Case presentation
A 40-year-old obese black woman developed abdominal pain with progressive generalized weakness over several days. Physical examination was normal except for several small bruises on her extremities. Laboratory data revealed the following: hemoglobin, 5.0 g/dL; platelet count, 4000/µL; creatinine, 0.8 mg/dL; lactate dehydrogenase, 1364 U/L. Peripheral blood smear revealed many schistocytes. Acquired thrombotic thrombocytopenic purpura (TTP) was diagnosed, and treatment was initiated with daily plasma exchange (PEX) and prednisone (1 mg/kg/day). The subsequent report of ADAMTS13 (a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13) activity <5% with an inhibitor titer of 3 Bethesda Units supported the diagnosis of TTP. After 6 days of PEX, she was asymptomatic, and her platelet count had been normal for 2 days, reaching 178 000/µL; PEX was stopped.
Should rituximab have been given as initial treatment of TTP, in addition to PEX and corticosteroids?
If her platelet count had decreased to 13 000/µL 3 days after PEX was stopped (an exacerbation, indicating refractory TTP), should rituximab be used in addition to resuming daily PEX?
The patient remained well following discontinuation of PEX and corticosteroids. Three years after her first episode, routine measurement of ADAMTS13 activity while she was asymptomatic documented activity of 57%. One year later, while still asymptomatic with normal laboratory evaluation, her ADAMTS13 activity was 4% with an inhibitor titer of 1 Bethesda Unit.
Should rituximab be given to prevent a relapse of TTP?
Introduction
In 1991, a new era of effective treatment of acquired TTP began with documentation of the efficacy of PEX, reducing mortality of acute episodes from 90% to 28%.1 In 1998, acquired TTP was found to be associated with a deficiency of ADAMTS13 caused by an inhibitor,2,3 suggesting an autoimmune etiology and providing the basis for corticosteroids as conventional initial treatment in addition to PEX.4 In 1997, rituximab (Rituxan, MabThera, Zytux) was approved by the US Food and Drug Administration for the treatment of non-Hodgkin lymphomas. Although rituximab is not approved for treatment of TTP, it has been used off-label with increasing frequency since 2002.5,6 The appropriate role of rituximab in the management of patients with TTP remains uncertain.
This review focuses on 3 periods during the course of TTP for which rituximab has been advocated: (1) for initial treatment of an acute episode, together with PEX and corticosteroids; (2) for treatment of a refractory episode (unsatisfactory response to initial treatment with PEX and corticosteroids), and (3) for prophylaxis in asymptomatic patients with severe ADAMTS13 deficiency following recovery but no clinical evidence of TTP to prevent relapse.
Methods
Clinical definitions
Response, exacerbation, remission, and relapse of TTP have been previously defined.7 Refractory TTP may be defined as failure to achieve a satisfactory response with PEX and corticosteroids, a decreased platelet count after an initial increase, the occurrence of new neurologic abnormalities while continuing treatment with PEX and corticosteroids, or an exacerbation after stopping PEX. We defined initial treatment of TTP with rituximab (together with PEX and corticosteroids) as beginning rituximab within the first 3 days of admission and diagnosis.8 We used these definitions to classify the studies included in this review into the 3 periods described above, although the individual studies may have used slightly different definitions.
Literature search
We searched seven databases on October 1, 2014 to identify articles describing treatment of TTP with rituximab. We used the Ovid interface to search (1) MEDLINE, (2) EMBASE, and (3) Cochrane Database of Systematic Reviews. We used the Web of Knowledge interface to search (4) Current Contents and (5) Web of Science. The EBSCO interface was used to search the (6) Cumulative Index to Nursing and Allied Health Literature database. We searched the (7) PubMed interface, which includes MEDLINE and additional databases. Articles were identified by using the MeSH terms or keywords thrombotic thrombocytopenic purpura and rituximab (or its trade names, Rituxan, MabThera, Zytux). The references of reviewed articles and the authors’ files were also reviewed.
Article selection
English language articles that administered ≥1 doses of rituximab for patients with TTP in the setting of initial TTP treatment, treatment of a refractory TTP episode, or in asymptomatic patients with prior TTP and decreased ADAMTS13 activity, and that measured ≥1 clinical outcome were included. Although documentation of severe ADAMTS13 deficiency (activity < 10%) with an ADAMTS13 inhibitor is the defining feature of acquired TTP, we did not exclude articles in which ADAMTS13 was not measured, because initial reports of rituximab treatment were published when ADAMTS13 measurements were not always available. We were aware of numerous case reports using rituximab for refractory TTP and decided a priori to exclude articles reporting <10 patients with rituximab used in this setting. For the other 2 indications (initial treatment and prophylaxis), we included all articles, including single patient case reports. We excluded single patient case reports when the patients had an additional diagnosis (eg, systemic lupus erythematosus, HIV infection, post-transplantation). However, if such patients were included in selected case series, in which the majority of the patients did not have additional diagnoses, we did not exclude these individual patients but noted this in our supplemental Tables available on the Blood Web site. We excluded articles that did not present patient data for any of the 3 indications that were the focus of this review. Article selection and data extraction were performed independently by each author.
Grading the evidence
The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system was used to classify recommendations as strong (grade 1) or weak (grade 2) based on the balance of benefits and risks and the confidence in these estimates.9 We recognize that patient values and preferences may influence the interpretation of our management recommendations. The quality of evidence was classified as high (grade A), moderate (grade B), or low (grade C) based on the study design, consistency of results, and directness of the evidence.
Results
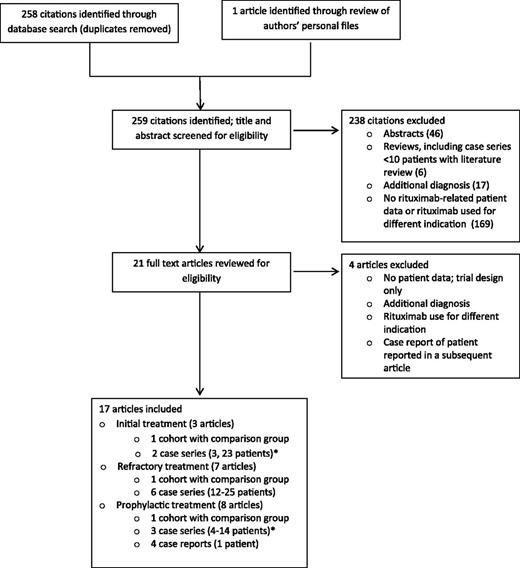
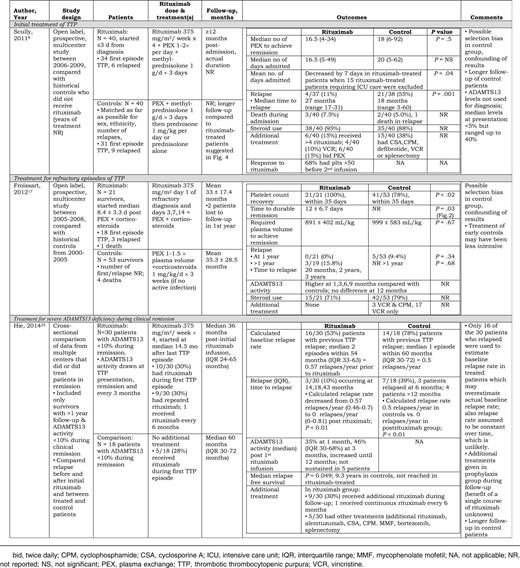
The literature search identified 258 citations and 1 additional article was identified through personal files; titles and abstracts were screened for eligibility. Of these, 238 were excluded and 21 underwent full text review. A total of 17 articles were included in our analysis (Figure 1): 3 articles (66 patients) evaluated rituximab for initial treatment together with PEX and corticosteroids8,10,11 ; 7 articles (119 patients) evaluated rituximab for refractory TTP12-18 ; and 8 articles (57 patients) evaluated rituximab for prophylaxis in asymptomatic patients with decreased ADAMTS13 activity following recovery from an acute episode but no clinical evidence for TTP.10,19-25 One article included 2 case series: patients receiving initial treatment with rituximab and asymptomatic patients receiving rituximab prophylaxis.10 One patient was reported in 3 articles19,22,26 ; for this review, we cite the most recent report.22 Patients from 2 cohorts appeared to be reported in multiple publications.8,10,12,17,19,25 Among the 17 included articles, 3 were observational studies that included a comparison group without rituximab treatment. These 3 articles provide the highest quality data available to assess the effect of rituximab for each of the 3 indications (Table 1).8,17,25 In 2 of these 3 articles,17,25 all patients in the rituximab-treated group and the historical controls had documented severe, acquired ADAMTS13 deficiency. In the third article, all patients had ADAMTS13 measurements; median ADAMTS13 activity was <5% for both groups, but values ranged up to 40%.8 All 17 included articles are described in greater detail in the supplemental Tables.
Literature search data. *One article included 2 case series: one case series of patients receiving initial treatment with rituximab and another case series of asymptomatic patients receiving rituximab prophylaxis.
Literature search data. *One article included 2 case series: one case series of patients receiving initial treatment with rituximab and another case series of asymptomatic patients receiving rituximab prophylaxis.
Rituximab for initial treatment of TTP
Three articles8,10,11 administered rituximab as initial treatment in addition to PEX and corticosteroids for patients with a first or relapsed episode of TTP. One observational study compared 40 rituximab-treated patients with 40 historical controls who did not receive rituximab (Table 1).8 The controls in this study were matched as far as possible for 3 variables: gender, ethnicity, and number of relapses; selection was based on the completeness of data. Six of the rituximab-treated patients and 9 of the control patients had ≥1 previous episode of TTP; these patients were not reported separately. This study reported that rituximab decreased the duration of hospitalization by 7 days when the 15 patients admitted to an intensive care unit (ICU) were excluded from the rituximab group. The number of patients in the control group that required ICU admission was not described. Ninety-five percent (38 of 40) of patients in the rituximab group received corticosteroids (typically methylprednisolone, 1000 mg/day for 3 days) in addition to PEX; 88% (35/40) of historical controls received corticosteroids (regimen not described). Fifteen control patients received treatments in addition to PEX and corticosteroids. The remission rate was 93% (37 of 40) among the patients treated with initial rituximab and was 95% (38 of 40) among the historical control patients. The frequency of relapse was 55% in the historical control patients and 11% in the rituximab-treated patients. Patients in the rituximab group were followed for ≥12 months after admission, but the actual duration of follow-up in both rituximab and control patients was not reported. The survival curve presented in the article suggests that the control patients were followed longer. In a second retrospective cohort where there was not a non-rituximab comparison group, the outcomes of 54 patients treated with rituximab administered ≤3 days from admission, which included 31 patients from the previous study,8 were compared with 32 patients treated with rituximab administered >3 days from admission.10 However, because patient selection for earlier or later rituximab administration was not described and 31 patients from the early rituximab group were enrolled in the previous trial,8 these comparisons are uninterpretable due to numerous potential confounders.
Summary.
Rituximab treatment of refractory episodes of TTP
Seven articles reported 119 patients treated with rituximab for a refractory first or relapsed episode of TTP.12-18 One observational study compared the outcome of 21 rituximab-treated patients with refractory TTP (defined as a platelet count increase less than twofold after 4 days of PEX) with 53 historical control patients not treated with rituximab, from the years prior to common rituximab use (Table 1).17 Three of the 21 rituximab-treated patients had a previous history of TTP but had not received rituximab. Previous episodes of TTP were not described for the control patients. Patients treated with rituximab all had platelet count recovery within 35 days in contrast to 78% of control patients (P < .02), and the time to achieve a normal platelet count was decreased compared with control patients (P = .03). Although there was a nonsignificant decrease in relapse at 1 year in the rituximab-treated patients and no difference in long-term (>1 year) relapse between groups, the occurrence of relapse may have been delayed by rituximab treatment. Data from 5 other articles similarly demonstrated, that following administration of rituximab, complete responses were achieved in 83% to 100% of patients with refractory TTP.12-16 One article only reported patients who achieved remission.18 Relapse rates after rituximab treatment ranged from 0% with a median follow-up of 10 months12 to 33% with a median follow-up of 73 months.18
Summary.
In patients with an episode of refractory TTP, addition of rituximab to PEX and corticosteroids increases platelet counts in >80% of patients and may decrease the time required to achieve a platelet count response. The frequency of relapse in rituximab-treated patients may be decreased compared with control patients in the short term, but may also represent a delay in relapse and not differ from control patients in long-term follow-up.
Rituximab treatment of asymptomatic patients in remission who have ADAMTS13 deficiency
Eight articles10,19-25 reported 57 asymptomatic patients following recovery from an acute episode with no clinical evidence of TTP who were treated with rituximab for the observation of severely decreased ADAMTS13 activity (typically <10%). One observational cross-sectional study compared outcomes in 30 patients treated with rituximab (and other treatments) to 18 patients who were managed prior to the era of rituximab treatment or in centers where treatment of patients who were in remission with rituximab and other treatments was not the standard of care (Table 1).25 This study reported that rituximab and other treatments in this setting resulted in longer relapse-free survival compared with patients who did not receive rituximab (P = .049). The length of follow-up was longer in the control patients (median, 60 months) than in the treated patients (median, 36 months after the first prophylactic infusion of rituximab). Thirty percent (9/30) of the rituximab-treated patients received additional courses of rituximab; some patients were treated additional immunosuppressive agents and/or splenectomy; 1 patient received continuous rituximab infusions every 6 months, and 4 patients did not have a durable increase of ADAMTS13 activity with multiple treatments.
In the 2 largest studies,10,25 median ADAMTS13 activity was 35% at 1 month and 46% at 3 months,25 and in 16 of 17 episodes where ADAMTS13 was 6% or less prior to the first dose, activity increased to >21% by 3 months.10 In other case reports and series, increases in ADAMTS13 ranged from 20% to 100% measured from 4 weeks to 9 months after rituximab.19,21-24 However, approximately one-third of patients in the 2 largest studies did not achieve durable ADAMTS13 recovery with 1 course of rituximab.10,25
Summary.
Prophylactic treatment with rituximab may result in fewer TTP relapses, although follow-up was longer in control patients, favoring detection of relapse.25 In the largest study, 30% of patients had asymptomatic decreased ADAMTS13 activity during follow-up after initial prophylactic rituximab and received additional rituximab or other treatments, some of which have greater risks than rituximab.25 In some asymptomatic patients, sustained ADAMTS13 activity recovery does not occur even with multiple rituximab treatments. The effect of a single course of rituximab cannot be assessed.
Discussion
There are few publications addressing the 3 indications for rituximab treatment of TTP that were the focus of this review. There were no publications with high quality evidence; there were no randomized controlled trials and no observational studies with a well-matched, concurrent control group. Cohort studies can provide useful information when randomized trial data are not available. However, cohort studies are subject to selection bias and confounding due to differences in the baseline characteristics between the groups. For each of the 3 indications, there was 1 observational study with a comparison group, but the comparison groups in these studies had important limitations. First, the patients were retrospectively selected from a time period preceding the patient group receiving rituximab, introducing the potential for selection bias. Second, the frequency of corticosteroid use and other treatments for TTP were not controlled in these studies, which can confound the reported response rates. Third, shorter duration of follow-up in the treatment groups compared with the control groups potentially biased the results to observe fewer relapses in the treatment group. B-cell depletion after rituximab treatment is apparent for 9 to 18 months,12 and patient follow-up in studies documenting relapse rates needs to be sufficiently long to observe relapses following B-cell recovery. Rituximab may only delay, not prevent, relapse.
The limitations of the control group are of lesser importance when evaluating rituximab for refractory TTP, because additional treatment is required for patients unresponsive to standard initial treatments. The available studies suggest that >80% of refractory patients receiving rituximab have a satisfactory platelet count response with few serious side effects, but these studies observe no difference in long-term relapse rates. While a comparison group would control for the possibility that these refractory patients may have responded without rituximab, these patients are often critically ill with limited additional treatment options. The balance of risks and benefits in this setting supports rituximab use, because it appears to be effective in achieving a remission within a shorter time (Table 2).
Rituximab for the treatment of patients with TTP and for treatment of ADAMTS13 deficiency during remission: levels of evidence and interpretation
| Indication . | Key citation . | Grade of recommendation and evidence* . | Interpretation . |
|---|---|---|---|
| Initial treatment of TTP | Scully, 20118 | 2C | We suggest rituximab be considered for this indication. Rituximab may decrease the time to achieve remission and may delay subsequent relapse. |
| Treatment of refractory episodes of TTP | Froissart, 201217 | 1C | We recommend rituximab be considered for this indication. Patients with refractory TTP require treatment in addition to PEX and conventional corticosteroid regimens, and rituximab appears to be effective. |
| Treatment of severe ADAMTS13 deficiency during clinical remission | Hie, 201425 | 1C | We recommend against the use of rituximab for this indication. The benefit for relapse-free survival is marginal (P = .049). Patients in the rituximab group received multiple different treatments. The benefit of a single course of rituximab is not known. The natural history of ADAMTS13 activity following recovery from acquired TTP is not known. High-quality evidence is required before treatment of patients with no clinical evidence of TTP can be recommended. |
| Indication . | Key citation . | Grade of recommendation and evidence* . | Interpretation . |
|---|---|---|---|
| Initial treatment of TTP | Scully, 20118 | 2C | We suggest rituximab be considered for this indication. Rituximab may decrease the time to achieve remission and may delay subsequent relapse. |
| Treatment of refractory episodes of TTP | Froissart, 201217 | 1C | We recommend rituximab be considered for this indication. Patients with refractory TTP require treatment in addition to PEX and conventional corticosteroid regimens, and rituximab appears to be effective. |
| Treatment of severe ADAMTS13 deficiency during clinical remission | Hie, 201425 | 1C | We recommend against the use of rituximab for this indication. The benefit for relapse-free survival is marginal (P = .049). Patients in the rituximab group received multiple different treatments. The benefit of a single course of rituximab is not known. The natural history of ADAMTS13 activity following recovery from acquired TTP is not known. High-quality evidence is required before treatment of patients with no clinical evidence of TTP can be recommended. |
Grade 1 represents a strong recommendation; grade 2 represents a weak recommendation; and grade C represents the lowest quality of evidence.
The presence of a well-matched control group is of moderate importance in studies evaluating rituximab as initial treatment of TTP together with PEX and corticosteroids, because additional treatment of an acutely ill patient may provide additional benefit. Multiple observations document that some patients recover promptly from their acute episode of TTP with only PEX and corticosteroids and do not relapse with long-term follow-up. Therefore, the routine initial use of rituximab may not benefit all patients. When rituximab is used as initial treatment, patients who are destined to become refractory may receive earlier adjunctive treatment and may benefit. However, for patients who would have responded promptly to PEX and corticosteroids alone, a benefit is less certain, particularly regarding subsequent relapse. Because of the limitations of the comparison group in the principal study,8 the value of routine use of rituximab in the initial treatment of patients with an acute episode of TTP is uncertain (Table 2).
A well-matched control group, preferably in a randomized study, is critically important to evaluate rituximab for prophylaxis in asymptomatic patients with low ADAMTS13 activity following recovery from an acute episode but no clinical evidence of TTP. It remains uncertain if decreased ADAMTS13 activity in asymptomatic patients following recovery from TTP is a reliable predictor of future relapse. One case series of consecutive patients reported that relapse was more frequent among patients who have lower ADAMTS13 activity during remission,27 but the probability of future relapses and their time of occurrence remain uncertain. Furthermore, ADAMTS13 activity measurements performed with different techniques may not be equivalent. For example, ADAMTS13 activity measured in the same sample in the same laboratory by different assay techniques may report ADAMTS13 activity <10% in one assay and 30% in another.28 Therefore, even if severe ADAMTS13 activity was a reliable predictor of future relapse, one measurement using one technique may not be sufficient evidence for intensive treatment of a patient with no clinical evidence of TTP.
We believe that ADAMTS13 activity <10% may not be a reliable predictor of relapse. Unpublished data from the Oklahoma Registry document that among 52 asymptomatic patients who have had ≥3 annual measurements of ADAMTS13 activity during remission, 20 (38%) have had 1 to 8 annual measurements with ADAMTS13 activity <10%; none were treated. Fourteen (70%) of these 20 patients have not relapsed during 1 to 10 (median, 4) years of follow-up. Finally, the natural history of ADAMTS13 activity in patients following recovery from acquired TTP is not known. Among the 20 patients who have had ADAMTS13 activity <10% during remission, ADAMTS13 activity spontaneously increased to >10% in 15 patients and returned to >50% in 9 patients.
Important concerns remain regarding the use of prophylactic rituximab. (1) Duration of follow-up was likely insufficient to observe relapses in some studies. (2) Higher relapse rates were observed in the control patients in the cohort study with a comparison group,25 but control patients had longer follow-up than rituximab-treated patients. (3) Thirty percent of the rituximab-treated patients in this cohort study received additional treatments, including prolonged rituximab treatment, which were likely to influence the observed relapse rates.25 (4) In this cohort study, 13% of patients never achieved durable ADAMTS13 responses.25 In contrast to the refractory setting, these patients are clinically well and may need no treatment. Therefore, evidence that the benefits of rituximab exceed the risks must be much stronger. Given these limitations, rituximab in this setting is not recommended (Table 2).
Recommendations
We suggest rituximab be considered for initial treatment with PEX and corticosteroids in patients who present with an acute episode of TTP (grade 2C).
We recommend rituximab for patients who have a refractory episode of TTP despite PEX and corticosteroids (grade 1C).
We recommend against the use of rituximab in asymptomatic patients who have a severe deficiency of ADAMTS13 activity but no clinical evidence of TTP (grade 1C).
Patients’ values and preferences
Patients who have experienced multiple severe episodes of TTP may place a higher value on rituximab treatment that may decrease the duration of acute episodes or decrease the risk of recurrent episodes. These patients may also place a lower value on the inconvenience and potential risks of rituximab treatment. These values and preferences should be taken into account when assessing the role of rituximab.
There is an Inside Blood Commentary on this article in this issue.
Acknowledgments
This work was supported in part by National Institute of General Medical Sciences of the National Institutes of Health award U54GM104938.
Authorship
Contribution: W.L., S.K.V., and J.N.G. designed the study; performed the article selection, data abstraction, and analysis; and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James N. George, The University of Oklahoma Health Sciences Center, Room CHB-237, PO Box 26901, Oklahoma City, OK 73126-0901; e-mail: james-george@ouhsc.edu.