7.9) Arm B: 7.1 (95% CI: 4.3-11.6) Arm C: 8.8 (95% CI: 4.3-17.3), P = .63 Death: 11 vs 11 vs 10 (P values NR) | CR/Cru at the end of chemotherapy: 69 vs 64 vs 64 CR/Cru at the end of treatment: 83 vs 85 vs 86 | 89 mo | 25 |
| Arm B: 4 × MOPP-ABV + IFRT (n = 333) | |||||||||
| Arm C: 4 × MOPP-ABV + STNI (n = 327) | |||||||||
| INCI | Arm A: ABVD + STNI (n = 66) | 96 (95% CI: 91-100) vs 94 (95% CI: 89-100) at 12 y | NR | 93 (95% CI: 83-100) vs 94 (95% CI: 88-100) at 12 y | 87 (95% CI: 85-98) vs 91 (95% CI: 85-98) at 12 y | NR | CR: 100 vs 97 | 116 mo | 26 |
| Arm B: ABVD + IFRT (n = 70) | |||||||||
| DNHSG | Arm A: MFRT + 6 × MOPP (n = 163) | For patients surviving >15 y, OS was better for arm A than for arm B (P < .02); estimate: 62 vs 50 at 30 y | NR | NR | NR | SC at 20 y: NS | NR | 25 y | 16 |
| Arm B: (S)TNI (n = 164) |
ABV, doxorubicin, bleomycin, vinblastine; COPP, cyclophosphamide, vincristine, procarbazine, prednisone; CR, complete response; CRu, complete response unconfirmed; DNHSG, Danish National Hodgkin Study Group; EF, extended field; IF, involved field; INCI, Italian National Cancer Institute; MFRT, mantle-field radiotherapy; MOPP, mechlorethamine, vincristine, procarbazine, prednisone.
Primary outcome.
Source of PFS data.
Results of RCTs comparing the efficacy of smaller field radiotherapy plus chemotherapy to larger field radiotherapy
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| EORTC-GELA H7 (favorable) | Arm A: STNI (n = 165) | *92 (95% CI: 85-95) vs 92 (95% CI: 84-95) at 10 y; P = .79 | NR | NR | *78 (95% CI: 70-83) vs 88 (95% CI: 82-92) at 10 y; P = .0113 | SC at 10 y: 2.3 (95% CI: 0.7-7.4) vs 2.9 (95%CI: 0.9-9.0) | CR: 94 vs 91; P = NS | 105 mo | 24 |
| Arm B: 6 × EPBV + IFRT (n = 168) | |||||||||
| EORTC-GELA H8 (favorable) | Arm A: STNI (n = 272) | *92 (95% CI: 87-95) vs 97 (95% CI: 92-99) at 10 y; P = .001 | NR | NR | *68 (95% CI: 64-76) vs 93 (95% CI: 85-97) at 10 y; P < .001 | SC at 10 y: 3.4 (95% CI: 1.3-8.4) vs 3.2 (95% CI: 1.2-8.0); P = .75 Death: 7 vs 1 | CR/CRu at the end of chemotherapy: NA vs 76 | 92 mo | 25 |
| Arm B: combination of 3 × MOPP-ABV + IFRT (n = 270) | CR/Cru at the end of treatment: 95 vs 93 |
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| EORTC-GELA H7 (favorable) | Arm A: STNI (n = 165) | *92 (95% CI: 85-95) vs 92 (95% CI: 84-95) at 10 y; P = .79 | NR | NR | *78 (95% CI: 70-83) vs 88 (95% CI: 82-92) at 10 y; P = .0113 | SC at 10 y: 2.3 (95% CI: 0.7-7.4) vs 2.9 (95%CI: 0.9-9.0) | CR: 94 vs 91; P = NS | 105 mo | 24 |
| Arm B: 6 × EPBV + IFRT (n = 168) | |||||||||
| EORTC-GELA H8 (favorable) | Arm A: STNI (n = 272) | *92 (95% CI: 87-95) vs 97 (95% CI: 92-99) at 10 y; P = .001 | NR | NR | *68 (95% CI: 64-76) vs 93 (95% CI: 85-97) at 10 y; P < .001 | SC at 10 y: 3.4 (95% CI: 1.3-8.4) vs 3.2 (95% CI: 1.2-8.0); P = .75 Death: 7 vs 1 | CR/CRu at the end of chemotherapy: NA vs 76 | 92 mo | 25 |
| Arm B: combination of 3 × MOPP-ABV + IFRT (n = 270) | CR/Cru at the end of treatment: 95 vs 93 |
NA, not available.
Primary outcome.
Results of RCTs comparing FDG-PET scanning to direct therapy
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| EORTC/LYSA/ FIL H10 (favorable) | Arm A (control) (n = 223): 3 × ABVD + 30-Gy INRT (FDG-PET completed only for comparison in control arm) Arm B (intervention) (n = 221): 2 × ABVD + FDG-PET If FDG-PET negative: 2 × ABVD and no radiotherapy If FDG-PET positive: 2 × BEACOPP + 30-Gy INRT | NR | NR | *100 vs 94.9 at 1 y | NR | NR | NR | 1.1 y | 17, 27 |
| EORTC/LYSA/ FIL H10 (unfavorable) | Arm A (control) (n = 346): 4 × ABVD + 30-Gy INRT (FDG-PET completed only for comparison) Arm B (intervention) (n = 347): 2 × ABVD + FDG-PET If FDG-PET negative: 4 × ABVD If FDG-PET positive: 2 × BEACOPP + 30-Gy INRT | NR | NR | *97.3 vs 94.7 at 1y | NR | NR | NR | 1.1 y | 17, 27 |
| RAPID | 3 × ABVD + FDG-PET If FDG-PET negative: Arm A: IFRT (n = 209) Arm B: no further intervention (n = 211) If FDG-PET positive: 1 × ABVD + IFRT | 97 vs 99.5 at 3 y (P values NR) | NR | *93.8 vs 90.7 at 3 y (risk difference of 2.9%; 95% CI: −10.7 to 1.4 (this exceeds the margin for noninferiority of −7%) | NR | NR | NR | 48 mo | 18 |
| Trial . | Intervention control participants . | OS, % . | FFTF, % . | PFS, % . | EFS, % . | Late AE, % . | Response, % . | Median follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| EORTC/LYSA/ FIL H10 (favorable) | Arm A (control) (n = 223): 3 × ABVD + 30-Gy INRT (FDG-PET completed only for comparison in control arm) Arm B (intervention) (n = 221): 2 × ABVD + FDG-PET If FDG-PET negative: 2 × ABVD and no radiotherapy If FDG-PET positive: 2 × BEACOPP + 30-Gy INRT | NR | NR | *100 vs 94.9 at 1 y | NR | NR | NR | 1.1 y | 17, 27 |
| EORTC/LYSA/ FIL H10 (unfavorable) | Arm A (control) (n = 346): 4 × ABVD + 30-Gy INRT (FDG-PET completed only for comparison) Arm B (intervention) (n = 347): 2 × ABVD + FDG-PET If FDG-PET negative: 4 × ABVD If FDG-PET positive: 2 × BEACOPP + 30-Gy INRT | NR | NR | *97.3 vs 94.7 at 1y | NR | NR | NR | 1.1 y | 17, 27 |
| RAPID | 3 × ABVD + FDG-PET If FDG-PET negative: Arm A: IFRT (n = 209) Arm B: no further intervention (n = 211) If FDG-PET positive: 1 × ABVD + IFRT | 97 vs 99.5 at 3 y (P values NR) | NR | *93.8 vs 90.7 at 3 y (risk difference of 2.9%; 95% CI: −10.7 to 1.4 (this exceeds the margin for noninferiority of −7%) | NR | NR | NR | 48 mo | 18 |
RAPID, PET Scan in Planning Treatment in Patients Undergoing Combination Chemotherapy For Stage IA or Stage IIA Hodgkin Lymphoma.
Primary outcome.
Study design, quality, and outcomes
All included studies were RCTs and fully published except 4 that were conference abstract publications.15-18 Supplemental Figure 2 shows the results of quality assessment of the included studies performed using the Cochrane Risk of Bias tool13 and the quality of each outcome measure evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation tool.14 None of the studies reported blinding, and 2 studies specifically declared that they were not blinded; the quality of the included studies was otherwise high. Across all 4 comparisons, study outcomes that were considered critical or important included overall survival, event-free survival, PFS, freedom from treatment failure, late adverse events, secondary malignancies, and response. Supplemental Tables 1A-5A present the results of the outcome by outcome assessment and the summary of results.
What are the results of radiation in the treatment of early HL?
Only 1 published trial in patients with early HL compared chemotherapy plus radiation to therapy with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) alone.19 The radiation arm of the study by Meyer et al used subtotal nodal irradiation, with or without 2 cycles of ABVD depending on risk factors. This study showed superior PFS rates at 12 years for those who received radiation (92% vs 87%; HR: 1.91; P = .05). However, overall survival favored the use of ABVD alone: the chemotherapy-alone arm was associated with a significant reduction in the risk of death (HR: 0.50) as well as a reduction in second cancers and cardiovascular events (Table 2). The extent of the radiation administered represented the standard of care for stage I-II HL at the time the study was designed and resulted in a much greater radiation dose to normal tissues; other, more recent studies have employed much smaller treatment volumes using IFRT or INRT. This trial highlights that PFS is not a reliable surrogate for overall survival in patients with early HL because of mortality from late complications and the effectiveness of salvage therapy for those who relapse. The benefits of the addition of radiation to chemotherapy are realized early, and the possible development of second cancers or cardiovascular disease appreciated years after treatment makes this discussion of trade-offs all the more challenging in the clinic.
A number of other prospective trials have informed the use of radiotherapy in early HL through testing radiation dose as well as extent of radiation field required to optimize therapy. Cooperative group trials by the French GOELAMS group and the GHSG have both demonstrated that lower doses of radiation to involved fields are as effective for disease control as higher doses and extended fields.20-22 The clearest example of this is from the GHSG HD10 trial,22 which demonstrated that for patients with early favorable HL (those without the risk factors listed in Table 1), excellent results can be achieved with only 20-Gy IFRT compared to 30 Gy after 2 cycles of ABVD, with a PFS rate close to 90% and an overall survival rate of 95% (Table 3). The GHSG HD11 trial21 demonstrated that for patients with early unfavorable HL (those with any of the risk factors in Table 1), the optimum dose of IFRT is 30 Gy when given with ABVD; similar outcomes could also be attained using escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) plus 20-Gy IFRT, although the acute toxicity of the latter regimen is greater. The follow-up of these trials is too short to fully understand the impact of radiation dose on late second cancers and cardiovascular disease.
Earlier trials have convincingly demonstrated that similar outcomes with regard to disease control can be achieved with therapy employing involved-field radiation compared to extended-field radiation, either alone or in combination with anthracycline-based chemotherapy23-26 (Tables 4 and 5). Acute toxicity from EFRT in these trials, when reported, was significantly greater than for IFRT, including gastrointestinal toxicity, myelosuppression, and treatment-related death. Furthermore, in the GHSG HD10 and HD11 studies, patients experienced significantly more severe grade 3 or 4 toxicity when treated with 30 Gy compared to 20 Gy: 8.7% vs 2.9% (P < .001) in the HD10 study22 ; 12% vs 5.7% (P < .001) in the HD11 study.21 We did not perform a meta-analysis of these trials because of the clinical heterogeneity of the treatments applied. In our opinion, the GHSG trials HD10 and HD11 were not suitable for a meta-analysis because these 2 studies evaluated patients with HL who were mutually exclusive with regard to clinical risk factors (outlined in Table 1) and differed in the number of cycles of chemotherapy received in the arms that were ultimately judged to be optimal treatment. In particular, HD10 enrolled early favorable HL patients and found that a lower radiation dose (20 Gy) was noninferior to a standard dose (30 Gy), whereas HD11 enrolled early unfavorable HL patients and found the opposite result: the higher radiation dose was better. Moreover, although the treatments used (ABVD and IFRT) were similar, the studies differed importantly with regard to radiation dose and number of cycles of therapy.
However, taken together, reported trials in this systematic review clearly indicate that IFRT (20 Gy for patients with early favorable HL; 30 Gy for those with early unfavorable HL) provides the optimum balance of disease control and short-term toxicity. A meta-analysis of trials of radiation field size across all stages of HL and recent observational data (neither included in our systematic review) suggest that IFRT may reduce the risk of secondary breast cancers in women10,11 ; however, individual trials do not have sufficient follow-up or statistical power to demonstrate a reduction in second cancers in patients treated with IFRT.8 There were no trials comparing INFT to IFRT identified through our systematic review.
What is the role of FDG-PET in guiding use of radiation for patients with early HL?
Two trials were identified by this systematic review that have examined the role of FDG-PET in identifying an early favorable HL patient population who, after ABVD chemotherapy, may have radiation omitted without compromising PFS.17,18,27 Both of these trials used noninferiority designs, asking whether radiation could be omitted from the treatment plan if an early (EORTC/LYSA/FIL H10 trial)27 or end-of-treatment (RAPID trial)18 FDG-PET scan was negative (Table 6).
In the experimental arm of the EORTC/LYSA/FIL H10 trial, INRT was omitted in patients with a negative scan after 2 cycles of ABVD compared to continuing with standard CMT employing INRT; the latter differed in treatment intensity depending on risk factors (favorable or unfavorable) at diagnosis.17,27 A planned interim analysis for futility led to the closure of the experimental no-radiation arms in both the favorable and unfavorable cohorts based on an increased number of progression events when radiation was omitted. Although this analysis was based on a small number of events (12 of the 26 required by the protocol in the favorable cohort, and 22 of 63 in the unfavorable group), it is highly unlikely that a strategy omitting radiation from primary treatment could be noninferior to CMT for PFS.
The RAPID trial reported by Radford et al18 randomized patients with stage I or II HL who had a negative FDG-PET scan after 3 cycles of ABVD to receive additional IFRT or no further therapy. After a median follow-up of nearly 4 years, the 3-year PFS rate was 93.8% for the CMT arm vs 90.7% for those in the no-radiation arm. The upper bound of the 95% CI for the difference between arms (−1.4% to 10.7%) exceeded the study’s noninferiority margin of 7%.28 In addition, in the per-protocol analysis (evaluating those who actually received their assigned treatment [26 of 209 patients randomized to CMT did not receive radiation]), the difference in PFS between arms was greater and the risk of progression was significantly higher in the experimental, no-radiation arm.
These 2 trials showed that omission of radiation from the treatment of early HL with a negative FDG-PET scan after 2 or 3 cycles of ABVD results in a higher rate of lymphoma progression of ∼5%, similar to that observed in the trial by Meyer et al,19 and that the use of PET to identify patients who may not need radiation is not currently recommended. The overall survival rate is excellent in the RAPID trial (97.1% after CMT and 99.5% after ABVD alone),18 and was not reported by Raemaekers et al27 for the EORTC/LYSA/FIL H10 trial; the follow-up for both studies, however, is too short to provide definitive information about long-term outcomes.
Are there subgroups of patients who benefit from or who do not require radiation following chemotherapy?
The 2 trials above using FDG-PET to inform the use of radiation represent the best-available quality prospective data to identify a subgroup of patients who may have radiation omitted without compromising disease control outcomes; as performed in those studies, FDG-PET does not identify a group of patients for whom radiation can be omitted without a reduction in PFS. The NCIC CTG HD.6 trial included a subset analysis of patients with a CR or CRu by computed tomographic (CT) scanning29 after 2 cycles of ABVD: these patients have an excellent outcome following 2 additional cycles of chemotherapy without radiation.19 Investigators from the NCIC CTG and GHSG reported an analysis of patients eligible for GHSG HD10 and NCIC CTG HD.6 (those without risk factors at presentation) and observed that among those with a CR or CRu by CT scan after 2 cycles of therapy, time to progression was similar, but PFS was inferior for those receiving IFRT compared to ABVD alone due to a greater number of deaths not attributable to progressive HL.30 These subset analyses are hypothesis generating (as acknowledged by the authors) and, although consistent within those trials, cannot be considered definitive. It is possible that use of both CT and PET response may identify patients who do not benefit from radiation; additional studies addressing this question are underway (see supplemental appendices).
The question of management of patients with early favorable or early unfavorable HL who have a positive FDG-PET scan at the end of chemotherapy is currently being addressed in the GHSG H10 trial, in which patients with a positive scan are randomized to continuing standard therapy or escalation of chemotherapy with the addition of 2 cycles of escalated BEACOPP. No other randomized studies were identified by our literature search. Patients in the RAPID trial with a positive PET scan after 3 cycles of ABVD received 1 additional cycle followed by IFRT (30 Gy); the PFS rate in this group of patients is 90.1% with a median follow-up of 48 months.18 Currently, patients with a positive FDG-PET scan after 2 or 3 cycles of ABVD should proceed to IFRT.
Discussion
The decision to include or omit radiation from the primary treatment of early-stage HL may be influenced by a number of factors, most of which cannot be addressed directly by data from currently available randomized trials. These factors include anatomic distribution of disease, expected normal tissue exposure, age-specific risks of secondary cardiovascular disease and second cancers, risk of treatment failure or relapse of HL, and toxicities from salvage therapy, which in the majority of cases will include high-dose chemotherapy and autologous stem cell transplant (ASCT).31,32 For Anne, CMT would involve radiation to what would approximate a mantle field: for her, this would be predicted to be accompanied by superior PFS but a higher risk of second cancers, in particular breast cancer. Although radiation fields that exclude the axilla and infraclavicular lymph nodes are associated with an approximately two-thirds lower risk of secondary breast cancer,10 this would not be feasible for Anne, and treatment with chemotherapy alone would be a reasonable alternative. For John, in light of the potential for higher risk of relapse due to his age (>40 years), treatment with CMT using 2 cycles of ABVD and 20-Gy IFRT for his early favorable HL would be the most appropriate strategy for him to maximize disease control.
Although strategies that omit IFRT or INRT from initial therapy for early HL are associated with a lower rate of PFS than that obtained with CMT, overall survival does not appear worse, likely a result of the effectiveness of second-line treatment. The outcome of patients with early HL who relapse after IFRT and 2 cycles of ABVD remains relatively poor, with a freedom-from-second-treatment-failure rate of only 52%31 ; currently, salvage therapy followed by ASCT is recommended for such patients.32 Patients who undergo ASCT for progressive or relapsed HL are at higher risk of infertility and second cancers including acute myeloid leukemia, and often encounter significant obstacles with regard to employment, education, and social engagement as a consequence of intensive therapy.33,34 A discussion of treatment options therefore needs to include the prospect of additional treatment with ASCT (although ultimately needed in only ∼5% to 8% of patients), which carries with it many of the same late effects that patients and physicians wish to avoid with omission of radiation from primary therapy. Patients who have experienced treatment of HL may assign different importance to short-term and long-term side effects than would be predicted by their treating physicians. HL survivors have perceived early, acute side effects as being at least as important in determining choice of treatment as later, more permanent effects; nonetheless, disease relapse and the development of a second cancer and late cardiovascular disease are highly important as related by experienced surrogates.35
There are some weaknesses of this evidence-based review. We chose to focus on randomized trials because these have been integral in shaping modern treatment practices and are the least biased level of evidence. However, few of these trials compared chemotherapy treatment alone to CMT for patients with early-stage disease. The follow-up of these studies, although extending up to 10 years from randomization, is still too short to fully capture the impact of late cardiovascular events and second cancers on overall outcomes. At present, long-term estimates of risk, up to 20 years after therapy, are derived from large cohort studies of patients receiving predominantly EFRT.36-39 Late effects of more modern approaches can ideally be captured in the context of the cooperative group trials that informed this review.40,41 Meta-analysis of the studies included in this review would have increased statistical power to compare rates of late events between treatment approaches, but these trials were too clinically heterogeneous for this to be undertaken. Although many patients present with bulky mediastinal adenopathy (≥10 cm, or more than one-third of the maximum thoracic diameter on chest radiograph), a circumstance in which IFRT is often routinely included, randomized trials comparing chemotherapy alone vs CMT have not been undertaken, and these patients were generally excluded from the trials identified in our literature review17,18,27 ; at present, inclusion of radiation in treatment of this patient subset is appropriate.
Studies to date have established chemotherapy and IFRT as standard: 2 cycles of ABVD followed by 20 Gy for early favorable HL; 4 cycles of ABVD plus 30 Gy for those with risk factors. It is recognized that in the context of CMT, techniques to further reduce the radiation field to only the nodal areas affected with a small margin are being explored (INRT and involved-site radiotherapy) in attempts to further reduce toxicity.42 These therapies are likely to be incorporated into future clinical trials of combined modality approaches. Longer follow-up of the RAPID and EORTC/LYSA/FIL H10 trials is needed, because late events such as second malignancies and other toxicities still have the potential to change the relative risks and benefits of the approaches tested in those trials, as was observed in the NCIC CTG HD.6 study by Meyer et al.19 Additional studies of functional imaging and the identification of biomarkers that may identify benefit from inclusion/exclusion of radiation from primary therapy are awaited. Until then, the inclusion of radiation in the primary treatment of early HL remains standard, and treatment with chemotherapy alone should be undertaken only after thoughtful discussion with patients regarding risks, both near-term and in the distant future.
The online version of this article contains a data supplement.
Acknowledgment
This work was supported in part by the Cancer Care Ontario Program in Evidence-Based Care.
Authorship
Contribution: M.C. participated in collecting and reviewing the data and wrote the first draft of the manuscript; J.H. reviewed the data and revised the manuscript; F.B. performed the literature search, collected and organized the data, and assisted in revising the manuscript; J.S., J.M., and D.H. reviewed the data and assisted in revising the manuscript; M.C.C. participated in collecting and reviewing the data and revising the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Crump, Princess Margaret Cancer Center, 610 University Ave, Room 5-209, Toronto, ON, Canada M5G 2M9; e-mail: michael.crump@uhn.ca.