Abstract
Multiple myeloma (MM) is a plasma cell malignancy leading to significant life-expectancy shortening. Although the incorporation of the novel agents thalidomide, bortezomib, and lenalidomide in the front-line therapy has resulted in significant improvement, almost all patients relapse, making the treatment of relapse a real challenge. In the present article, when and how to treat relapsed MM is discussed. Treatment can be safely delayed in a subset of patients with asymptomatic relapse, whereas those with symptomatic relapse, advanced disease at diagnosis, or significant paraproteinemic increase require prompt rescue therapy. The benefit of retreatment and the use of a sequential approach for successive relapses considering drug synergism are highlighted. For patients with aggressive relapses and for those who have exhausted all available options, continued therapy until disease progression is recommended, particularly when using regimens with a long-term safety profile. Patients with a duration response to a first autologous stem cell transplantation (ASCT) longer than 2 years may benefit from a second ASCT. Patients with aggressive disease and/or poor cytogenetics at diagnosis relapsing within the first 2 years from ASCT should be considered for an allogeneic transplantation. Finally, a number of newer promising drugs are being actively investigated and the enrolment of patients in clinical trials is encouraged.
Introduction
The incorporation of the novel drugs thalidomide, bortezomib, and lenalidomide has resulted in a significant survival prolongation in patients with multiple myeloma (MM).1-3 However, MM remains incurable with an important life-expectancy shortening.4 Autologous stem cell transplantation (ASCT) is the gold standard in younger patients and the incorporation of novel drugs in the induction phase has improved the post-ASCT complete remission (CR) rate and progression-free survival (PFS).5-9 However, most patients ultimately relapse. Concerning elderly patients, melphalan and prednisone (MP) or dexamethasone-based regimens have been the standard of care for many years. The novel drugs thalidomide, bortezomib, and lenalidomide have been associated with MP (MPT, MPV, and MPR) or with low-dose dexamethasone (Rd) resulting in superior PFS in almost all studies and in a significant overall survival (OS) prolongation in some of them.10-14 Although the improvement achieved is clinically relevant, it is far from satisfactory.
Despite bortezomib, pegylated doxorubicin, lenalidomide, carfilzomib, and pomalidomide having been recently approved, the treatment of patients with relapse or refractory MM remains a challenge.3,15,16 Unfortunately, the duration of responses is limited and all patients will develop progressive disease (PD). In patients with relapsed MM, the choice of salvage therapy should be individualized and must depend on the considerations summarized in Table 1. The most frequently asked questions in the treatment of relapsed patients are listed in Table 2. In this How I Treat article, we use a case-and-comments approach with our decision processes illustrated in the context of real-world patients seen at our clinic.
General considerations in relapsed myeloma
| Variable . | Considerations . |
|---|---|
| Components of the initial therapy | Novel agents? |
| ASCT? | |
| Degree and duration of response to primary therapy | PR, VGPR, CR? |
| ≥6 mo, ≥1 y? | |
| Previous toxicities | Myelosuppression |
| Peripheral neuropathy | |
| Type of relapse | Aggressive |
| Indolent | |
| Age and performance status | Elderly |
| Frail |
| Variable . | Considerations . |
|---|---|
| Components of the initial therapy | Novel agents? |
| ASCT? | |
| Degree and duration of response to primary therapy | PR, VGPR, CR? |
| ≥6 mo, ≥1 y? | |
| Previous toxicities | Myelosuppression |
| Peripheral neuropathy | |
| Type of relapse | Aggressive |
| Indolent | |
| Age and performance status | Elderly |
| Frail |
Frequently asked questions in relapsed patients with myeloma
| Questions . | Considerations . |
|---|---|
| 1. To treat or not to treat asymptomatic relapses | When treatment can be safely delayed? |
| When early treatment should be administered? | |
| 2. When retreatment should be considered | What should be the response duration cutoff? |
| 3. Which are the best drug associations? | Additive effect? |
| Synergistic effect? | |
| 4. How to use available drugs | Sequential approach? |
| Multidrug combination approach? | |
| 5. For how long should a rescue treatment be continued | Limited number of cycles? |
| Indefinite? | |
| 6. When to consider a rescue second ASCT? | What should be the minimal response duration from the first ASCT? |
| 7. Is there a role for allogeneic transplantation? | When should it be considered? |
| Questions . | Considerations . |
|---|---|
| 1. To treat or not to treat asymptomatic relapses | When treatment can be safely delayed? |
| When early treatment should be administered? | |
| 2. When retreatment should be considered | What should be the response duration cutoff? |
| 3. Which are the best drug associations? | Additive effect? |
| Synergistic effect? | |
| 4. How to use available drugs | Sequential approach? |
| Multidrug combination approach? | |
| 5. For how long should a rescue treatment be continued | Limited number of cycles? |
| Indefinite? | |
| 6. When to consider a rescue second ASCT? | What should be the minimal response duration from the first ASCT? |
| 7. Is there a role for allogeneic transplantation? | When should it be considered? |
Case 1. A young patient with primary refractory myeloma to VTD
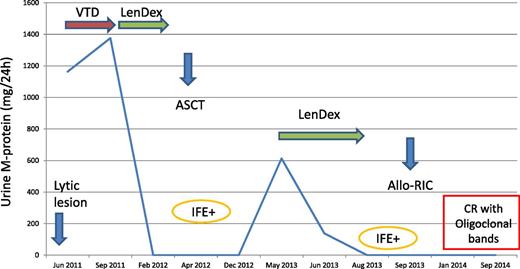
A 52-year-old woman presented with a pathological femoral fracture in June 2011. She was diagnosed with immunoglobulin A-λ (IgA-λ) MM with a serum M-protein of 4.5 g/L and a λ light-chain urine protein excretion of 1163 mg per 24 hours. Her bone marrow contained 57% plasma cells with no cytogenetic abnormalities by fluorescence in situ hybridization. A hip replacement was performed and treatment with VTD (bortezomib, thalidomide, and dexamethasone) was initiated. After 3 cycles, urine light-chain protein excretion increased to 1376 mg per 24 hours and rescue therapy with lenalidomide and dexamethasone (LenDex) was started. After 4 courses, the patient achieved very-good-partial response (VGPR) with a urine M-protein <100 mg per 24 hours. In April 2012, an ASCT with MEL-200 was performed. The patient remained in VGPR until May 2013 when PD with a urine light-chain protein excretion of 613 mg per 24 hours was documented. An identical sibling donor was available and rescue therapy with LenDex retreatment followed by a reduced-intensity conditioning (RIC) allogeneic transplantation (Allo-RIC) was planned. After 3 LenDex cycles, the patient achieved VGPR. The Allo-RIC procedure was performed in September 2013. The patient did not develop graft-versus-host disease (GVHD) and she is asymptomatic in stringent and immunophenotypic CR 1 year after Allo-RIC (Figure 1).
A young patient with primary refractory myeloma to VTD. IFE, immunofixation.
Comments
This patient had IgA-λ MM with predominant light-chain urine protein excretion, as the IgA serum level was below the measurability threshold (5 g/L).17,18 Concerning initial therapy, outside clinical trials and besides patients with ultra-high risk (see next paragraph), we select our best option for both standard- and high-risk myeloma. In this regard, VTD is a highly effective induction regimen prior to ASCT. However, 15% of patients fail to respond.6 In this situation, LenDex is our preferred rescue regimen.19-21 Our patient achieved VGPR and her response status was not improved with ASCT, developing PD 1 year later. We have shown that in patients presenting with predominant light-chain urine protein excretion, the first indicator of relapse is the reappearance of the light chains in the urine.22 The patient with IgA-λ had both intact immunoglobulin and light-chain urine protein production at diagnosis, and light-chain escape at relapse. This phenomenon portends a poor prognosis and is usually seen in advanced phases of the disease when the myeloma cells become undifferentiated with a shift from intact immunoglobulin to free-light-chains-only secretion.23 Of interest, the urine progression was preceded by increasing levels in serum free light-chain from 10.7 after ASCT to 433 mg/L at the time of PD. Our patient was resistant to bortezomib and also to high-dose melphalan with early relapse after ASCT, so an Allo-RIC from her identical sibling donor was considered. Taking into account the previous response to LenDex, retreatment with the same regimen was given resulting in VGPR.
Allogeneic stem-cell transplantation (Allo-SCT) is a potentially curative approach for patients with MM even in advanced disease.24-28 The transplant-related mortality (TRM) of about 20% higher with myeloablative conditioning has resulted in a shift to Allo-RIC. The final outcome of the 2 conditioning approaches seems similar because the higher TRM with myeloablative conditioning is compensated by a higher relapse rate with Allo-RIC. We have just reported our experience showing a trend toward a better PFS and significantly longer OS with Allo-RIC when compared with myeloablative conditioning.28 Although the role of allotransplantation in MM is controversial, there are 2 situations in clinical practice in which the expected survival of patients with MM is very limited: (1) early relapse after an optimal induction followed by ASCT and (2) the recently recognized ultra-high-risk myeloma with poor-risk cytogenetics plus either high lactate dehydrogenase (LDH) or International Staging System III (ISS III) who soon become refractory to all the available treatments.29 Our patient fulfilled our indication for Allo-SCT in early chemosensitive progression: aggressive presentation with bone fracture and light-chain proteinuria escape and resistant disease to an optimal induction with VTD and high-dose melphalan with early relapse (>2 years) after ASCT. In this situation, the probability of long-term disease control with the currently available antimyeloma agents is very unlikely.28 No GVHD was observed, and 1 year after Allo-RIC she is in stringent CR with the hope for a long-term remission and eventual cure.
Case 2. A patient with primary resistance to alkylating agents and to bortezomib
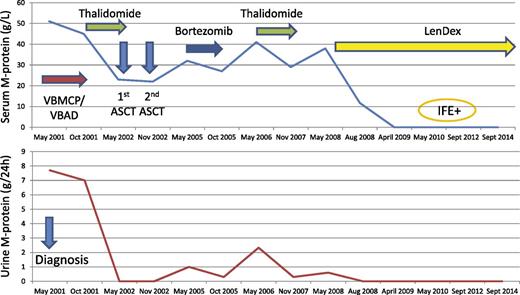
A 52-year-old man presented with nephrotic range proteinuria in May 2001. His only complaint was moderate fatigue; his hemoglobin was 11.4 g/dL with a serum IgG-κ monoclonal protein of 51 g/L and a urine protein excretion of 7.7 g per 24-hour glomerular pattern. His serum creatinine was 1.1 mg/dL, bone marrow contained 46% plasma cells, and skeletal survey showed lytic skull lesions. Subcutaneous fat was negative for amyloid. The patient was treated with 6 courses of alternating vincristine, carmustine, cyclophosphamide, melphalan, prednisone/vincristine, carmustine, doxorubicin, dexamethasone (VBCMP/VBAD) chemotherapy30 with no response. In October 2001, the patient received rescue therapy with single-agent thalidomide at a dose up to 400 mg per day achieving minimal response (MR) (serum M-protein, 23 g/L; urine M-protein, 90 mg per 24 hours) to proceed to ASCT with a lower tumor burden. No further response improvement to tandem ASCT (the first with MEL-200 performed in May 2002 and the second with cyclophosphamide, etoposide, carmustine (BCNU) in November 2002) was achieved.31 In March 2005, the patient developed PD and failed rescue therapy with bortezomib.32 In May 2006, serum M-protein increased to 41 g/L and the glomerular proteinuria was 2.33 g per 24 hours. Retreatment with thalidomide up to 200 mg per day was started resulting again in MR, but the drug was discontinued due to intolerance. In May 2008, the serum M-protein increased to 38 g/L and the glomerular proteinuria to 0.6 g per 24 hours. At that time, lenalidomide was approved in Europe and LenDex was initiated. The patient achieved VGPR. Because of poor tolerance, doses of Len and Dex were subsequently reduced to 15 mg and to 20 mg only on days 1 to 4, respectively. This patient enjoys a long-lasting VGPR and remains on continued therapy in September 2014 (Figure 2).
A patient with primary resistance to alkylating agents and to bortezomib. VBMCP/VBAD, vincristine, carmustine, cyclophosphamide, melphalan, prednisone alternated with vincristine, carmustine, doxorubicin, dexamethasone.
A patient with primary resistance to alkylating agents and to bortezomib. VBMCP/VBAD, vincristine, carmustine, cyclophosphamide, melphalan, prednisone alternated with vincristine, carmustine, doxorubicin, dexamethasone.
Comments
This patient presented with MM and glomerular nephrotic range proteinuria. The most common cause of nephrotic proteinuria in MM is associated amyloid light-chain (AL) amyloidosis.33,34 This patient had no other features consistent with AL and subcutaneous fat was negative for amyloid. It was thought that amyloid kidney involvement was unlikely and treatment of his MM was initiated. Interestingly, the amount of proteinuria (Figure 2) significantly decreased or disappeared when a response was obtained and increased with myeloma progression. This patient, refractory to both conventional and high-dose cytotoxic agents and to bortezomib, had an exquisite sensitivity to the immunomodulatory drugs (IMiDs) thalidomide and lenalidomide. In fact, he reached a MR 2 times with single-agent thalidomide (Figure 2). However, the drug was discontinued due to fatigue, constipation, and peripheral neuropathy.35 The durable MR responses achieved 2 times in this patient with single-agent thalidomide are not only a clear indication of his exquisite sensitivity to IMiDS but also highlight the importance of MR attainment in patients with refractory or relapsed myeloma.36 At the next progression, the treatment of choice was the more potent and less toxic IMiD lenalidomide. There is evidence that lenalidomide and glucocorticoids have a synergistic effect with a significant increase in the response rate and on the duration of response of the combination19,20 compared with lenalidomide alone.37 For this reason, we always use lenalidomide associated with glucocorticoids. In contrast, there is no evidence of a synergism between thalidomide and glucocorticoids, and we used single-agent thalidomide avoiding dexamethasone exposure in this patient who previously failed high-dose dexamethasone included in the initial VBAD regimen. The patient achieved partial response (PR) after 3 cycles of LenDex and subsequently VGPR. At this point, the question is for how long treatment should be maintained. The choice of some physicians, particularly in Europe, is to discontinue therapy after 1 or 2 years of treatment in responding patients in order to avoid potential toxicity and to reduce cost, re-treating at the time of a subsequent progression. Although this option seems reasonable, there are no studies on retreatment in patients with previous long-term exposure to lenalidomide. With the lack of data on lenalidomide retreatment and, particularly, in a patient with refractory disease to all the currently available antimyeloma agents, we favor continued therapy with a regimen with acceptable tolerance until disease progression.
This patient has now received treatment with LenDex for over 6 years. It is almost certain that our patient will end-up developing progressive disease. At that time, we believe that in a patient who showed a so exquisite sensitivity to IMiDs, the best option would be pomalidomide and dexamethasone.38-41 Other options could be: (1) the addition of a synergistic drug to LenDex, such as elotuzumab,42,43 (2) proteasome inhibitors (PIs) such as carfilzomib44 or ixazomib,45-47 despite his previous resistance to bortezomib, or (3) monoclonal antibodies such as anti-CD38 (daratumumab or SAR650984) as single agents or in combination. Concerning the efficacy of drug combination, when 2 or more drugs are given simultaneously, the result can be the expected by the addition of the efficacy of each compound (additive) or higher than the expected only by the combination of drugs (synergistic). Ideally, the additive or synergistic effect should be demonstrated in preclinical studies. Unfortunately, laboratory findings are not always translated into clinical results. For this reason, the additive or synergistic effects are, in most instances, clinically inferred by comparing outcomes across clinical trials rather than on a laboratory basis.
Case 3. A patient successfully retreated with bortezomib
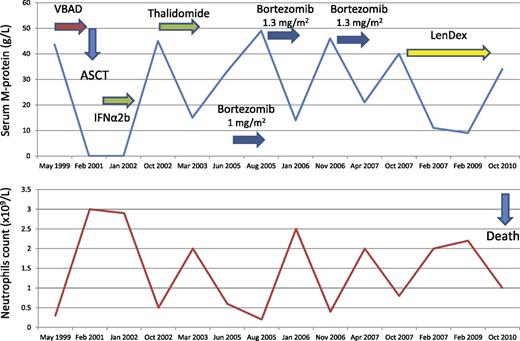
A 57-year-old woman presented with bone pain in May 1999. She was diagnosed with IgG-κ MM with a serum M-protein of 43.6 g/L, urine κ light chains of 116 mg per 24 hours, 12% bone marrow plasma cells, and lytic bone lesions. Her blood counts showed a neutrophil count of 0.3 × 109/L, with normal hemoglobin and platelet count. Due to severe neutropenia, no alkylating agents were administered and the patient was treated with 6 courses of VBAD and ASCT followed by α2b-interferon maintenance achieving CR in February 2001, with normalization of her neutrophil count. In October 2002, the patient developed PD with spontaneous rib fracture and neutropenia of 0.5 × 109/L. The patient received rescue therapy with single-agent thalidomide at a dose up to 400 mg per day achieving a decrease in her serum M-protein from 45 to 15 g/L and normalization in her neutrophil count. However, 10 months later, thalidomide was discontinued due to grade 2 peripheral neuropathy. In June 2005, a new progression was documented with a serum M-protein of 33 g/L and a neutrophil count of 0.6 × 109/L. Single-agent IV bortezomib at a reduced dose of 1 mg/m2 was initiated because of thalidomide-related grade 1 peripheral neuropathy. After 2 cycles, the serum M-protein increased to 48.6 g/L with decreasing neutropenia to 0.13 × 109/L with no worsening in her peripheral neuropathy. The dose of bortezomib was increased at 1.3 mg/m2 and granulocyte colony-stimulating factor (G-CSF) 3 times per week was started. The patient achieved PR with a decrease in her serum M-protein to 14 g/L and normal white cell count at bortezomib discontinuation after 6 full-dose cycles. Nine months later, in November 2006, a new increase in her serum M-protein to 46 g/L as well as recurrent neutropenia were observed. Retreatment with 8 courses of full-dose bortezomib plus G-CSF resulted in a new PR with M-protein decrease to 21 g/L and neutropenia resolution. Seven months later, in October 2007, the patient experienced a new progression with a serum M-protein increase to 40 g/L. At this time, the patient was treated with LenDex achieving PR with an M-protein decrease to 11 g/L. The patient was continued on LenDex therapy for 3 years and remained in PR until October 2010 when she developed PD and died in May 2011 (Figure 3).
Comments
Although neutropenia not due to heavy bone marrow involvement is exceedingly rare in MM, we have seen a few cases. Of interest, the neutrophil count normalized with all responses to therapy and recurred at each relapse (Figure 3). At first relapse, our patient was successfully treated with single-agent thalidomide which should be discontinued due to peripheral neuropathy. The presence of residual toxicity, particularly peripheral neuropathy, should always be considered because it may influence the treatment at relapse. In our patient, the thalidomide-related peripheral neuropathy led to the initiation of the next salvage therapy with bortezomib at reduced dose. Concerning bortezomib therapy in our patient, we want to highlight the decision on single-agent administration, the dose-dependent effect, and the retreatment benefit. Concerning the single-agent administration, our results in a phase 2 trial strongly supported that bortezomib and dexamethasone have only an additive rather than a synergistic effect.48 In addition, in bortezomib-retreatment studies, the benefit of bortezomib/dexamethasone vs single-agent bortezomib was marginal.49-51 In relapsed patients (candidates to a rescue ASCT), a short induction period with 3 to 4 cycles of bortezomib plus dexamethasone (in order to obtain the benefit from both drugs) is most reasonable. However, when a rescue ASCT is not planned, as in our patient, we prefer the use of single-agent bortezomib and, if there is no response, to switch to a different rescue regimen. Although the CREST study52 showed that bortezomib at 1 mg/m2 can still be effective, our patient showed evidence of a dose-dependent effect with PD after 2 cycles at 1 mg/m2 and PR when the dose was increased at 1.3 mg/m2. This highlights the importance of full-dose administration whenever possible.53-55 In this regard, subcutaneous bortezomib at 1.6 mg/m2 deserves investigation. Finally, our patient illustrates the benefit of bortezomib retreatment with 1 year gain before a new line of therapy was required. In this regard, it has been shown in a prospective phase 2 study that retreatment with bortezomib is an effective treatment option, with no significant cumulative toxicities.49 The decrease of peripheral neuropathy with the subcutaneous administration56 is an additional argument in favor of retreatment. Classically, bortezomib has been administered for a fixed number of cycles. Whether an extended treatment could be beneficial in relapsed patients is unknown. In the front-line setting, maintenance with IV bortezomib at 1.3 mg/m2 every 2 weeks for 2 years57 or 1 complete cycle in combination with thalidomide every 3 months for 3 years6 has been beneficial with acceptable toxicity.
Case 4. A patient successfully retreated with a second ASCT
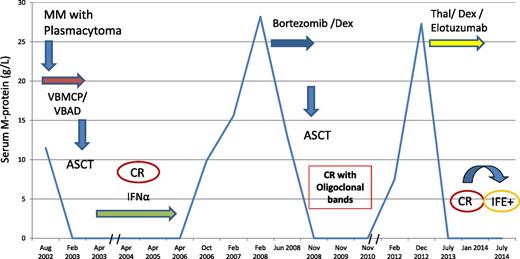
A 42-year-old-man presented with a skull mass in August 2002. A biopsy was consistent with plasmacytoma. He was diagnosed with IgA-κ MM with a serum M-protein size of 11.5 g/L, 6% bone marrow plasma cells, and multiple lytic bone lesions. Treatment with 6 courses of VBMCP/VBAD31 resulted in CR. The induction treatment was followed by ASCT with MEL-200 and maintenance with α-2b interferon plus prednisone.31 The patient remained in CR until October 2006 when he had an asymptomatic relapse with slowly increasing serum M-protein until February 2008 when the M-protein reached 28.2 g/L. At that time, 4 courses of salvage therapy with bortezomib/dexamethasone were given and a PR was achieved. A second ASCT with MEL-200 in August 2008 resulted in CR. In February 2012, a second asymptomatic relapse occurred, with the serum M-protein progressively increasing up to 27.3 g/L in December 2012. A third-line therapy with thalidomide, dexamethasone, and elotuzumab in the context of a phase 2 clinical trial was initiated.58 The patient achieved a third CR in July 2013 and remains on treatment, but the original IgA-κ M-protein was detected by immunofixation in July 2014 with no criteria for PD as of September 2014 (Figure 4).
A patient successfully retreated with a second ASCT. Thal/Dex, thalidomide and dexamethasone.
A patient successfully retreated with a second ASCT. Thal/Dex, thalidomide and dexamethasone.
Comments
This patient had a skull soft-tissue plasmacytoma. The extraosseous spread in MM is associated with poor outcome in patients treated with conventional dose chemotherapy.59,60 Concerning transplant candidates, the PETHEMA group showed that the PD rate during induction was significantly higher in patients with extramedullary disease.6 Although there were not significant differences in PFS, the OS was shorter in patients with extramedullary plasmacytomas. In contrast, 2 studies showed that patients who underwent ASCT had similar outcome irrespective of the presence or absence of extramedullary disease, indicating that high-dose therapy can overcome the poor prognosis of extramedullary involvement.59,60 In fact, in our patient, the extramedullary plasmacytoma disappeared with the use of VBMCP/VBAD chemotherapy; he subsequently received ASCT and the plasmacytoma never reappeared during the course of his disease. After 42 months in CR, the patient developed asymptomatic progression. In our experience, 50% of patients relapsing after upfront ASCT develop asymptomatic relapse with a median time to require treatment of 6 months.22 Of interest, in the present case, there was a progressive increase in the serum M-spike with no need of therapy for 18 months. In fact, in up to one-fourth of patients with asymptomatic relapse after ASCT treatment it can be safely delayed even for >2 years.22 We identified patients relapsing from CR, particularly those with ISS stage I or II and with no significant light-chain proteinuria at diagnosis, as the most likely to enjoy long periods until clinical relapse or significant paraproteinemic progression develop.22 The rescue treatment of choice in patients with late relapse after ASCT is a second high-dose procedure.61-64 We have shown that the features associated with prolonged OS in relapsing patients were the time to relapse, the type of relapse (asymptomatic vs symptomatic), and the use of salvage second auto-SCT or allogeneic-SCT.22 This patient fulfilled all the above criteria and achieved a second CR after the second rescue ASCT. The greatest benefit from a rescue ASCT is achieved in patients relapsing beyond 3 years from the first ASCT.64 It is of note that in our patient the duration of the second CR, lasting for >3 years, was almost as long as his first CR. The patient subsequently developed other asymptomatic relapse and he was given a completely new approach combining thalidomide and dexamethasone with the monoclonal antibody elotuzumab resulting in a new CR of 1 year duration followed by other asymptomatic relapse. Although elotuzumab is not active as single agent,65 encouraging results with its combination with Len/Dex have been reported suggesting a synergistic effect.42 This case illustrates how long-term survival in a patient with a biologically indolent and chemosensitive disease can be achieved despite recurrent disease. Possible available future options for this patient are: (1) lenalidomide/dexamethasone, (2) carfilzomib with or without dexamethasone, or (3) pomalidomide/dexamethasone. Considering the response duration to the second autologous transplant, a third ASCT could also be considered. Although this patient is still 56 years old, we do not believe that an Allo-RIC is an option for a patient with late and nonaggressive relapses with highly chemosensitive disease.
Conclusions
Treatment can be safely delayed in patients with asymptomatic serological relapse, particularly in those with stage I/II at diagnosis and relapsing from CR. In contrast, early treatment should be considered in patients with aggressive disease at diagnosis and in those with significant paraproteinemic relapse with any of the following features in 2 consecutive measurements separated by 2 months: doubling of the serum M-protein, increase in serum and/or urine M-protein by at least >10 g/L or 500 mg per 24 hours, respectively.36 Patients who achieve at least PR with primary therapy and who relapse beyond 1 year after front-line therapy discontinuation or beyond 6 months after a rescue therapy may benefit from retreatment with the same option. We favor the use of a sequential approach for successive relapses rather than the use of multiple-agent combination. Whether a synergistic or only an additive effect exists should be taken into account when selecting rescue treatment regimens. In this regard, dexamethasone has only an additive effect when combined with bortezomib whereas it shows synergism with lenalidomide. If the association of LenDex with bortezomib or new proteasome inhibitors (carfilzomib or ixazomib) or with monoclonal antibody (elotuzumab or anti-CD38)42,66-69 demonstrates a clinically relevant superiority to LenDex, these triple combinations should be seriously considered in the relapse setting. If a patient is considered candidate for SCT, a short induction with bortezomib/dexamethasone or LenDex is most appropriate. If the patient fails proteasome-inhibitors and IMiD-based regimens, debulking chemotherapy with PACE (cisplatin, doxorubicin, cyclophosphamide, and etoposide) could be helpful.70 For patients with indolent relapses, when long-term use of the rescue therapy is not feasible because of drug toxicity (ie, causing peripheral neuropathy) or for those in whom future effective options are still possible we administer a fixed number of cycles. In contrast, in patients with aggressive relapses and in those in whom all the effective available options have been exhausted, we favor continued therapy until PD provided that the regimen has an acceptable long-term safety profile. A rescue ASCT should be considered in patients in whom the duration of response to the first ASCT has been ≥2 years, although the greatest benefit is obtained when the response duration to the first ASCT has been ≥3 years. We plan an Allo-RIC in patients relapsing within the first 2 years after ASCT, particularly in those with aggressive disease and/or poor cytogenetics at diagnosis.
Future directions
Despite significant advances in recent years, treatment of relapsed myeloma remains unsatisfactory. New generations of powerful agents from consolidated drug families, such as proteasome inhibitors (carfilzomib, ixazomib) or IMiDs (pomalidomide) are most promising. In 2 studies, the histone deacetylase inhibitors vorinostat and panobinostat added to bortezomib have resulted in statistically significant PFS prolongation. However, more studies aimed at improving the toxicity profile are needed.71,72 The monoclonal antibodies FRMF7 (elotuzumab)42 and anti-CD38 (daratumumab, SAR650984),66,73 particularly when associated with bortezomib/dexamethasone or lenalidomide/dexamethasone, may result in a relevant improvement. A newer exciting approach is immunotherapy with modified virus use,74 natural killer cell therapy,75,76 or chimeric antigen receptor strategies,77 combining activity against malignant plasma cells and T-cell receptors. The above and other potential effective drugs in MM78-86 are listed in Table 3. However, all of these newer agents are only used in clinical trials and are not yet available for most practitioners.
Newer generations of antimyeloma drugs
| Mechanism/Target . | Drugs . |
|---|---|
| Proteasome inhibitors | Carfilzomib |
| Ixazomib | |
| Immunomodulatory drug | Pomalidomide |
| PI3K/AKT/mTOR inhibitors | Temsirolimus |
| Everolimus | |
| Perifosine | |
| Histone deacetylase inhibitor | Panobinostat |
| Vorinostat | |
| Alkylating plus purine analog | Bendamustine |
| p38/JNK activators | Plitidepsin |
| Hypoxia-activated alkylator | TH-302 |
| DNA-damaging agents | Zalypsis |
| Kinesin spindle protein inhibitor | Arry-520 |
| Monoclonal antibodies | Elotuzumab |
| Daratumumab | |
| SAR650984 |
| Mechanism/Target . | Drugs . |
|---|---|
| Proteasome inhibitors | Carfilzomib |
| Ixazomib | |
| Immunomodulatory drug | Pomalidomide |
| PI3K/AKT/mTOR inhibitors | Temsirolimus |
| Everolimus | |
| Perifosine | |
| Histone deacetylase inhibitor | Panobinostat |
| Vorinostat | |
| Alkylating plus purine analog | Bendamustine |
| p38/JNK activators | Plitidepsin |
| Hypoxia-activated alkylator | TH-302 |
| DNA-damaging agents | Zalypsis |
| Kinesin spindle protein inhibitor | Arry-520 |
| Monoclonal antibodies | Elotuzumab |
| Daratumumab | |
| SAR650984 |
JNK, c-Jun N-terminal kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase.
For the time being, we can anticipate the introduction of the currently investigated recent treatment combinations as well as the progressive incorporation of a new generation of drugs with more specific molecular targets in our myeloma treatment programs.
Acknowledgments
This work has been supported in part by grants RD12/0036/0046 and PI12/01093 from Instituto de Salud Carlos III (Ministerio de Economía y Competitividad, Cofinanciado por Fondo Europeo de Desarrollo Regional [FEDER], Unión Europea, Una Manera de Hacer Europa).
Authorship
Contribution: J.B., L.R., and C.F.d.L. collected data, wrote and critically reviewed the manuscript, and gave final approval.
Conflict-of-interest disclosure: J.B. has received honoraria from Janssen, Celgene, Amgen, and The Binding Site, as well as grant support from Janssen. L.R. and C.F.d.L. have received honoraria from Janssen and Celgene.
Correspondence: Joan Bladé, Servei d’Hematologia, Hospital Clínic de Barcelona, Villarroel 170, 08036 Barcelona, Spain; e-mail: jblade@clinic.ub.es.
References
Author notes
J.B. and C.F.d.L. contributed equally to this manuscript.